Open Access Article
Open Access ArticleFacile fabrication of raspberry-like composite microspheres for the construction of superhydrophobic films and applications in highly efficient oil–water separation†
Mingguang Yu a,
Qing Wang*b,
Min Zhanga,
Qianjun Denga and
Dongchu Chena
a,
Qing Wang*b,
Min Zhanga,
Qianjun Denga and
Dongchu Chena
aSchool of Materials Science and Energy Engineering, Foshan University, Foshan 528000, China
bState Key Laboratory of Pulp & Paper Engineering, South China University of Technology, Guangzhou 510640, China. E-mail: wangqing_1216@126.com
First published on 14th August 2017
Abstract
Inspired by the “lotus effect”, we proposed a facile synthetic route toward raspberry-like PS@SiO2 microspheres, which further lead to superhydrophobic surfaces. In this approach, monodispersed polystyrene (PS) microspheres were first synthesized via dispersion polymerization using polyvinylpyrrolidone (PVP) as stabilizer. The obtained PS microspheres were then used as template microspheres for biomimetic silification using tetraethyl orthosilicate (TEOS) as precursor. Upon adjusting the molecular weight of PVP and the concentration of NH3·H2O, the surface roughness of PS@SiO2 microspheres can be well controlled. Furthermore, after hydrophobization treatment, by drop-casting the raspberry-like PS@SiO2 microspheres onto a glass slide, dual-scale films were obtained, which had a similar surface morphology to that of the lotus leaf, exhibiting a water contact angle of 163.3° and water contact angle hysteresis of 4°. In addition, the oil–water separation ability of hydrophobic raspberry microsphere treated steel mesh was investigated. The results demonstrated excellent oil–water separation efficiency and reusability. This facile and robust synthesis technique for constructing a superhydrophobic surface hold great potential application in versatile and large-scale oil–water separation.
Introduction
Materials with superwetting ability have attracted wide attention from both academia and industry due to their great potential applications in self-cleaning,1–7 combatting bacteria,8,9 agriculture,10 separation of liquids,11–19 printing and reprography,20,21 etc. In many scientific studies, the “lotus effect” has been reported as a non-wetting phenomenon, which is governed by a specific nano-micro structured surface topography and low surface energy. Therefore, a superhydrophobic surface can be obtained by lowering the surface energy and adjusting the surface roughness.20Inspired by superhydrophobic biomaterials, many synthesis strategies have been established to mimic the dual-scale rough hierarchical morphology for the fabrication of superhydrophobic surfaces,22–32 such as plasma polymerization/etching,33,34 chemical vapor deposition,31 electro spinning,35,36 etc. However, the high cost materials, relatively complicated production equipment or operation procedures largely affect the actual application of the superhydrophobic materials.
Recently, raspberry-like colloidal microspheres with dual-sized hierarchical structures have gained increasing attention for their potential applications in the construction of superhydrophobic films.36–43 In most cases, inorganic/organic raspberry-like microspheres were used in assemble superhydrophobic coatings.44–48 A variety of methods have been developed for the successful fabrication of these samples.47,49,50 The silica-coated or titania-coated raspberry-like microspheres was usually obtained by acid–base interaction, electrostatic interaction between the silanol groups and the functional groups on templates. Zhang et al.51 reported a method for the preparation of raspberry microspheres using non-covalent interactions between organic and inorganic components. They first introduced the functional group of acrylic acid (AA) in the preparation of polystyrene (PS) microspheres to prepare polyacrylic acid functionalized PS microspheres. Due to the strong hydrogen bonding between the carboxyl groups in the polyacrylic acid and the siloxanes possessed by the ethyl orthosilicate (TEOS), TEOS is adsorbed to the PS microspheres surface, and then in situ hydrolysis into nano-silica on the surface of PS microspheres to obtain the raspberry-like PS@SiO2 microspheres. Caruso et al.52 reported the method of lays of self-assembly (LBL) to prepare PS@SiO2 raspberry-like structural microspheres utilized non-covalent interactions of the negatively charged SiO2 microspheres and positively charged polydimethylenediammonium chloride (PDDA) modified PS microspheres. Raspberry-like microspheres can also obtained via surface modification/polymerization method, in which silica microspheres were first surface treated with methacryloxypropyltrimethoxysilane then copolymerized with vinyl monomers.53 However, many of these methods used toxic or high cost materials, wild reaction conditions, or multistep processes.33
Poly(vinylpyrrolidone) (PVP) was an amphiphilic, hypotoxicity, nonionic polymer which was widely used in scientific research and industry. In dispersion polymerization system, PVP was a very commonly used stable dispersant. After nucleation, it can be adsorbed or grafted on the surface to stabilize the nucleus of the polymer microspheres.54,55 Moreover, the tertiary amine groups presented in the molecular structure of PVP had excellent coordination and adsorption ability to adsorb a broad range of different materials such as metals, metal oxides and inorganic source precursors. They can be adsorbed on the surface of microspheres and in situ induced or hydrolysed into nanoparticles to obtain organic and inorganic hybrid materials.
Herein, we present a general, simple and fast method to fabricate raspberry-like PS@SiO2 composite microspheres, which was based on the use of PVP. In detail, monodispersed PS microspheres were first synthesized via dispersion polymerization using PVP as stabilizer. Then the PS microspheres were used as templates for the biomimetic silification using tetraethyl orthosilicate (TEOS) as precursor. During the process, PVP on the one hand stabilized styrene monomer forming monodispersed PS microspheres, on the other hand worked as the linkers to bridge PS microspheres and TEOS to induce the formation of the raspberry-like PS@SiO2 composite microspheres. In addition, surface roughness of the as-prepared raspberry-like composite microspheres could be well controlled upon adjusting the molar mass of PVP and the concentration of ammonia. Furthermore, steel mesh treated with hydrophobized raspberry-like PS@SiO2 microspheres showed excellent oil–water separation efficiency and reusability. This facile synthesis technique for construction the superhydrophobic surface hold great potential application in versatile and large-scale oil–water separation.
Experimental
Materials
Styrene (St, 99.9%, Aldrich) was freshly distilled under vacuum and stored under freeze prior to use. PVP with an average molecular weight of 10 kg mol−1, 40 kg mol−1, 58 kg mol−1 and 130 kg mol−1 were purchased from Aldrich and used as received. Azobisisobutyronitrile (AIBN, Aldrich, 99%) was purified by recrystallization and stored in a refrigerator prior to use. Hexadecyltrimethoxysilane (HDTMS, >99%) were purchased from Aldrich and used as received. Tetraethyl orthosilicate (TEOS, >99%), anhydrous ethanol, and aqueous ammonia solution (NH3·H2O, 25 wt%) were purchased from Guangzhou Chemical Reagent Co. and used as received. Polyurethane (PU) sponges and steel mesh (Φ = 30 mm, 200 mesh sieve) were purchased from a local store, which were cleaned by ethanol and deionized water, respectively.Preparation of monodispersed PS microspheres
Monodispersed polystyrene (PS) microspheres were performed in ethanol–water mixture (80/20, w/w) at 70 °C via dispersion polymerization. In a typical procedure, styrene (2.0 g, 10 wt% relative to the reaction system), stabilizer PVP (0.3 g, 15 wt% relative to monomer) and AIBN (0.04 g, 2 wt% relative to monomer) were dissolved in EtOH/H2O mixture (18.0 g, 80/20, w/w). The mixture was purged with nitrogen for 30 minutes under stirring, sealed, and then conducted at 70 °C for 8 hours.The reaction mixture was separated by centrifugation, rinsed with ethanol/water mixture and centrifuged repeatedly.
Preparation of raspberry-like PS@SiO2 microspheres
The raspberry-like PS@SiO2 microspheres were prepared by a modified Stöber method.51 Typically, 100 mg of monodispersed PS microspheres were dispersed in reaction mixture in a 250 mL flask containing 100 mL ethanol and 20 mL deionized water and a certain amount of ammonium hydroxide (25 wt%) under gentle magnetic stirring at room temperature. Then 0.2 mL of TEOS was slowly added to the solution. The mixture was stirred vigorously for 24 h at room temperature. After the hydrolysis reaction of TEOS, the mixture was centrifuged and rinsed with ethanol/water mixture and centrifuged repeatedly to get the raspberry-like PS@SiO2 microspheres.Fabrication of the superhydrophobic surfaces
The raspberry-like PS@SiO2 microspheres (1.0 g) were dispersed into 20 mL ethanol solution containing HDTMS (1 wt%) and the solution was magnetic stirring for 2 hours. Then a certain amount of sample emulsion was carefully drop-cast onto glass, paper, cotton fibres, polyurethane (PU) sponge and steel mesh in order to generate the dual-scale films, and then these films were heated for 5 min at 60 °C in an oven to form superhydrophobic surfaces.Characterization
FT-IR spectra were recorded on a Spectrum 2000 Perkin Elmer FT-IR spectrometer. X-Ray photoelectron spectroscopy (XPS) spectra were recorded on a Thermo Electron Corporation Escalab 250 spectrometer with Li Kα radiation (20 eV) as the exciting source. All the samples were ground into powder and dried under infrared lamp before examination. TEM measurements were carried out on FEI Tecnai G2 Spirit transmission electron microscopy. Samples were diluted with ethanol and ultrasonicated for 10 min to make the suspensions with a concentration of 0.1 wt% and then dried onto carbon-coated copper grids before examination. SEM measurements were carried out on a Termal field-emission scanning electron microscope (FESEM, Quanta 400F) at 15 kV. Samples were dispersed into ethanol with a concentration of 0.1 wt% via ultrasonication for 10 min. The dispersions were dip-casting onto the surface of glass and coated with a thin layer of gold before examination. Thermogravimetric analysis (TGA) was performed on a Q600 instrument (TA Instruments, New Castle, USA). All samples were heated from 40 °C to 600 °C at a heating rate of 20 °C min−1. Contact angles (CAs) were measured on a Dataphysics OCA20 Contract-Angle System with liquid droplets of 5 μL. And the reported values represented the averages of at least 5 different positions' measurements. The separation efficiency was calculated according to θ = (m1/m0) × 100%, where m0 and m1 are the mass of the residual liquid before and after the separation process, respectively.17Results and discussion
Composition characterization of monodispersed PS microspheres and raspberry-like PS@SiO2 composite microspheres
As is well known, TEOS can be used to prepare a variety of nano-structured silicon nanoparticles. Polymers containing secondary amines and tertiary amines could catalyse the hydrolysis and condensation polymerization of TEOS, which may strongly adsorbed TEOS by the charge effect of the amine sites. Here, the monodispersed PS microspheres were prepared via one-pot dispersion polymerization using PVP as stabilizer (Scheme 1). The presence of PVP ensured strong static adsorption of the PS microspheres with the TEOS, which arose from the presence of a highly polar amide group within the pyrrolidone ring of PVP.52 Under alkaline condition (NH3·H2O as catalyst), TEOS was in situ hydrolysed into silica nanoparticles on the surface of the PS microspheres.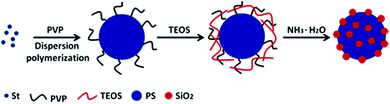 | ||
| Scheme 1 Schematic illustration of procedure for preparation of raspberry-like PS@SiO2 composite microspheres. | ||
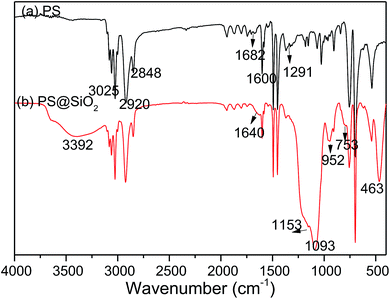
FT-IR spectra was used to record the structure of the PS and raspberry-like PS@SiO2 composite microspheres. As shown in Fig. 1a, absorption peaks at 3025, 1600, 1492, 1450, 757, 697 cm−1 corresponded to the characteristic absorption peaks of phenyl group, the peaks at 2920 and 2848 cm−1 can be attributed to the methylene and methenyl groups of polystyrene main chain. The peak at 1682 cm−1 and the peak at 1291 cm−1 attributed to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibration and the C–N stretching vibration of the pyrrolidone ring in PVP, which means PVP was located at the surface of PS spheres. After coated with SiO2, there were several new absorbance signals appearing in FT-IR spectra. As shown in Fig. 1b, 463 cm−1 and 753 cm−1 were the antisymmetric stretching vibration peak and symmetric stretching vibration peak of Si–O respectively, and the Si–O–Si stretching vibration band was split into two peaks (1153 and 1093 cm−1). The absorption peak of Si–OH was at 952 cm−1. The peaks at 1640 cm−1 and 3392 cm−1 were the bending vibration peak of H–O–H and the antisymmetric stretching vibration peak of O–H. These features indicated that SiO2 were successfully coated to the PS microspheres.
O stretching vibration and the C–N stretching vibration of the pyrrolidone ring in PVP, which means PVP was located at the surface of PS spheres. After coated with SiO2, there were several new absorbance signals appearing in FT-IR spectra. As shown in Fig. 1b, 463 cm−1 and 753 cm−1 were the antisymmetric stretching vibration peak and symmetric stretching vibration peak of Si–O respectively, and the Si–O–Si stretching vibration band was split into two peaks (1153 and 1093 cm−1). The absorption peak of Si–OH was at 952 cm−1. The peaks at 1640 cm−1 and 3392 cm−1 were the bending vibration peak of H–O–H and the antisymmetric stretching vibration peak of O–H. These features indicated that SiO2 were successfully coated to the PS microspheres.
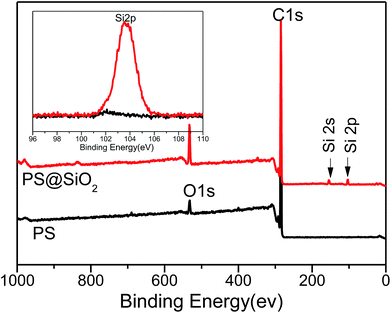
XPS was further employed to determine the surface composition of PS and raspberry-like PS@SiO2 composite microspheres, shown in Fig. 2. The typical information depth of XPS was 5 nm. The spectra of PS microspheres revealed the presence of carbon (284.8 eV) and oxygen (532.82 eV). The peak of oxygen was corresponding to the stabilizer PVP, which proved PVP was adsorption onto the surface of PS microspheres during dispersion polymerization. In the XPS spectra of the raspberry-like PS@SiO2 composite microspheres, the relative atomic concentration of oxygen increased from 3.62% to 10.07%, together with the appearance of two typical peaks with binding energies of 155.24 eV (Si 2s) and 103.58 eV (Si 2p), strongly confirmed the presence of silica nanoparticles on the surface of the PS microspheres.
Effect of the concentrations of NH3·H2O on raspberry-like PS@SiO2 composite microspheres
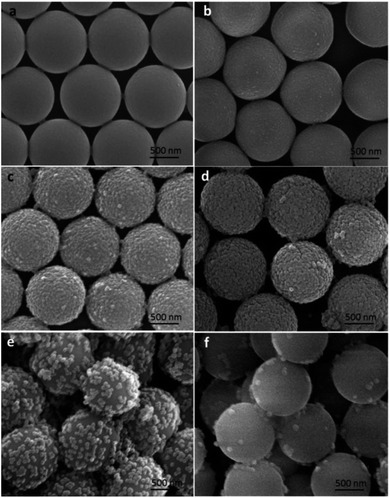
According to the classic Stöber process, the surface morphologies and roughness of the silica coated PS microspheres could be easily tuned by varying the reaction conditions, such as the catalyst, the temperature, medium, and time. As is well known, ammonia was often used as the catalyst causing the formation of spherical silica nanoparticles. So in this study, the influence of the amount of ammonia on the surface roughness of the silica modified PS microspheres was studied. The nano-SiO2 diameter and coverage degree on the surface of PS microspheres could be tailored simply by varying the concentration of NH3·H2O.Monodispersed PS microspheres synthesized using PVP-40 as stabilizer were first used as templates to fabricate raspberry-like PS@SiO2 composite microspheres in the one-step sol–gel process. Fig. 3 showed SEM images of raspberry-like PS@SiO2 composite microspheres obtained at varied NH3·H2O concentrations. When the NH3·H2O volume was 0.5 mL, the PS template was densely and uniformly covered by the silica nanoparticles. The PS surface roughness had no intuitionistic improvement. When the NH3·H2O volume increased to 1.0 mL, the roughness of the PS surface became more pronounced and small humps with size distribution of ca. 50 nm were presented on the PS template surface (Fig. 3c). With the content of NH3·H2O increased from 1.0 to 2.0 mL, the roughness of the PS surface became more pronounced because of the growth of the nodules. The diameter of SiO2 nanoparticles on the surface of PS microspheres increased to ca. 100 nm (Fig. 3d). Although the diameter of silica nanoparticles increased with the addition of NH3·H2O, further increase the volume of NH3·H2O would decreased the coverage degree of silica on the PS microspheres, such as 3.0 mL and 4.0 mL (Fig. 3e and f). This may due to that with the speeded up of the hydrolysis, it became harder for the PVP adsorbing TEOS onto the surface of PS microspheres.
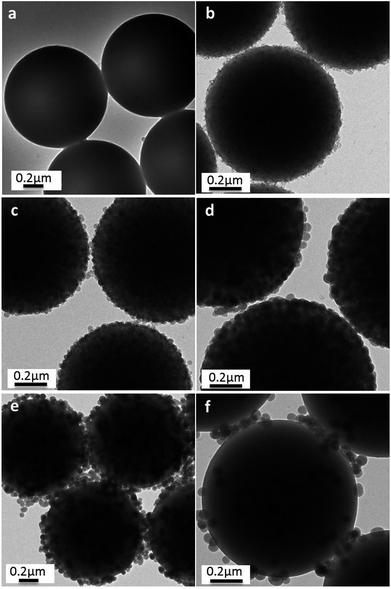
This raspberry-like structure had also been confirmed by TEM, as shown in Fig. 4. In comparison with PS templates (Fig. 4a), these PS@SiO2 microspheres with relatively rough surfaces and slightly larger sizes were observed after the silification. The average diameter measured from the TEM images of PS@SiO2 microspheres (Fig. 4b–d) increased from 837 to 928 nm with the content of NH3·H2O increased from 0.5 to 2.0 mL during the silification process. But further increase the contents of NH3·H2O would decrease the cover density of silica, which was confirmed by Fig. 4e and f.
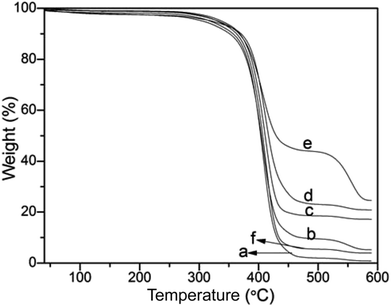
Thermogravimetric analysis was used to quantitate the amount of silica nanoparticles deposited on the PS surface (Fig. 5). The PS template was decomposed completely with residue content of 0.825% (Fig. 5a). And the residue content slightly increased to 5.21% after hydrolysis of TEOS with 0.5 mL NH3·H2O (Fig. 5b), illustrating that the silica nanoparticles were incorporated onto the PS surface. As shown in Fig. 5c–e, with increasingly employing NH3·H2O from 1.0 mL, 2.0 mL to 3.0 mL, the final residue weight of the PS@SiO2 were 16.96%, 20.82% and 24.55%, demonstrating that more silica was incorporated onto PS template. But as the content of NH3·H2O increased to 4.0 mL (Fig. 5f), the final residue weight of the raspberry like PS@SiO2 decreased to 3.91%. The results of thermogravimetric analysis were consistent with those of SEM and TEM in Fig. 3 and 4. The use of NH3·H2O with 2.0–3.0 mL appeared to be an optimum for this system, for it provided sufficient surface roughness and silica coverage.
Effect of the molar mass of PVP
The mass weight of the PVP used played an important role in the stability of the microspheres during the hydrolysis process, since it provided the affinity of tetraethyl orthosilicate with PS template and led TEOS hydrolysis into silica nanoparticles in situ on the surfaces of PS microspheres to obtain raspberry-like PS@SiO2 composite microspheres. So the PVP molar mass had an important impact on the surface roughness of raspberry-like microspheres and silica coverage. In this study, the PVP with average molar masses of 10 kg mol−1 (PVP-10), 40 kg mol−1 (PVP-40), 58 kg mol−1 (PVP-58), and 130 kg mol−1 (PVP-130) were not only used as stabilizer to prepared PS microspheres, but also used as an anchor to adsorb and in situ hydrolysis of TEOS into silica nanoparticles on the surface of PS microspheres, and to examine how the molar mass of PVP affected the surface morphology of raspberry-like PS@SiO2 composite microspheres.Fig. 6 showed the SEM images of raspberry-like PS@SiO2 composite microspheres prepared with different molar mass of PVP as stabilizer. It could be clearly observed that all the four runs yielded the raspberry-like PS@SiO2 composite spheres and the SiO2 nanoparticles were evenly distributed on the surface of PS microspheres. Meanwhile, it was found that with the increase of chain length of PVP, the mean diameter of the PS spheres decreased from 2.2 μm to less than 1 μm and the monodispersity deterioration which was similar with other reported dispersion polymerization system to prepare polymer microspheres.56 In Fig. 6a, raspberry-like PS@SiO2 composite microsphere fabricated with PVP-10 stabilized PS microsphere templates, it had a relatively smooth silica-covered aggregates and homogeneous coating thickness. When the molar mass of PVP increased to PVP-40 and PVP-58, the surface of raspberry-like PS@SiO2 composite microsphere turned to more roughness and densest (Fig. 6b and c). The microspheres coated with PVP-130 had a much more inhomogeneous coating thickness and some silica microspheres with multiple layers were observed, but both the PS microspheres and raspberry-like PS@SiO2 composite microsphere showed polydispersity, which were shown in Fig. 6d and S1d.†
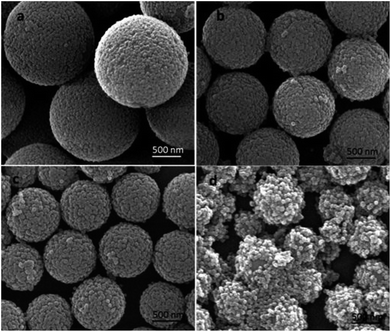 | ||
| Fig. 6 SEM images of silica coated PS with: (a) PVP-10; (b) PVP-40, (c) PVP-58 and (d) PVP-130. NH3·H2O = 2 mL. | ||
These changes have also been confirmed by TEM, as shown in Fig. 7, in which a close inspection among the raspberry-like PS@SiO2 microspheres was observed. The thickness of the silica shell were about 40 nm, 57 nm, 60 nm and 100 nm, respectively, with the chain length of PVP increased from 10 kg mol−1, 40 kg mol−1, 58 kg mol−1 and 130 kg mol−1. The results indicated that silica was growing onto the PS microspheres directly. Moreover, longer chain length of PVP can provide more tertiary amine sites which can strongly adsorbed TEOS on the surface of the PS particles and subsequently in situ hydrolysis into silica nanoparticles. In this process, the abundant amount of PVP was not only involving in dispersion polymerization forming monodispersed PS microspheres, but also playing a pivotal role in the biomimetic silification, since the tertiary amine sites may strongly adsorbed TEOS and catalyze the hydrolysis and condensation polymerization. Both of which are key factors for the formation of raspberry-like PS@SiO2 composite microspheres.
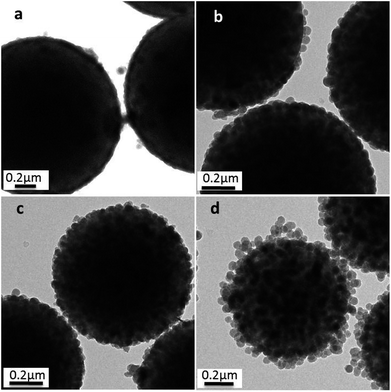 | ||
| Fig. 7 TEM images of silica coated PS with: (a) PVP-10; (b) PVP-40, (c) PVP-58 and (d) PVP-130. NH3·H2O = 2 mL. | ||
Thermogravimetric analysis was also used to quantitatively determine the effects of the molecular weight of PVP on the silica nanoparticles deposited onto PS surface (Fig. 8). As shown in Fig. 8a–d, with increasing molecular weight from 10 kg mol−1, 40 kg mol−1, 58 kg mol−1 to 130 kg mol−1, the final residue weight ratio of the composite microspheres was 12.21 wt%, 20.82 wt%, 24.51 wt% and 38.71 wt%, respectively. This result demonstrated that more silica was incorporated onto the PS microspheres, which was accordance with the analyses above.
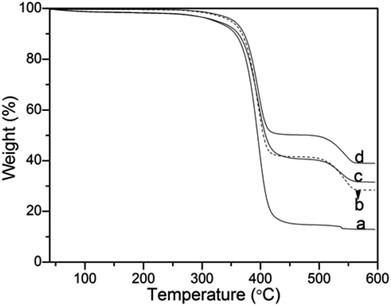 | ||
| Fig. 8 Thermogravimetric analysis (TGA) curves of (a) PVP-10; (b) PVP-40, (c) PVP-58 and (d) PVP-130. NH3·H2O = 2 mL. | ||
Wettability of coatings assembly from dual sized PS@SiO2 microspheres
As is well known that superhydrophobic surface can be obtained by lowering the surface energy and adjusting the surface roughness. Raspberry-like composite microspheres which had uniform sizes and regular hierarchical surface roughness were the ideal building blocks for constructing superhydrophobic films. The schematic illustration of procedure for construction of superhydrophobic surfaces based on hydrophobic raspberry-like PS@SiO2 composite microspheres was shown in Scheme 2.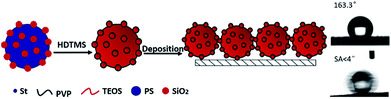 | ||
| Scheme 2 Schematic illustration of procedure for construction of superhydrophobic surfaces based on hydrophobic raspberry-like PS@SiO2 composite microspheres. | ||
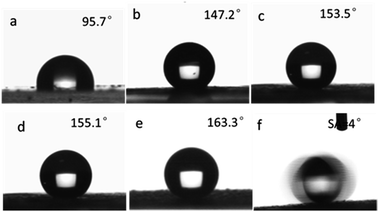
So in this study, the influence of the surface roughness on the superhydrophobic property was investigated. The hydrophobic property can be reflected by the water contact angle (WCA) of a water droplet on the as-prepared surface. The film which was made from pure micro-sized PS microspheres showed a water CA of 95.7°, which was a little higher than that of smooth PS film. This was because the PS microspheres were prepared via dispersion polymerization using PVP as stabilizer, the surface of the microspheres were anchored by hydrophilic PVP that reduce the surface hydrophobicity which was also accordance with other reports.57 After hydrophobization treatment with HDTMS, the raspberry-like PS@SiO2 composite microspheres were used to fabricate hydrophobic film forming a 3D multi-scales regular hierarchical surface. Accordingly, the particulate films comprised of the as-prepared raspberry-like PS@SiO2 microspheres all showed enhanced hydrophobic feature. In detail, the water CA increased from 147.2° (Fig. 9c) to 153.5° (Fig. 9d) and 155.1° (Fig. 9e), and further to 163.3° (Fig. 9f), with the corresponding roughened of the raspberry-like PS@SiO2 microspheres. Among them, the samples showed in Fig. 9d and e exhibited superhydrophobic properties against water, on which the water droplet is in Cassie–Baxter state.
The results mentioned above indicated that the water contact angles of the particulate films were closely related to the surface roughness of the raspberry-like PS@SiO2 composite microspheres. With increasing the molecular weight of the PVP used, the surface roughness of the microspheres obtained correspondingly increased (Fig. 3d and 6), which altered the geometry of their built films. According to the Wenzel and Cassie–Baxter model, magnified hydrophobicity could be approached by increasing the surface roughness. Therefore, adjusting the rough structure of the particles can effectively tune the wettability of the film. Enhanced roughness can endow film with the ability to trap a very large fraction of air, which can repel the permeation of water as a result forming more hydrophobic surface. Moreover, the water droplet could easily slide on the lifted surface with a very low tilt angle of 4°, as shown in the time-lapse images in Fig. 9f and Movie S1.†
The above mentioned method paved an avenue for further biomimic the lotus leaves surface structure. And this kind of raspberry-like particles could be used in many porous materials such as paper, cotton, sponge and steel mesh (shown in Fig. 10). These superhydrophobic/superoleophilic porous substrates can further be utilized for oil–water separation.
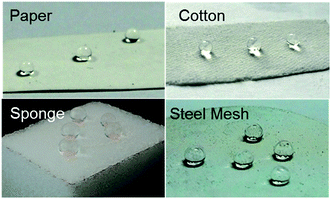 | ||
| Fig. 10 Optical images of water droplets on microspheres treated (a) paper, (b) cotton, (c) sponge and (d) steel mesh. | ||
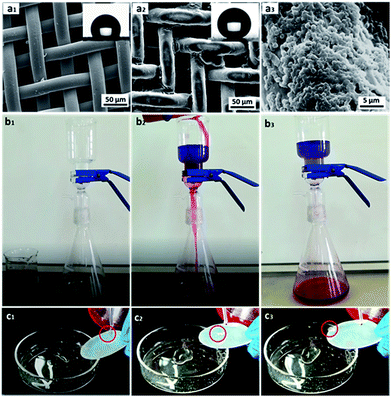
In this study, hydrophobicity-modified steel mesh with rigid frame was representatively selected for oil/water separation. The surface morphologies of the pristine and modified steel meshes were characterized using FE-SEM (Fig. 11a). Fig. 11a1 showed that the pristine steel mesh had a smooth surface. After modified with raspberry-like particles, it could be seen that the particles were closely attached to the surface of the steel mesh forming the typical micro/nano multi-scale hierarchical structure (Fig. 11a2 and a3). Meanwhile, the pore size of the modified mesh retained almost the same, which ensures free passage of liquids through the modified mesh. Moreover, the water contact angle of the modified steel mesh was 160.6°, while the water contact angle of pristine steel mesh was only 121.7°. This demonstrated that the modified of raspberry-like particles had endowed the steel mesh excellent superhydrophobic/superoleophilic property.58
Then, chloroform–water mixture (200 mL, 1/1, v/v) was chosen as a representative separation test to evaluate the oil–water separation efficiency, which was shown in Fig. 11b. When the blue-dyed water was added to the funnel, it retained on the upper part of the mesh while the red-dyed chloroform quickly spread and permeated through the as prepared steel mesh and rapidly dropped into the conical flask. And there was no visible blue dyed water in the chloroform, since water was retained on the upper part of the mesh due to its excellent water repellence (see Movie S2 in the ESI†). Moreover, after the oil–water separation experiment, the mesh still showed excellent superhydrophobic properties, which implied that the steel mesh modified with raspberry-like microspheres showed robust superhydrophobicity even under oil immersion and could be used recycled (see Fig. 11c and Movie S3 in the ESI†).
The separation efficiency of the superhydrophobic/superoleophilic steel mesh was also investigated. The separation efficiency was calculated by the weight ratio between the water before and after the separation process. The separation efficiency of the steel mesh was calculated up to 95.4% for the chloroform–water mixture after 5 recycle times, as shown in Fig. 12a.
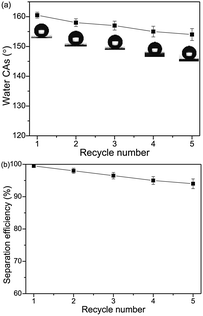 | ||
| Fig. 12 Variation of the water CAs (a) and the separation efficiencies (b) of the steel mesh versus the recycle numbers by taking chloroform–water as an example. | ||
As important criteria for practical applications, the recyclability of the steel mesh were also investigated. As shown in Fig. 12b, the water contact angles of modified steel mesh decreased slightly from 160.6° to 153.8°. The steel mesh still retained superhydrophobicity after five separation cycles.
Besides, when the oil density is smaller than that of water (ρoil < ρwater), the PU sponges can be used for the removal oil from an oil/water mixture (see Fig. S2 and Movie S4 in the ESI†). These results suggested that the as-prepared porous materials had excellent superhydrophobicity and can rapidly separate oil–water mixture at large scale.
Conclusions
In conclusion, we presented a facile route for the synthesis of raspberry-like PS@SiO2 composite microspheres which could further used for the fabrication of superhydrophobic surfaces. In this procedure, the PVP used are not only involving in dispersion polymerization forming monodispersed PS microspheres, but also playing a pivotal role in the biomimetic silification, since the tertiary amine sites can strongly adsorb TEOS and the biomimetic silification. The surface geometry of these raspberry-like PS@SiO2 microspheres can be well tailored by simply adjusting the molecular weight of PVP and the NH3·H2O concentration. Furthermore, superhydrophobic surfaces can be subsequently obtained via a hydrophobization modification of these dual-scaled raspberry-like PS@SiO2 microspheres, exhibiting a water contact angle of 163.3° and water contact angle hysteresis of 4°. Furthermore, steel mesh treated with hydrophobized raspberry-like PS@SiO2 microspheres showed excellent oil–water separation efficiency and reusability. It is believed that this facile and robust method for construction the superhydrophobic surface hold many potential application in self-cleaning and oil–water separation in large-scale.Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
This work was supported by the High-Level Talent Start-Up Research Project of Foshan University (gg040945).Notes and references
- X. Ding, S. Zhou, G. Gu and L. Wu, J. Mater. Chem., 2011, 21, 6161–6164 RSC.
- M. G. Salvaggio, R. Passalacqua, S. Abate, S. Perathonera, G. Centib, M. Lanzac and A. Stassi, Sol. Energy, 2016, 12, 5227–5242 Search PubMed.
- S. Liu, S. S. Latthe, H. Yang, B. Liu and R. Xing, Ceram. Int., 2015, 41, 11719–11725 CrossRef CAS.
- Y. Lai, Y. Tang, J. Gong, D. Gong, L. Chi, C. Lin and Z. Chen, J. Mater. Chem., 2012, 22, 7420–7426 RSC.
- Y. Wang, L. Zhang, Y. Hu and C. Li, J. Mater. Sci. Technol., 2015, 31, 901–906 Search PubMed.
- K. Ellinas, S. P. Pujari, D. A. Dragatogiannis, C. A. Charitidis, A. Tserepi, H. Zuilhof and E. Gogolides, ACS Appl. Mater. Interfaces, 2014, 6, 6510–6524 CAS.
- Y. Lu, S. Sathasivam, J. Song, C. R. Crick, C. J. Carmalt and I. P. Parkin, Science, 2015, 347, 1132–1135 CrossRef CAS PubMed.
- L. Shen, B. Wang, J. Wang, J. Fu, C. Picart and J. Ji, ACS Appl. Mater. Interfaces, 2012, 4, 4476–4483 CAS.
- Z. Wang, Y. Su, Q. Li, Y. Liu, Z. She, F. Chen, L. Li, X. Zhang and P. Zhang, Mater. Charact., 2015, 99, 200–209 CrossRef CAS.
- B. Bai, N. Quici, Z. Li and G. L. Puma, Chem. Eng. J., 2011, 170, 451–456 CrossRef CAS.
- Z. Zhou and X. Wu, Mater. Lett., 2015, 160, 423–427 CrossRef CAS.
- M. W. Lee, S. An, S. S. Latthe, C. Lee, S. Hong and S. S. Yoon, ACS Appl. Mater. Interfaces, 2013, 5, 10597–10604 CAS.
- Q. Wang, M. Yu, G. Chen, Q. Chen and J. Tai, BioResources, 2017, 12, 643–654 CrossRef CAS.
- X. Zhou, Z. Zhang, X. Xu, F. Guo, X. Zhu, X. Men and B. Ge, ACS Appl. Mater. Interfaces, 2013, 5, 7208–7214 CAS.
- W. Zhang, Y. Zhu, X. Liu, D. Wang, J. Li, L. Jiang and J. Jin, Angew. Chem., Int. Ed., 2014, 53, 856–860 CrossRef CAS PubMed.
- J. Li, L. Yan, X. Tang, H. Feng, D. Hu and F. Zha, Adv. Mater. Interfaces, 2016, 3, 1500770–1500777 CrossRef.
- J. Li, D. Li, Y. Yang, J. Li, F. Zha and Z. Lei, Green Chem., 2016, 18, 541–549 RSC.
- J. Li, R. Kang, X. Tang, H. She, Y. Yang and F. Zha, Nanoscale, 2016, 8, 7638–7645 RSC.
- J. Li, C. Xu, Y. Zhang, R. Wang, F. Zha and H. She, J. Mater. Chem. A, 2016, 4, 15546–15553 CAS.
- Y. Wang, X. Li, H. Hu, G. Liu and M. Rabnawaz, J. Mater. Chem. A, 2014, 2, 8094–8102 CAS.
- L. Zhang, J. Wu, M. N. Hedhili, X. Yang and P. Wang, J. Mater. Chem. A, 2015, 3, 2844–2852 CAS.
- L. Li, L. Liu, J. Lei, J. He, N. Li and F. Pan, J. Mater. Chem. A, 2016, 4, 12334–12340 CAS.
- P. Zhang, H. Chen, L. Zhang, Y. Zhang, D. Zhang and L. Jiang, J. Mater. Chem. A, 2016, 4, 12212–12220 CAS.
- M. Tuominen, H. Teisala, J. Haapanen, J. M. Mäkelä, M. Honkanen, M. Vippola, S. Bardage, M. E. P. Wålinder and A. Swerin, Appl. Surf. Sci., 2016, 389, 135–143 CrossRef CAS.
- N. Wang, D. Xiong, S. Pan, Y. Deng, Y. Shi and K. Wang, Appl. Surf. Sci., 2016, 389, 354–360 CrossRef CAS.
- I. O. Arukalam, E. E. Oguzie and Y. Li, J. Colloid Interface Sci., 2016, 484, 220–228 CrossRef CAS PubMed.
- Y. Si, F. Yang and Z. Guo, J. Colloid Interface Sci., 2016, 484, 173–182 CrossRef CAS PubMed.
- W. Wang, K. Lockwood, L. M. Boyd, M. D. Davidson, S. Movafaghi, H. Vahabi, S. R. Khetani and A. K. Kota, ACS Appl. Mater. Interfaces, 2016, 8, 18664–18668 CAS.
- Y. Lin, G. J. Ehlert, C. Bukowsky and H. A. Sodano, ACS Appl. Mater. Interfaces, 2011, 3, 2200–2203 CAS.
- A. Davis, Y. H. Yeong, A. Steele, I. S. Bayer and E. Loth, ACS Appl. Mater. Interfaces, 2014, 6, 9272–9279 CAS.
- U. Manna and D. M. Lynn, ACS Appl. Mater. Interfaces, 2013, 5, 7731–7736 CAS.
- S. Pana, N. Wang, D. Xiong, Y. Deng and Y. Shi, Appl. Surf. Sci., 2016, 389, 547–553 CrossRef.
- F. Li, Y. Tu, J. Hu, H. Zou, G. Liu, S. Lin, G. Yang, S. Hu, L. Miao and Y. Mo, Polym. Chem., 2015, 6, 6746–6760 RSC.
- J. H. Yim, V. Rodriguez-Santiago, A. A. Williams, T. Gougousi, D. D. Pappas and J. K. Hirvonen, Surf. Coat. Technol., 2013, 234, 21–32 CrossRef CAS.
- R. Jafari, S. Asadollahi and M. Farzaneh, Plasma Chem. Plasma Process., 2013, 33, 177–200 CrossRef CAS.
- Y. Sun, Y. Yin, M. Chen, S. Zhou and L. Wu, Polym. Chem., 2013, 4, 3020–3027 RSC.
- C. Zhao and A. P. J. Middelberg, RSC Adv., 2013, 3, 21227–21230 RSC.
- X. Fan, X. Jia, Y. Liu, B. Zhang, C. Li, Y. Liu, H. Zhang and Q. Zhang, Polym. Chem., 2015, 6, 703–713 RSC.
- S. Mehlhase, C. G. Schafer, J. Morsbach, L. Schmidt, R. Klein, H. Frey and M. Gallei, RSC Adv., 2014, 4, 41348–41352 RSC.
- F. Li, Y. Tu, J. Hu, H. Zou, G. Liu, S. Lin, G. Yang, S. Hu, L. Miao and Y. Mo, Polym. Chem., 2015, 6, 6746–6760 RSC.
- T. Ren, J. Wang, J. Yuan, M. Pan, G. Liu, G. Zhang, G. Zhong and Z. Li, RSC Adv., 2015, 5, 36845–36857 RSC.
- C. Du, N. Zhang, S. Ding, X. Gao, P. Guan and X. Hu, Polym. Chem., 2016, 7, 4531–4541 RSC.
- R. Guo, X. Chen, X. Zhu, A. Dong and J. Zhang, RSC Adv., 2016, 6, 40991–41001 RSC.
- X. Du, X. Liu, H. Chen and J. He, J. Phys. Chem. C, 2009, 113, 9063–9070 CAS.
- Z. Qian, Z. Zhang, L. Song and H. Liu, J. Mater. Chem., 2009, 19, 1297–1304 RSC.
- H. Zhu, Q. Zhang and S. Zhu, Dalton Trans., 2015, 44, 16752–16757 RSC.
- Q. Shang, M. Wang, H. Liu, L. Gao and G. Xiao, Polym. Compos., 2013, 34, 51–57 CrossRef CAS.
- Y. Cai, H. Xie, J. Sun, H. Liu, J. Wang, Y. Zhou, W. Nie and L. Son, Mater. Chem. Phys., 2013, 137, 796–801 CrossRef CAS.
- Z. Li, C. Wu, K. Zhao, B. Peng and Z. Deng, Colloids Surf., A, 2015, 470, 80–91 CrossRef CAS.
- D. Xu, M. Wang, X. Ge, M. H. Lamb and X. Ge, J. Mater. Chem., 2012, 22, 5784–5791 RSC.
- X. Fan, X. Jia, Y. Liu, B. Zhang, C. Li, Y. Liu, H. Zhang and Q. Zhang, Polym. Chem., 2015, 6, 703–713 RSC.
- F. Caruso, R. A. Caruso and H. Mohwald, Science, 1998, 282, 1111–1114 CrossRef CAS PubMed.
- Q. Shang, M. Wang, H. Liu, L. Gao and G. Xiao, Polym. Compos., 2013, 34, 51–57 CrossRef CAS.
- C. Graf, D. L. J. Vossen, A. Imhof and A. V. Blaaderen, Langmuir, 2003, 19, 6693–6700 CrossRef CAS.
- L. Jia, L. Tong, Y. Liang, A. Petretic, G. Guerin, I. Manners and M. A. Winnik, J. Am. Chem. Soc., 2014, 136, 16676–16682 CrossRef CAS PubMed.
- J. Song, F. Tronc and M. A. Minnik, J. Am. Chem. Soc., 2004, 126, 6562–6563 CrossRef CAS PubMed.
- R. Wang, H. Liu and F. Wang, Langmuir, 2013, 29, 11440–11448 CrossRef CAS PubMed.
- Q. Wang, M. Yu, G. Chen, Q. Chen and J. Tian, J. Mater. Sci., 2017, 52, 2549–2559 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7ra07250c |
| This journal is © The Royal Society of Chemistry 2017 |