Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Algal biomass from the stable growth phase as a potential biosorbent for Pb(II) removal from water
Yinta Li abc,
Ling Xia*ad,
Rong Huanga,
Chenggong Xiaa and
Shaoxian Song*ad
abc,
Ling Xia*ad,
Rong Huanga,
Chenggong Xiaa and
Shaoxian Song*ad
aSchool of Resources and Environmental Engineering, Wuhan University of Technology, Luoshi Road 122, Wuhan, Hubei 430070, China. E-mail: xialing@whut.edu.cn; ssx851215@whut.edu.cn
bDoctorado Institucional de Ingeniería y Ciencia de Materiales, Universidad Autonoma de San Luis Potosi, Av. Sierra Leona 530, San Luis Potosi, C.P. 78210, Mexico
cDepartment of Food Engineering, Weihai Ocean Vocational College, Haiwan South Road 1000, Weihai, Shandong 264300, China
dHubei Key Laboratory of Mineral Resources Processing and Environment, Luoshi Road 122, Wuhan, Hubei 430070, China
First published on 11th July 2017
Abstract
In this study, the effect of the growth phase on the Pb(II) removal performance from water using Chlorella sp. QB-102 dry biomass was investigated. Optimum biosorption conditions were determined as a function of initial solution pH and contact time. Freundlich isotherm and pseudo-second-order kinetics models were found to be most applicable to Pb(II) adsorption on biomass from each growth phase. The maximum adsorption capacities of Pb(II) were found to be 205.5, 298.5 and 171.9 mg g−1 for logarithmic, stable and decline phases of biosorbents, respectively. The FT-IR, XPS and potentiometric titration results showed that the lack of phosphoryl groups caused the lowest adsorption efficiency for the decline phase, and the more effective active sites in carboxyl and higher site concentrations of hydroxyl and phosphoryl functional groups led to most of the bioadsorption occurring during the stable phase. Thus, algal biomass from the stable phase can be used as a potential adsorbent for heavy metal removal from water.
1 Introduction
With the rapid development of industrialization, plenty of industrial effluents containing heavy metals are discharged into unpolluted natural water bodies.1,2 These heavy metals can be accumulated in living organisms, including humans, animals and plants. Unlike organic pollutants, heavy metals are non-biodegradable and remain in the environment available to cause pollution.1,3 Lead is one of the common contaminants, which causes various health hazards. It is harmful to nervous, digestive, blood, endocrine and reproductive systems.2 Therefore, the permissible limit of lead in potable water should not exceed 0.01 mg L−1 according to the regulation of the WHO.Conventional methods for heavy metal removal from wastewater include chemical precipitation, complexation, ion exchange, reverse osmosis, filtration, and coagulation. Nevertheless, these processes are limited by high capital and operational cost.1,4 Biosorption has emerged as a potential alternative to conventional methods for the heavy metals removal due to its cost effectiveness, high selectivity and efficiency, and good removal performance.5 In the last decades, different sources of biomass, such as bacteria, fungi, and algae have been screened and studied extensively.1,6,7 Among them, algae including macroalgae and microalgae have been proven to be highly effective, reliable and predictable in the heavy metals removal from aqueous solutions.6,8–10 It has been found that algal biomass are rich in organic ligands or functional groups, such as carboxyl, hydroxyl, phosphate, amine groups, and so on. These functional groups play dominant roles in the heavy metals removal. However, most researches mainly concern the potential of algal biomass as adsorbents for heavy metal removal, or the modification of algal biomass for better biosorption of heavy metals.6,8–10 Well, with the growth time of algal cells, the biochemical component and also surface properties as well as the biomass concentration from different growth phases can be changed. To the best of our knowledge, there are rare reports focus on the effect of growth phase on the surface properties of algal biomass and further effect on the removal performances of heavy metal.
The objective of this study was to screen the best harvest stage for algal biomass as adsorbent for efficient lead removal from water. Chlorella sp. QB-102, isolated form a lead–zinc tailing wasteland was selected as the research subjective. The study gave detailed analysis of lead adsorption behaviors on algal dry biomass from different growth stages in terms of adsorption isotherms and kinetics experiments. Meanwhile, the effect of growth stage on biomass surface characterization has been investigated. The explanations for adsorption mechanism have been supported by FT-IR, XPS and potentiometric titration.
2 Materials and methods
2.1 Algal-based adsorbents preparation
Chlorella sp. QB-102 was isolated from the tailing wasteland in Qibo mountain lead–zinc mine, Shanggao, Jiangxi provence in China (28°09′N, 114°52′E) and identified by classical morphological methods. Stock cultures were grown in BG-11 medium containing 1.5 g NaNO3, 30 mg K2HPO4, 36 mg CaCl2·2H2O, 6 mg ammonium citrate monohydrate, 6 mg ammonium ferric citrate, 1 mg EDTA, 2.86 μg H3BO3, 1.81 μg MnCl2·4H2O, 0.222 μg ZnSO4·7H2O, 0.39 μg NaMoO4·5H2O, 0.079 μg CuSO4·5H2O, 0.050 μg CoCl2·6H2O in 1 L sterile distilled water.11For trials, Chlorella sp. QB-102 cells were cultivated in BG11 medium at 25 °C with a light/dark cycle of 14 h/10 h. Samples were taken every day to measure the biomass concentration. The biomass from exponential (day 14), stable (day 18) and declined phase (day 29) as presented in Fig. 1 was centrifuged harvested, washed three times and then freeze-dried (FD-1B-50, Ouman International Industry Co., Ltd., China) for the subsequential biosorption experiments.
2.2 Reagents
A stock solution containing 1000 mg L−1 Pb(II) was prepared by Pb(NO3)2 in deionized water. All chemicals were of analytical grade and obtained from the Xinyang chemical reagent (China). The pH of the test solution was adjusted with 0.1 M HNO3 or 0.1 M NaOH. Deionized water was used throughout this study (Millipore Q, USA).2.3 Biosorption experiments
The adsorption experiments of Pb(II) were implemented in triplicate in 100 mL Erlenmeyer flasks with 50 mL of Pb(II) solution combined with 10 mg of adsorbents in a mechanical shaker at the speed of 180 rpm at 25 °C. The influence of pH on Pb(II) adsorption was conducted within a pH range of 2 to 6 at an initial Pb(II) concentration of 100 mg L−1 for 6 h. Biosorption isotherm experiments were performed by ranging the initial concentration of Pb(II) from 10 to 300 mg L−1 at pH 5.5 for 6 h. The kinetic experiments were studied by varying contact time intervals in the range of 0.5–300 min at an initial Pb(II) concentration of 100 mg L−1 at pH 5.5. Afterwards, the mixture was filtered through 0.22 μm filter membrane and the supernatant was used for Pb(II) determination.2.4 Analytical methods
| BC = 0.255 × OD680 (R2 = 0.9986) | (1) |
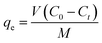 | (2) |
2.5 Isotherm and kinetics modelling
Langmuir (eqn (3)),17 Freundlich (eqn (5))18 and Temkin (eqn (6))13 models were used to fit the biosorption data.
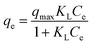 | (3) |
RL, a dimensionless constant as separation factor or equilibrium parameter, can determine whether the essential biosorption characteristics of the Langmuir isotherm is favorable or unfavorable, defined as following:17
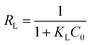 | (4) |
| qe = KFCe1/n | (5) |
qe = A + B![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ce Ce
| (6) |
The kinetic parameters were evaluated by using the pseudo-first order (eqn (7)),19 pseudo-second-order (eqn (8)),20 intra-particle diffusion (eqn (9))21 models, the initial sorption rate (eqn (10)) and the half-sorption time (eqn (11))22,23 expressed as follows:
ln(qe − qt) = ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) qe − k1t qe − k1t
| (7) |
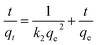 | (8) |
| qt = C + knt0.5 | (9) |
| h = k2qe2 | (10) |
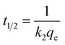 | (11) |
3 Results and discussion
3.1 Influences of growth phase on Pb(II) biosorption
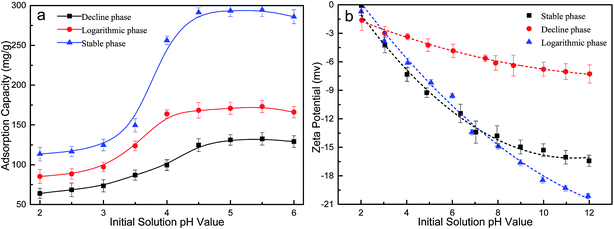
Fig. 2b shows the zeta potential of algal biomass from three growth phases as a function of pH. The zeta potential of algal biomass from all the growth phases was negatively charged within the pH range from 2.0 to 7.0, and the net zeta potential increased with pH increasing. Thus, at pH 2.0 where the ionization degree of superficial functional groups of biomass in terms of zeta potential is lower, the biosorption capacity of Pb(II) was lower. Meanwhile, the increase in the initial solution resulted in the increase of net negatively charged biomass surface, in consequence the number of electrostatic interactions increased. The stable phase biomass showed the lowest net negative surface charge when pH was above 2, which can explain that the lowest biosorption capacity of Pb(II) compared with that from other two growth phases. In addition, at pH values higher than 5.5, the decrease in biosorption capacity probably due to conversion of Pb(II) ions to hydroxylated complexes of Pb(II), resulting into hydroxide precipitation. Consequently, an initial solution pH of 5.5 was considered as the optimum value and was used in all the following experiments.
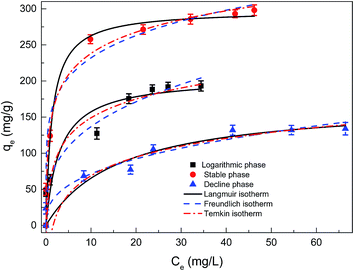 | ||
| Fig. 3 Adsorption isotherms of dried Chlorella sp. QB-102 biomass of each growth phase, the experimental data were fitted with Langmuir, Freundlich and Temkin models (25 °C, pH 5.5 for 6 h). | ||
| Langmuir | Freundlich | Temkin | |||||||
|---|---|---|---|---|---|---|---|---|---|
| qe (mg g−1) | KL (L g−1) | R2 | KF (L g−1) | 1/n | R2 | A | B | R2 | |
| Logarithmic phase | 205.5 | 0.326 | 0.9672 | 64.83 | 0.324 | 0.9729 | 60 | 37.55 | 0.8715 |
| Stable phase | 298.2 | 0.738 | 0.9912 | 153.13 | 0.181 | 0.9957 | 100 | 42.56 | 0.8939 |
| Decline phase | 171.9 | 0.061 | 0.9761 | 30.71 | 0.367 | 0.9861 | −20 | 37.35 | 0.8861 |
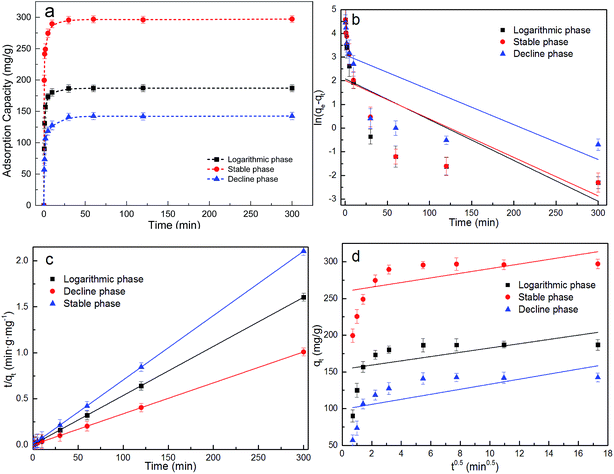
As shown in Table 1, the KF value (153.1 L g−1) of Pb(II) adsorbed on the dried Chlorella sp. QB-102 biomass from stable phase was significantly higher than that from logarithmic phase (61.8 L g−1) or decline phase (30.7 L g−1). Correspondingly, the highest maximum biosorption capacity was also obtained at stable phase from Langmuir fitting, valued at 298.2 mg g−1, which was 1.5 times higher against that at exponential phase (205.5 mg g−1) and 1.8 times against that at declining phase (171.9 mg g−1). The largest biosorption capacity was higher than most biosorbents as listed in Table 2. These results indicated that the biomass from stable growth phase showed the best performances as an adsorbent for Pb(II) removal from water.
| Adsorbent | pH | Adsorption capacity (mg g−1) | Contact time | References |
|---|---|---|---|---|
| Pelvetia canaliculata | 5.0 | 300.1 | 100 h | (Costa, Vilar, Botelho, da Silva, & Boaventura, 2010) |
| Algal strains | 3.0–5.0 | 50–208 | 30 min | (Romera, González, Ballester, Blázquez, & Muñoz, 2007) |
| Sargassum sp. | 5 | 240.3 | 60 min | (Sheng, Ting, Chen, & Hong, 2004) |
| Lactarius scrobiculatus | 5.5 | 56.2 | 60 min | (Anayurt, Sari, & Tuzen, 2009) |
| Ulva sp. | 5 | 287.6 | 60 min | (Sheng et al., 2004) |
| Bacillus sp. L14 | 5.0–5.5 | 280.02 | 5 min | (Luo et al., 2014) |
| Cabbage waste | 6.0–6.5 | 40.963–50.216 | 3 h | (Hossain et al., 2014) |
| Fe3O4-treated waste litchi peel | 6.0 | 78.74 | 120 min | (R. Jiang et al., 2015) |
| Chlorella sp. QB-102 | 5.5 ± 0.3 | 297.1 | 5 min | This study |
The experimental data were further fitted to the pseudo-first-order, pseudo-second order and intra-particle diffusion models. Linear plots of log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (qe − qt) versus t, t/qt versus t and qt against t0.5 were illustrated in Fig. 4b–d, and the associated kinetic parameters and the corresponding linear regression R2 were summarized in Table 3. It showed that the experimental data were best fitted with the pseudo-second-order model according to R2 for biomass from all three growth phases. It was suggested that the biosorption of Pb(II) on the algal biomass was chemisorption.
(qe − qt) versus t, t/qt versus t and qt against t0.5 were illustrated in Fig. 4b–d, and the associated kinetic parameters and the corresponding linear regression R2 were summarized in Table 3. It showed that the experimental data were best fitted with the pseudo-second-order model according to R2 for biomass from all three growth phases. It was suggested that the biosorption of Pb(II) on the algal biomass was chemisorption.
| Pseudo-first-order kinetics | Pseudo-second-order kinetics | Intra-particle diffusion model | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| qe,exp (mg g−1) | qe,cal (mg g−1) | k1 (min−1) | R2 | qe,cal (mg g−1) | k2 (min−1) | R2 | h (mg g−1 min−1) | t1/2 (min) | Kn (mg g−1 min−1) | C | R2 | |
| Logarithmic phase | 187.1 | 182.3 | 1.222 | 0.6736 | 188.8 | 0.0111 | 1 | 395.6 | 0.4772 | 16.55 | 12.074 | 0.2294 |
| Stable phase | 297.1 | 287.8 | 2.001 | 0.7217 | 295.7 | 0.0128 | 1 | 1119 | 0.2642 | 25.63 | 19.71 | 0.2722 |
| Decline phase | 142.5 | 136.7 | 0.813 | 0.6970 | 142.6 | 0.0088 | 1 | 179.0 | 0.7968 | 11.47 | 7.51 | 0.3841 |
In addition, it was worth mentioning that the biosorptive capacity of Pb(II) on dried Chlorella sp. QB-102 biomass of the stable phase is faster than those of the logarithmic and decline phase based on the h and t1/2 values (Table 3). The h and qe values of dried Chlorella sp. QB-102 biomass of the stable phase were more than 3 times and 1.5 times higher than those of the logarithmic and decline phase, respectively.
Given that the algal biomass from stable phase for Pb(II) removal showed the best performances in terms of higher biosorption capacity and shorter reaction time, it was recommended that the harvesting operation can be implemented when the algal cells grow to a stable stage when using as algal-based adsorbents.
3.2 FT-IR spectroscopy
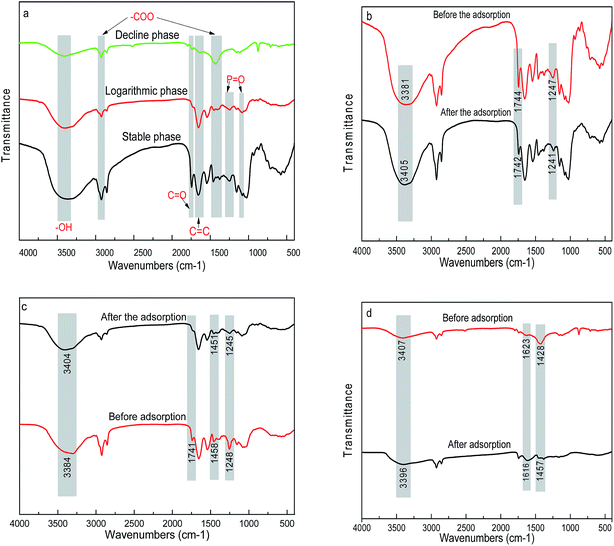
The FT-IR spectra of biosorbents from three growth stages are shown in Fig. 5a. The broad and intense adsorption band at around 3303 cm−1 was attributed to the bending vibration of the water molecule and –OH stretching frequency in carbohydrates, protein and lipids.4 The peaks at around 2924 and 1458 cm−1 were assigned to the asymmetric stretching bands of carboxylates groups.20 The peaks at 1610–1634 cm−1 was assigned to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching mode from peptide of amide I.25 The above functional groups were all present in the biosorbents from the three growth stages. However, it was worth mentioning that the characteristic features were the peaks at 1248 and 1080 cm−1 only presented in the biomass from logarithmic and stable phases, attributed to the P
C stretching mode from peptide of amide I.25 The above functional groups were all present in the biosorbents from the three growth stages. However, it was worth mentioning that the characteristic features were the peaks at 1248 and 1080 cm−1 only presented in the biomass from logarithmic and stable phases, attributed to the P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching bands.15,26
O stretching bands.15,26
After biosorption of Pb(II), the broad peaks shifted from 3387 to 3405 cm−1 for the biomass from all three growth phases, which might be attributed to the complexation of Pb(II) with free or bonded –OH groups (Fig. 5b–d). The variation in peaks at 1744 and 1247 cm−1 in algal biomass of logarithmic growth phase represented –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups in carboxylic acids and phosphate groups stretching vibration due to complexation of Pb(II) with these sites. As for the stable phases, besides variations of –C
O groups in carboxylic acids and phosphate groups stretching vibration due to complexation of Pb(II) with these sites. As for the stable phases, besides variations of –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and –P
O and –P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups, a shift of peak at 1458 cm−1 was observed, which considerably might be due to the complexation between Pb(II) and –COOH groups. In contrast, the decline phase lacking phosphate groups showed a variation peaks located at 1623 and 1428 cm−1, which might be attributed to the reaction between Pb(II) with –C
O groups, a shift of peak at 1458 cm−1 was observed, which considerably might be due to the complexation between Pb(II) and –COOH groups. In contrast, the decline phase lacking phosphate groups showed a variation peaks located at 1623 and 1428 cm−1, which might be attributed to the reaction between Pb(II) with –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and –COOH groups. It can be concluded that the growth stages significantly influenced the surface functional groups of algal biomass, which can be attributed to the gradually depletion of the nutrients in the medium with the cultivation time of the serial growth phases. The changes in the functional groups at different growth phases further affected the biosorption capacity of heavy metals.
O and –COOH groups. It can be concluded that the growth stages significantly influenced the surface functional groups of algal biomass, which can be attributed to the gradually depletion of the nutrients in the medium with the cultivation time of the serial growth phases. The changes in the functional groups at different growth phases further affected the biosorption capacity of heavy metals.
3.3 X-ray photoelectron spectroscopy
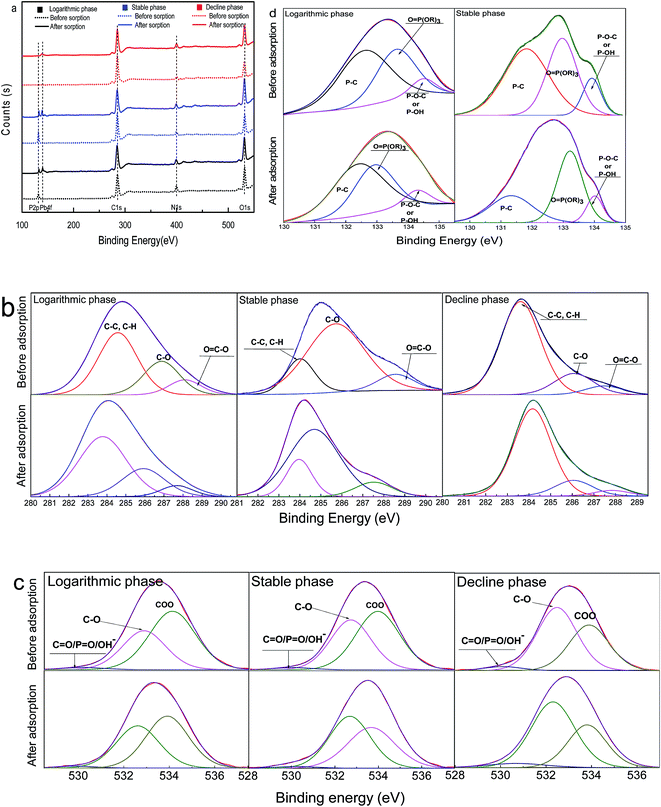
To further explore the Pb(II) biosorption mechanism, the biomass of dried Chlorella sp. QB-102 at three growth phase before and after Pb(II) loading were analyzed by XPS and shown in Fig. 6. It can be seen from Fig. 6a, the main compositions of the biosorbents for all three stages were C, O and N, however, P for only stable and decline phase, which was consistent with the observation in FT-IR (Fig. 6a). After Pb(II) was adsorbed, two new peaks appeared at the binding energy of 141.4 eV and 136.2 eV for all the samples, which belonged to Pb 5s and Pb 4f, respectively.27 Hence, it can be concluded that Pb was successfully absorbed onto the surface of biomass from all three growth stages.The high-resolution XPS spectra of C 1s and O 1s for dried Chlorella sp. QB-102 biomass of each growth phase before and after Pb(II) biosorption was shown Fig. 6b and c, respectively. As summarized in Table 4, C1 peaks were resolved into the following components peaks: and C–C and C–H, around 284 eV; C–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and C–OH, around 286 eV; O
O and C–OH, around 286 eV; O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–OR and O
C–OR and O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–OH, around 287 eV. O1 peaks were also resolved into three component peaks: the small peak at around 529 eV represented O
C–OH, around 287 eV. O1 peaks were also resolved into three component peaks: the small peak at around 529 eV represented O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) P–O− or O
P–O− or O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–O− from phosphate or carboxylate; the strong peak at around 532 eV was associated with C–O–(C, H, P) in alcohols, acetal, or esters; and the peak at around 534 eV was attributed to C–O (carboxyl and ether).28–30 In addition, the intensity of carbon peak was considerably decreased while oxygen peak was increased on surface of the metal loaded biosorbents of each growth phase. The quantity of C in C–C
C–O− from phosphate or carboxylate; the strong peak at around 532 eV was associated with C–O–(C, H, P) in alcohols, acetal, or esters; and the peak at around 534 eV was attributed to C–O (carboxyl and ether).28–30 In addition, the intensity of carbon peak was considerably decreased while oxygen peak was increased on surface of the metal loaded biosorbents of each growth phase. The quantity of C in C–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O/C–OH and O
O/C–OH and O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–OR/O
C–OR/O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–OH was considerably decreased for the biosorbents of each growth phase after Pb(II) was adsorbed, so did the quantity of O in O
C–OH was considerably decreased for the biosorbents of each growth phase after Pb(II) was adsorbed, so did the quantity of O in O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) P–O−/O
P–O−/O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–O−. The shift in the binding energies and the decreased area ratio in these groups after Pb(II) biosorption for the samples of each growth stage was likely to be caused by the binding of lead ions onto the oxygen atoms. It was worth mentioning that the carboxyl groups (COO−) at around 534 eV was found decreased in the biosorbent-Pb(II) of stable and decline phase, however, not the logarithmic phase. The observation was consistent with the results of FT-IR.
C–O−. The shift in the binding energies and the decreased area ratio in these groups after Pb(II) biosorption for the samples of each growth stage was likely to be caused by the binding of lead ions onto the oxygen atoms. It was worth mentioning that the carboxyl groups (COO−) at around 534 eV was found decreased in the biosorbent-Pb(II) of stable and decline phase, however, not the logarithmic phase. The observation was consistent with the results of FT-IR.
| Logarithmic phase | Stable phase | Decline phase | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Biomass | Biomass + Pb(II) | Biomass | Biomass + Pb(II) | Biomass | Biomass + Pb(II) | ||||||||
| EB (eV) | % | EB (eV) | % | EB (eV) | % | EB (eV) | % | EB (eV) | % | EB (eV) | % | ||
| C 1s | C–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O/C–OH O/C–OH |
286.4 | 31.83 | 286.1 | 20.31 | 285.6 | 41.72 | 285.1 | 23.67 | 286.5 | 17.31 | 286.3 | 10.38 |
O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–OR/O C–OR/O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–OH C–OH |
287.9 | 12.79 | 287.7 | 9.46 | 288.2 | 11.30 | 287.6 | 7.22 | 288.3 | 6.82 | 288.0 | 4.11 | |
| O 1s | C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O/P O/P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O/OH– O/OH– |
529.7 | 2.91 | 529.7 | 1.27 | 529.8 | 4.90 | 529.6 | 2.87 | 529.9 | 4.84 | 530.2 | 3.51 |
| C–O | 532.8 | 43.41 | 532.6 | 44.52 | 532.6 | 42.54 | 532.5 | 47.09 | 532.5 | 63.53 | 532.3 | 67.63 | |
| COO− | 534.0 | 54.68 | 534.1 | 52.21 | 533.8 | 53.56 | 533.8 | 46.04 | 534.2 | 32.63 | 534.0 | 30.86 | |
| P 2p | P–C | 132.5 | 27.28 | 132.4 | 15.97 | 131.7 | 40.91 | 131.3 | 13.57 | ||||
O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) P(OR)3 P(OR)3 |
133.6 | 11.66 | 133.6 | 8.65 | 133.3 | 42.05 | 133.3 | 12.99 | |||||
| P–O–C/P–OH | 134.51 | 5.29 | 134.47 | 2.19 | 134.11 | 17.04 | 134.0 | 5.83 | |||||
Fig. 6d was the P 2p XPS spectra for the samples from logarithmic and stable phases before and after Pb(II) loading. It was noted that both intensity and relative area of P 2p obviously decreased after biosorption of Pb(II). It was observed that after Pb(II) adsorption, the position of P 2p peak shifted to a lower energy, reducing by 0.89 and 0.80 eV respectively for logarithmic and stable phases, indicating that the relevant chemical reaction occurred between Pb(II) and the biosorbents from stable and decline growth phase during the biosorption process. The P 2p spectra can be decomposed into three components 131.50, 133.00 and 134.20 eV, which can be assigned to P–C, O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) P(OR)3 and P–O–C (phosphate),31–33 respectively. The relative area ratio of the three P-binded functional groups considerably reduced for the biosorbents from both exponential and stable phases after Pb(II) was adsorbed, indicating that all the phosphorus functional groups involved in the complexation reactions with Pb2+.
P(OR)3 and P–O–C (phosphate),31–33 respectively. The relative area ratio of the three P-binded functional groups considerably reduced for the biosorbents from both exponential and stable phases after Pb(II) was adsorbed, indicating that all the phosphorus functional groups involved in the complexation reactions with Pb2+.
3.4 Potentiometric titration
The species and concentrations of functional groups on the surface of dried Chlorella sp. QB-102 biomass of each growth phase were determined by PT. The pK values, site densities (mol kgdw−1) and corresponding functional groups were summarized in Table 5. The first pK was attributed to the carboxyl groups (pK = 2–6).14,15 The pK2 and pK3 were at around pH 6.7 and pH 8.7, which can be attributed to the phosphoric (pK = 5.6–7.2) and amine groups (pK = 8.6–9.0) that exist in the form of bioorganic molecules on the cell wall of microalgae. The pK4 appeared at around pH 10 and this buffering capacity is usually attributed to hydroxyl functional groups on the algal surface. The predominant functional groups for three growth phases observed were in agreement with the result of FT-IR, and they are all the active sites for Pb(II) binding. It was worth noted that the phosphoryl functional groups were not found in biomass of decline phase, which was consistent with the observations of FT-IR and XPS. It might be because the depletion of phosphorus after the long-time incubation during the decline phase.| Growth phase | pK1 | C1 | pK2 | C2 | pK3 | C3 | pK4 | C4 | Ctot |
|---|---|---|---|---|---|---|---|---|---|
| a ND: not detected. | |||||||||
| Logarithmic phase | 6.78 ± 0.11 | 0.19 ± 0.11 | 4.04 ± 0.06 | 0.39 ± 0.07 | 8.73 ± 0.15 | 0.21 ± 0.03 | 10.37 ± 0.09 | 2.95 ± 0.06 | 3.74 ± 0.05 |
| Stable phase | 6.61 ± 0.13 | 0.22 ± 0.08 | 3.98 ± 0.05 | 0.27 ± 0.08 | 8.78 ± 0.10 | 0.23 ± 0.06 | 9.79 ± 0.20 | 3.07 ± 0.10 | 3.79 ± 0.11 |
| Decline phase | ND | ND | 3.43 ± 0.06 | 0.15 ± 0.05 | 8.92 ± 0.08 | 0.22 ± 0.04 | 11.22 ± 0.21 | 1.81 ± 0.09 | 1.55 ± 0.14 |
| Fundamental groups | Phosphoryl | Carboxyl | Amine | Hydroxyl | |||||
For the four sites, the binding site densities were estimated to be 0.39, 0.19, 0.21 and 2.95 mol kg−1 for logarithmic phase; 0.27, 0.22, 0.23, 3.07 mol kg−1 for stable phase, respectively. While for the decline phase, 0.15, 0.22, 1.81 mol kg−1 for carboxyl, amine and hydroxyl functional groups, respectively. In addition, the decline phase showed the lowest total sites concentrations, which was largely attributed to the lack of phosphoric functional groups and the lower hydroxyl functional groups. Although the carboxyl and hydroxyl functional groups were involved in the Pb(II) binding as aforementioned, the lowest total sites concentrations caused the lowest biosorption capacity of Pb(II) biosorption.
It was noted that the total surface functional groups showed no significant differences between the biomass of logarithmic phase and stable phase. However, as for the effective active sites for Pb(II) binding, the carboxyl groups were found more in stable phase than that in logarithmic phase as stated in FT-IR and XPS analysis. Indeed, the extra effective carboxyl groups contributing to the Pb(II) binding at the stable growth stage (Fig. 5, 6 and Table 4) might be attributed to the presence of galacturonic acid on the surface of microalgal biomass,14 which tended to increase with growth phase and decreased after stationary phase,34 leading to the higher biosorption capacity of biomass from stable growth phase. Also, the higher concentration of hydroxyl and phosphoryl in the surface of biomass from stable phase than that from logarithmic phase further contributed to the larger Pb(II) biosorption capacity. Given that the algal cells of stable growth stage had the highest biomass concentration, it was recommended that stable phase biomass can be harvested as biosorbents for heavy metal removal.
4 Conclusions
The algal dry biomass from each growth phase represented highly efficient removal of Pb(II) from water with the highest Pb(II) biosorption capacity of 298.17 mg g−1 at stable phase. The experimental data were well fitted with the pseudo-second-order kinetics and Freundlich isotherm models. The FT-IR, XPS and PT analysis showed that the more effective active sites in carboxyl, and higher sites concentrations of hydroxyl and phosphoryl functional groups contributed to the largest biosorption capacity of biomass from stable phase. Thus, the stable phase biomass can be used as an efficient, and inexpensive and environment-friendly biosorbent for heavy metal removal.Acknowledgements
This work was financially supported by the China Postdoctoral Science Foundation (Grant No. 2015M580671; 2016T90738), the National Natural Science foundation of China (Grant No. 51604207) and the Independent Innovation Foundation of Wuhan University of Technology (Grant No. 173208001).References
- M. V. Subbaiah, G. Yuvaraja, Y. Vijaya and A. Krishnaiah, J. Taiwan Inst. Chem. Eng., 2011, 42, 965–971 CrossRef CAS.
- Y. Liu, Q. Zhao, G. Cheng and H. Xu, Chem. Eng. J., 2011, 173, 792–800 CrossRef CAS.
- H. Chen, J. Zhao, G. Dai, J. Wu and H. Yan, Desalination, 2010, 262, 174–182 CrossRef CAS.
- A. Çelekli, M. Yavuzatmaca and H. Bozkurt, J. Hazard. Mater., 2010, 173, 123–129 CrossRef PubMed.
- F. V. Hackbarth, F. Girardi, S. M. A. G. U. de Souza, A. A. U. de Souza, R. A. R. Boaventura and V. J. P. Vilar, Chem. Eng. J., 2013, 242, 294–305 CrossRef.
- D. Bulgariu and L. Bulgariu, Bioresour. Technol., 2012, 103, 489–493 CrossRef CAS PubMed.
- M. Mohan and S. K. Dubey, Ecotoxicol. Environ. Saf., 2013, 98, 1–7 CrossRef PubMed.
- L. Bulgariu, M. Lupea, D. Bulgariu, C. Rusu and M. Macoveanu, Environ. Eng. Manage. J., 2013, 12, 183–190 CAS.
- D. Bulgariu and L. Bulgariu, J. Cleaner Prod., 2015, 1–9 Search PubMed.
- J. He, A. Liu and J. Paul Chen, J. Colloid Interface Sci., 2015, 439, 162–169 CrossRef CAS PubMed.
- R. Y. Stanier, R. Kunisawa, M. Mandel and G. Cohen-Bazire, Bacteriol. Rev., 1971, 35, 171–205 CAS.
- M. K. Ji, R. A. I. Abou-Shanab, S. H. Kim, E. S. Salama, S. H. Lee, A. N. Kabra, Y. S. Lee, S. Hong and B. H. Jeon, Ecological Engineering, 2013, 58, 142–148 CrossRef.
- W. Peng, H. Li, Y. Liu and S. Song, J. Mol. Liq., 2016, 221, 82–87 CrossRef CAS.
- S. Hadjoudja, V. Deluchat and M. Baudu, J. Colloid Interface Sci., 2010, 342, 293–299 CrossRef CAS PubMed.
- L. Xia, H. Li and S. Song, J. Appl. Phycol., 2016, 28, 391–407 Search PubMed.
- X. Zhang, P. Amendola, J. C. Hewson, M. Sommerfeld and Q. Hu, Bioresour. Technol., 2012, 116, 477–484 CrossRef CAS PubMed.
- H. B. Senturk, D. Ozdes and C. Duran, Desalination, 2010, 252, 81–87 CrossRef CAS.
- N. Masoudzadeh, F. Zakeri, T. b. Lotfabad, H. Sharafi, F. Masoomi, H. S. Zahiri, G. Ahmadian and K. A. Noghabi, J. Hazard. Mater., 2011, 197, 190–198 CrossRef CAS PubMed.
- U. F. Umar, M. A. Khan, M. Athar and J. A. Kozinski, Chem. Eng. J., 2011, 171, 400–410 CrossRef.
- N. Barka, M. Abdennouri, A. Boussaoud and M. EL Makhfouk, Desalination, 2010, 258, 66–71 CrossRef CAS.
- F. Deniz and S. D. Saygideger, Bioresour. Technol., 2010, 101, 5137–5143 CrossRef CAS PubMed.
- Y. Zhou, P. Lu and J. Lu, Carbohydr. Polym., 2012, 88, 502–508 CrossRef CAS.
- T. Y. Jiang, J. Jiang, R. K. Xu and Z. Li, Chemosphere, 2012, 89, 249–256 CrossRef CAS PubMed.
- P. Sarwa and S. K. Verma, Clean: Soil, Air, Water, 2014, 42, 1298–1303 CrossRef CAS.
- G. Blázquez, M. A. Martín-Lara, E. D. Ruiz, G. Tenorio and M. Calero, J. Ind. Eng. Chem., 2012, 18, 1741–1750 CrossRef.
- M. Mecozzi, M. Pietroletti and A. Tornambé, Spectrochim. Acta, Part A, 2011, 78, 1572–1580 CrossRef PubMed.
- H. Cui, J. Chen, H. Yang, W. Wang, Y. Liu, D. Zou, W. Liu and G. Men, Chem. Eng. J., 2013, 232, 372–379 CrossRef CAS.
- C. Bertagnolli, A. Uhart, J. C. Dupin, M. G. C. da Silva, E. Guibal and J. Desbrieres, Bioresour. Technol., 2014, 164, 264–269 CrossRef CAS PubMed.
- P. Sun, C. Hui, S. Wang, L. Wan, X. Zhang and Y. Zhao, Colloids Surf., B, 2016, 139, 164–170 CrossRef CAS PubMed.
- X. Xu, A. Schierz, N. Xu and X. Cao, J. Colloid Interface Sci., 2016, 463, 55–60 CrossRef CAS PubMed.
- J. Wu, C. Jin, Z. Yang, J. Tian and R. Yang, Carbon, 2015, 82, 562–571 CrossRef CAS.
- Z. Zheng, L. Qiang, T. Yang, B. Wang, X. Cui and H. Wang, J. Polym. Res. DOI:10.1007/s10965-014-0443-2.
- F. Xiao, K. Wang and M. S. Zhan, J. Mater. Sci., 2012, 47, 4904–4913 CrossRef CAS.
- R. L. Soon, R. L. Nation, S. Cockram, J. H. Moffatt, M. Harper, B. Adler, J. D. Boyce, I. Larson and J. Li, J. Antimicrob. Chemother., 2011, 66, 126–133 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2017 |