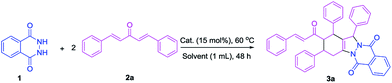Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Synthesis of tetrasubstituted 1H-indazolo[1,2-b]phthalazinedione derivatives bearing three-dimensional turbine-type structures via domino reaction of phthalhydrazide and vinylketones†
Ya-Lun Xu,
Ji-Ya Fu *,
Chun-Hui Liu and
Tao Ding*
*,
Chun-Hui Liu and
Tao Ding*
Henan Engineering Laboratory of Flame-Retardant and Functional Materials, College of Chemistry and Chemical Engineering, Henan University, Kaifeng, Henan 475004, China. E-mail: fujiya@henu.edu.cn; dingtao@henu.edu.cn
First published on 8th August 2017
Abstract
A domino reaction of phthalhydrazide and vinylketone in the presence of phosphotungstic acid was successfully established, and 3D turbine-type tetrasubstituted 1H-indazolo[1,2-b]phthalazinedione derivatives were conveniently obtained with moderate to excellent yields (up to 95%). The highly efficient catalytic system exhibited broad substrate scopes under mild conditions. A 78% yield of the desired product was obtained when the reaction was conducted on a multi-gram scale.
Molecules bearing special structures are versatile and fascinating building blocks that self-assemble.1 Particularly, those bearing special one-dimensional (1D), two-dimensional (2D) and three-dimensional (3D) structures have unique and tunable optical or electronic properties and are, therefore, widely used in supramolecular chemistry to create advanced functional materials.2 To the best of our knowledge, numerous molecules with 1D and 2D structures have been designed and developed in the past decades;3–6 however, except for porphyrin derivatives and their modifications, molecules with 3D structure have been rarely used,7 despite their better assembly capability and properties than those with 1D and 2D structures. Thus, designing and synthesizing novel molecules with 3D structure are highly significant for studying supramolecular chemistry and soft material science.
Phthalazine-fused cyclic skeletons are received considerable attention due to their pharmacological properties and applications in luminescence materials or fluorescence probes.8 Among these compounds, 1H-indazolo[1,2-b]phthalazinediones (IPDs) contain fused hydrazine-based pyrazole heterocycles and nearly planar structures that are widely distributed in self-assembly, molecular recognition, sensors, and optical/electronic materials.9 Their medicinal relevance and structural characteristic have led to the development of efficient synthetic protocols for the constructing such skeletons.10,11 The established strategies mainly involve cyclo-condensation of ketones10 or malononitrile,11 as well as aldehydes and phthalhydrazide, promoted by Brønsted acid (SiO2/SO3H,10a H2SO4,10b heteropolyacids,10c–e p-toluenesulfunic acid,10f (S)-CSA10g), or Lewis acid (Fe2(SO4)3·xH2O,10h Ce(SO4)2·4H2O,10i). However, these approaches mainly focus on the construction of disubstituted IPDs. Preparation methods for tetrasubstituted IPDs are limited. Likewise, tetrasubstituted IPDs have an interesting 3D turbine-type structure that can be used in supramolecular chemistry and functional materials. To the best of our knowledge, these novel 3D structural molecules have been rarely studied.
Though the developed cyclo condensation reaction has emerged as a powerful tool to synthesize diverse disubstituted IPDs,10,11 it does not work in the application for synthesis of tetrasubstituted IPDs. Therefore, it is still highly challenging and desirable to develop efficient strategies to access novel tetrasubstituted IPDs with 3D structures.
Solid acids have attracted much attention from chemists because of their easy recovery and recycling in organic synthesis applications. Among these acids, heteropolyacids are environment-friendly and have high stability towards humidity, easy recovery, and recyclability.12 Based on this background and our continuing interest in the development of useful synthetic methodologies,13 we envisioned that the aza-Michael addition reaction of phthalhydrazide and vinylketone could be achieved in the presence of an appropriate Brønsted acid, and subsequently Michael addition reaction with another vinylketones occurred. Then, intramolecular Michael addition and condensation reaction occurred to give the tetrasubstituted IPDs. This transformation involves two intermolecular Michael additions, namely, an intramolecular Michael addition and a condensation reaction. Herein, we wish to report our preliminary results on the first efficient synthesis of tetrasubstituted IPDs with phthalhydrazide and two vinylketones as reactants in the presence of phosphotungstic acid under mild conditions.
Phthalhydrazide (1a) and 2a were selected as model substrates to test the feasibility of our hypothesis. The reaction proceeded smoothly in the presence of phosphotungstic acid and successfully produced the desired product 3a (49% yield, Table 1, entry 1). The reaction of phthalhydrazide and vinylketone was performed in CH3CN without a catalyst at 60 °C for the comparative group. As expected, the reaction did not occur even after 48 h (Table 1, entry 2). Moreover, the use of different Lewis acids as catalysts was investigated to develop an efficient catalytic system. The results were dissatisfactory, and only AlCl3 produced the desired product 3a with a 13% yield (Table 1, entry 3). Furthermore, several Brønsted acids were screened. When the reaction was performed using PhCO2H, CF3CO2H, CF3SO3H, or HCl as the catalysts, only CF3SO3H produced the desired product with a moderate yield (49%, Table 1, entry 8). Among the screened catalysts, both phosphotungstic and trifluoromethanesulfonic acid produced the desired products in almost similar yields and were, therefore, selected as the catalysts for further optimization (entries 1 and 8).
| Entry | Catalyst | Solvent | Yieldb (%) |
|---|---|---|---|
| a Unless otherwise specified, all reaction carried out with phthalhydrazide (0.10 mmol, 1.0 equiv.), vinylketone (2a, 0.20 mmol, 2.0 equiv.), solvent (1 mL) and catalysts (15 mol%) at 60 °C.b Isolated yields.c The yields in parenthesis are the isolated yields using trifluoromethanesulfonic acid as the catalyst.d Carried out at 40 °C.e Carried out with 10 mol% phosphotungstic acid.f Carried out with phthalhydrazide (0.10 mmol, 1.0 equiv.), vinylketone (2a, 0.10 mmol, 1.0 equiv.), PhCN (1 mL) and catalyst (15 mol%) at 60 °C.g Carried out with phthalhydrazide (0.10 mmol, 1.0 equiv.), vinylketone (2a, 0.10 mmol, 1.0 equiv.) and PhCN (1 mL) at 60 °C. | |||
| 1 | Phosphotungstic acid | CH3CN | 49 |
| 2 | — | CH3CN | NR |
| 3 | FeCl3 | CH3CN | NR |
| 4 | CuCl2 | CH3CN | Trace |
| 5 | AlCl3 | CH3CN | 13 |
| 6 | PhCO2H | CH3CN | NR |
| 7 | CF3CO2H | CH3CN | Trace |
| 8 | CF3SO3H | CH3CN | 49 |
| 9 | HCl | CH3CN | Trace |
| 10 | Phosphotungstic acid | THF | 64 (14)c |
| 11 | Phosphotungstic acid | Dioxane | 10 (9)c |
| 12 | Phosphotungstic acid | DMC | 36 (trace)c |
| 13 | Phosphotungstic acid | PhCN | 92 (trace)c |
| 14 | Phosphotungstic acid | PhMe | 24 (trace)c |
| 15 | Phosphotungstic acid | CHCl3 | 11 (trace)c |
| 16 | Phosphotungstic acid | DMF | 10 (22)c |
| 17d | Phosphotungstic acid | PhCN | 60 |
| 18e | Phosphotungstic acid | PhCN | 87 |
| 19f | Phosphotungstic acid | PhCN | 68 |
| 20g | Phosphotungstic acid | PhCN | NR |
The solvents were examined using phosphotungstic or trifluoromethanesulfonic acid as the catalyst at 60 °C to further optimize the reaction conditions and the results described in Table 1 (entries 10–12). As it turned out, the results were significantly dependent on the reaction media. When phosphotungstic acid was used as the catalyst, PhCN produced the best yield among the screened solvents (92%, Table 1, entry 13).
The effects of reaction temperature on PhCN were examined using phosphotungstic acid as the catalyst. When the temperature was lowered to 40 °C, the yield was decreased significantly (60% yield, Table 1, entry 17). When the catalyst loading was reduced to 10 mol%, the yield was decreased as well (87%, Table 1, entries 18 vs. 13). Comparatively, when the reaction was carried out in the absence of the catalyst, no desired product was obtained (Table 1, entry 20). Thus, the optimized reaction conditions were found to be the reaction of 1.0 equiv. phthalhydrazide with 2.0 equiv. 2a in the presence of 15 mol% of phosphotungstic acid in cyanobenzene at 60 °C (Table 1, entry 13).
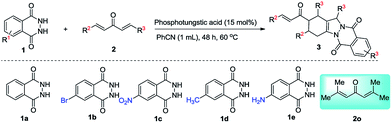
To broaden the substrate scopes, the reaction generality was examined with the domino reaction of phthalhydrazide (1) with various vinylketones (2) under optimized conditions, and the results are listed in Table 2. Various electron-withdrawing groups (4-F, 4-Cl, 4-Br, 4-CF3, Table 2, entries 2–5) and electron-donating groups (4-CH3O, 4-CH3, Table 2, entries 6 and 7) at the 4-position on the aromatic ring of 2 were completely tolerated in the transformation. And the electron-donating groups at the 4-position on the aromatic ring of 2 gave relatively higher yields than those with electron-withdrawing groups. Furthermore, the positions of the substituents on the aryl group of 2 were studied. Unfortunately, the vinylketones with electron-withdrawing or -donating substituents on the aromatic ring at the 2-position produced no desired products as the reaction mixture became merry (Table 2, entries 11 and 12). The position of the substituents on the aromatic ring of 2 was more important than the electronic property of the substituents. An excellent yield of 95% was obtained when 1-naphthyl was used to substitute the vinylketones (Table 2, entry 13).
| Entry | 1 | 2 | d.r.b | Yieldc (%) |
|---|---|---|---|---|
| a All reaction carried out with phthalhydrazide (0.10 mmol, 1.0 equiv.), vinylketone (2, 0.20 mmol, 2.0 equiv.), cyanobenzene (1 mL) and phosphotungstic acid (15 mol%) at 60 °C.b Diasteromeric ratio was determined by 1H NMR analysis.c Isolated yield.d The reaction mixture became messy and no desired product was obtained.e The d.r. value was not determined because that the main characteristic peaks of the 1H NMR spectra were not found. | ||||
| 1 | 1a | 2a: R2 = R3 = Ph | 2.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.8 1.8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.0 1.0 |
92 (3a) |
| 2 | 1a | 2b: R2 = R3 = 4-FPh | 1.8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.1 1.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.0 1.0 |
60 (3b) |
| 3 | 1a | 2c: R2 = R3 = 4-ClPh | 3.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.4 2.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.0 1.0 |
84 (3c) |
| 4 | 1a | 2d: R2 = R3 = 4-BrPh | 1.8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.3 1.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.0 1.0 |
89 (3d) |
| 5 | 1a | 2e: R2 = R3 = 4-CF3Ph | 1.6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.0 1.0 |
40 (3e) |
| 6 | 1a | 2f: R2 = R3 = 4-CH3OPh | 2.8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.0 1.0 |
91 (3f) |
| 7 | 1a | 2g: R2 = R3 = 4-CH3Ph | 1.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.0 1.0 |
90 (3g) |
| 8 | 1a | 2h: R2 = R3 = 3-ClPh | 1.8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.0 1.0 |
62 (3h) |
| 9 | 1a | 2i: R2 = R3 = 3-CH3OPh | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
89 (3i) |
| 10 | 1a | 2j: R2 = R3 = 3-CH3Ph | 1.7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.0 1.0 |
86 (3j) |
| 11 | 1a | 2k: R2 = R3 = 2-BrPh | n.d. | —d (3k) |
| 12 | 1a | 2l: R2 = R3 = 2-CH3Ph | n.d. | —d (3l) |
| 13 | 1a | 2m: R2 = R3 = 1-naphthyl | —e | 95 (3m) |
| 14 | 1a | 2n: R2 = Ph, R3 = 3-CH3OPh | —e | 85 (3n) |
| 15 | 1a | 2o | n.d. | Trace |
| 16 | 1b | 2a | n.d. | Trace |
| 17 | 1c | 2a | 2.8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.0 1.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.0 1.0 |
87 (3q) |
| 18 | 1d | 2a | 4.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4.4 4.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.0 1.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.0 1.0 |
63 (3r) |
| 19 | 1e | 2a | 4.9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.4 2.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.0 1.0 |
69 (3s) |
Delightedly, when the domino reaction of phthalhydrazide and unsymmetric vinylketone 2n was conducted under optimized reaction conditions, the desired product with a good yield was obtained (85%, Table 2, entry 14). When a multi-substituted 2o was used, only trace of the desired product was obtained because of the steric hindrance (Table 2, entry 15).
This method is compatible with phthalhydrazide which has diverse electronic properties substituent in aryl ring (Table 2, entries 16–19). The electron-deficient 4-NO2 substituted phthalhydrazide (2b) led to relatively high yield of the corresponding formation (87%, Table 2, entry 16). The reaction of electron-rich group substituted phthalhydrazides with 2a afforded moderate yields (63–69%, Table 2, entries 18 and 19).
For the open-chain N,N′-dibenzoylhydrazine, the domino reaction still proceeded smoothly and resulted in a corresponding product with 30% yield (Scheme 1).
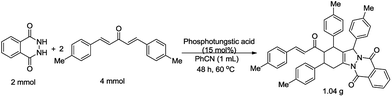
To demonstrate the synthetic potential of the transformation, a gram-scale experiment was conducted with 2.0 mmol 1a and 4.0 mmol 2g. This reaction proceeded smoothly and afforded the domino product 3g with 78% yield (Scheme 2).
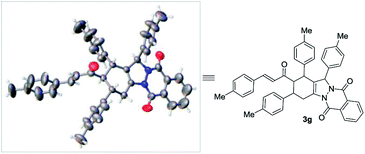
In order to clarify the structure of the product, we studied the X-ray crystallographic analysis of 3g (Fig. 1).
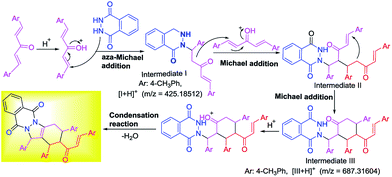
Based on Brønsted acids catalysis, the experimental results and HRMS analysis, a possible mechanism was proposed for synthesis of tetrasubstituted IPDs as shown in Scheme 3. Initially, vinylketone was activated by phosphotungstic acid, and then trapped by phthalhydrazide 1a to undergo aza-Michael addition reaction, leading to the formation of intermediate I. An intermolecular Michael addition reaction between intermediate I and another vinylketones affording intermediate II. And then intramolecular Michael addition reaction of intermediate II occurred giving intermediate III. Finally, the condensation reaction of intermediate III and dehydration occurred, which furnished the desired compound and released the catalyst. ESI-HRMS analysis and control experiments were conducted to support the proposed catalytic cycle. Firstly, the intermediates I and III were supported by ESI-HRMS. A characteristic signal at m/z 691.28589 was observed, which was consistent with [3g + Na]+, and [I + H]+ at 425.18512 as well as [III + H]+ at 687.31604 (Scheme 3 and see detail in ESI Fig. S1†).
Conclusions
In summary, we successfully developed a simple and efficient domino reaction for constructing 3D turbine-type tetrasubstituted IPDs. In the presence of phosphotungstic acid, the reaction between phthalhydrazide and vinylketone could proceed smoothly and produced a series of 3D turbine-type tetrasubstituted IPDs in excellent yields (up to 95%). This protocol provides a valuable access to novel tetrasubstituted IPDs with a 3D turbine-type structures, which would be applied in supramolecular chemistry, functional materials and medicinal chemistry. And the application of the tetrasubstituted IPDs in supramolecular chemistry is currently being studied in our laboratory.Notes and references
- (a) C. Zhang, P. Chen, H. Dong, Y. Zhen, M. Liu and W. Hu, Adv. Mater., 2015, 27, 5379 CrossRef CAS PubMed; (b) C. C. Lee, C. Grenier, E. W. Meijer and A. P. H. Schenning, Chem. Soc. Rev., 2009, 38, 671 RSC.
- Selected review on special structural moleculars: (a) M. Liu, L. Zhang and T. Wang, Chem. Rev., 2015, 115, 7304 CrossRef CAS PubMed; (b) L. Zhang, T. Wang, Z. Shen and M. Liu, Adv. Mater., 2016, 28, 1044 CrossRef CAS PubMed; (c) R. May, S.-S. Jester and S. Höger, J. Am. Chem. Soc., 2014, 136, 16732 CrossRef CAS PubMedS. Zhang, X. Liu, C. Li, L. Li, J. Song, J. Shi, M. Morton, S. Rajca, A. Rajca and H. Wang, J. Am. Chem. Soc., 2016, 138, 10002 CrossRef CAS PubMed.
- (a) J. Herrikhuyzen, P. Jonkheijm, A. P. H. J. Schenning and E. W. Meijer, Org. Biomol. Chem., 2006, 4, 1539 RSC; (b) M. H. C. Bakker, C. Lee, E. W. Meijer, P. Y. W. Dankers and L. Albertazzi, ACS Nano, 2016, 10, 1845 CrossRef CAS PubMed; (c) M. Garzoni, M. Baker, B. C. M. A. Leenders, I. K. Voets, L. Albertazzi, A. R. A. Palmans, E. W. Meijer and G. M. Pavan, J. Am. Chem. Soc., 2016, 138, 13985 CrossRef CAS PubMed; (d) E. Kim, D. Kim, Y.-A. Lee and O.-S. Jung, J. Coord. Chem., 2014, 67, 3532 CrossRef CAS; (e) S. Cantekin, T. F. A. Greef and A. R. A. Palmans, Chem. Soc. Rev., 2012, 41, 6125 RSC; (f) K. Pandurangan, J. A. Kitchen, S. Blasco, E. M. Boyle, B. Fitzpatrick, M. Feeney, P. E. Kruger and T. Gunnlaugsson, Angew. Chem., Int. Ed., 2015, 54, 4566 CrossRef CAS PubMed.
- (a) F. Aparicio, F. García and L. Sánchez, Chem.–Eur. J., 2013, 19, 3239 CrossRef CAS PubMed; (b) F. García, J. Buendia and L. Sánchez, J. Org. Chem., 2011, 76, 6271 CrossRef PubMed; (c) F. García and L. Sánchez, J. Am. Chem. Soc., 2012, 134, 734 CrossRef PubMed; (d) F. Wang, M. A. J. Gillissen, P. J. M. Stals, A. R. A. Palmans and E. W. Meijer, Chem.–Eur. J., 2012, 18, 11761 CrossRef CAS PubMed.
- A. M. Castilla, N. Ousaka, R. A. Bilbeisi, E. Valeri, T. K. Ronson and J. R. Nitschke, J. Am. Chem. Soc., 2013, 135, 17999 CrossRef CAS PubMed.
- (a) Y. Xu, H. Jiang, Q. Zhang, F. Wang and G. Zou, Chem. Commun., 2014, 50, 365 RSC; (b) Y. Xu, G. Yang, H. Xia, G. Zou, Q. Zhang and J. Gao, Nat. Commun., 2014, 5, 5050 CrossRef CAS PubMed.
- (a) F. Helmich, C. C. Lee, M. M. L. Nieuwenhuizen, J. C. Gielen, P. C. M. Christianen, A. Larsen, G. Fytas, P. E. L. G. Leclere, A. P. H. J. Schenning and E. W. Meijer, Angew. Chem., Int. Ed., 2010, 49, 3939 CrossRef CAS PubMed; (b) N. C. Maiti, S. Mazumdar and N. Periasamy, J. Phys. Chem. B, 1998, 102, 1528 CrossRef CAS; (c) V. V. Borovkov, J. M. Lintuluoto and Y. Inoue, J. Phys. Chem. B, 1999, 103, 5151 CrossRef CAS.
- (a) S. Grasso, G. DeSarro, N. Micale, M. Zappala, G. Puia, M. Baraldic and C. Demicheli, J. Med. Chem., 2000, 43, 2851 CrossRef CAS PubMed; (b) J. S. Kim, H. K. Rhee, H. J. Park, S. K. Lee, C. O. Lee and H. Y. P. Choo, Bioorg. Med. Chem., 2008, 16, 4545 CrossRef CAS PubMed; (c) J. Sinkkonen, V. Ovcharenko, K. N. Zelenin, I. P. Bezhan, B. A. Chakchir, F. Al-Assar and K. Pihlaja, Eur. J. Org. Chem., 2002, 2002, 2046 CrossRef; (d) L. Zhang, L. P. Guan, X. Y. Sun, C. X. Wei, K. Y. Chai and Z. S. Quan, Chem. Biol. Drug Des., 2009, 73, 313 CrossRef CAS PubMed; (e) C.-K. Ryu, R.-E. Park, M.-Y. Ma and J.-H. Nho, Bioorg. Med. Chem. Lett., 2007, 17, 2577 CrossRef CAS PubMed; (f) J. Li, Y. F. Zhao, X. Y. Yuan, J. X. Xu and P. Gong, Molecules, 2006, 11, 574 CrossRef CAS PubMed; (g) H. Wu, X. M. Chen, Y. Wan, H. Q. Xin, H. H. Xu, R. Ma, C. H. Yue and L. L. Pang, Lett. Org. Chem., 2009, 6, 219 CrossRef CAS.
- (a) A. S. Amarasekara and S. Chandrasekara, Org. Lett., 2002, 4, 773 CrossRef CAS PubMed; (b) C. Zhang, S. Li, L. Ji, S. Liu, Z. Li, S. Li and X. Meng, Bioorg. Med. Chem. Lett., 2015, 25, 4693 CrossRef CAS PubMed.
- (a) H. Veisi, A. Sedrpoushan, A. R. Faraji, M. Heydari, S. Hemmatia and B. Fatahia, RSC Adv., 2015, 5, 68523 RSC; (b) J. M. Khurana and D. Magoo, Tetrahedron Lett., 2009, 50, 7300 CrossRef CAS; (c) H. J. Wang, X. N. Zhang and Z. H. Zhang, Monatsh. Chem., 2010, 141, 425 CrossRef CAS; (d) A. Corma and H. Garcia, Catal. Today, 1997, 38, 257 CrossRef CAS; (e) G. Sabitha, C. Srinivas, A. Raghavendar and J. S. Yadav, Helv. Chim. Acta, 2010, 93, 1375 CrossRef CAS; (f) M. Sayyafi, M. Sayyedhamzeh, H. R. Khavasi and A. Bazgir, Tetrahedron, 2008, 64, 2375 CrossRef CAS; (g) G. Shukla, R. K. Verma, G. K. Verma and M. S. Singh, Tetrahedron Lett., 2011, 52, 7195 CrossRef CAS; (h) A. Choudhury, S. Ali and A. T. Khan, J. Korean Chem. Soc., 2015, 280 CrossRef CAS; (i) E. Mosaddegh and A. Hassankhani, Tetrahedron Lett., 2011, 52, 488 CrossRef CAS.
- C. B. Sangani, J. A. Makwana, Y.-T. Duan, N. J. Thumar, M.-Y. Zhao, Y. S. Patel and H.-L. Zhu, Res. Chem. Intermed., 2016, 42, 2101 CrossRef CAS.
- (a) T. Ueda and H. Kotsuki, Heterocycles, 2008, 76, 73 CrossRef CAS; (b) M. Dabiri and S. Bashiribod, Molecules, 2009, 14, 1126 CrossRef CAS PubMed.
- (a) J.-Y. Fu, X.-Y. Xu, Y.-C. Li, Q.-C. Huang and L.-X. Wang, Org. Biomol. Chem., 2010, 8, 4524 RSC; (b) J.-Y. Fu, Q.-C. Yang, Q.-L. Wang, X.-Y. Xu and L.-X. Wang, J. Org. Chem., 2011, 76, 4661 CrossRef CAS PubMed; (c) W.-Q. Hu, Y.-S. Cui, Z.-J. Wu, C.-B. Zhang, P.-H. Dou, S.-Y. Niu, J.-Y. Fu and Y. Liu, RSC Adv., 2015, 5, 70910 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: X-ray crystallographic data for compound 3g. Copies of 1H NMR and 13C NMR of products. CCDC 1554488. For ESI and crystallographic data in CIF or other electronic format see DOI:10.1039/c7ra06856e |
| This journal is © The Royal Society of Chemistry 2017 |