Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
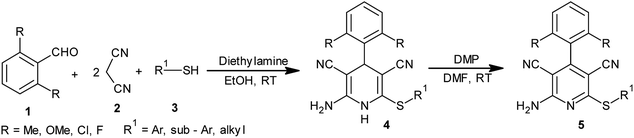
Diethylamine Dess–Martin periodinane: an efficient catalyst–oxidant combination in a sequential, one-pot synthesis of difficult to access 2-amino-3,5-dicarbonitrile-6-sulfanylpyridines at ambient temperature†
R. V. Kupwadea,
S. S. Khota,
M. A. Kulkarnia,
U. V. Desai a and
P. P. Wadgaonkarb
a and
P. P. Wadgaonkarb
aDepartment of Chemistry, Shivaji University, Kolhapur, Maharashtra, India. E-mail: uvdchem2011@gmail.com; uprabhu_desai@rediffmail.com
bPolymer Science and Engineering Division, CSIR National Chemical Laboratory, Pune, India
First published on 9th August 2017
Abstract
Herein, diethylamine Dess–Martin periodinane has been demonstrated for the first time as an efficient catalyst–oxidant combination in a sequential, one-pot synthesis of medicinally privileged but difficult to access 2-amino-3,5-dicarbonitrile-6-sulfanylpyridines via a pseudo-four component reaction between 2,6-disubstituted benzaldehydes, malononitrile, and thiols. Ambient reaction conditions, excellent yields, and total avoidance of conventional isolation as well as purification are the noteworthy merits of this developed protocol.
Introduction
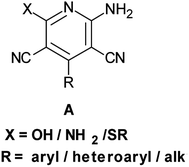
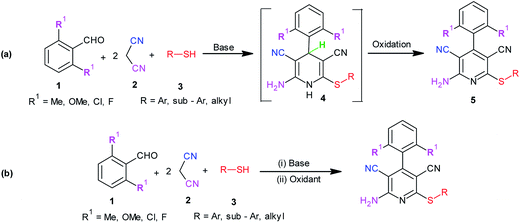
Rapid assembly of molecular diversity, obeying the demands of green chemistry is an important area of current research in organic synthesis. A rational pathway to this goal begins with the design and implementation of appropriate multicomponent reactions.1 Multicomponent reactions for the generation of complex products involve the formation of several bonds with concomitant elimination of simple molecules such as water, ammonia, and alcohol that can be very easily removed from the resultant product. The usefulness of multicomponent reactions increases many fold when they provide an easy access towards medicinally privileged scaffolds that can act as ligands for structurally diverse biological receptors and consequently assist in drug discovery.2 Pyridine-3,5-dicarbonitriles, possessing amino and sulfanyl moieties at the positions C2 and C6, respectively, and aryl or heteroaryl substitution at C4 have long been recognised as one such medicinally important scaffolds. Compounds belonging to this class are known to exhibit diverse pharmacological activities and are useful as anti-prion,3 anti-hepatitis B virus,4 anti-bacterial,5 and anticancer6 agents and as potassium channel openers in the treatment of urinary incontinence.7 A few compounds of this class have been reported to serve as high potency agonists for human adenosine receptors8 and are, therefore, used in the development of new drugs useful in the treatment of Parkinson's disease, hypoxia/ischemia, asthma, kidney disease, and epilepsy.9 This wide range of applications of pyridine-3,5-dicarbonitriles has inspired many researchers to develop efficient methods for their synthesis (Fig. 1).As far as synthesis of 2-amino-3,5-dicarbonitrile-6-sulfanylpyridines is concerned, the most preferred pathway is to perform a multicomponent reaction between an aldehyde, malononitrile, and thiol.10 The synthesis follows the Knoevenagel-carba–Michael-thia–Michael addition-oxidation pathway.11 Taking into account the fact that Knoevenagel as well as Michael addition reactions are typically base-catalyzed reactions, many protocols using the aforementioned substrates have been subsequently developed employing the catalysts such as Et3N, DABCO,10 piperidine, TBAH,11 DBU,12a {(bmim)OH},12b KF-Al2O3,12c nanocrystalline-MgO,12d ethanolic KOH,12e and diethylamine12f (Scheme 1). Although these reported protocols are efficient, they are associated with a common drawback that using 2,6-disubstituted benzaldehyde as an aldehyde component, instead of the desired 2-amino-3,5-dicarbonitrile-6-sulfanylpyridine 5, the corresponding 2-amino-3,5-dicarbonitrile-6-sulfanyldihydropyridine, 4 is obtained (Scheme 1a). A logical approach towards the synthesis of this difficult to access 2-amino-3,5-dicarbonitrile-6-sulfanylpyridines would be either to synthesize respective dihydropyridines, 4, and to oxidize them to the corresponding pyridine derivatives 5 in two separate steps (Scheme 1a) or to perform their sequential, one-pot synthesis involving a multicomponent condensation-oxidation approach (Scheme 1b). From a practical viewpoint, the latter approach is certainly advantageous; however, its success depends upon the correct choice of the catalyst–oxidant combination.
Literature studies have revealed that there are only three protocols available for the synthesis of pyridine-3,5-dicarbonitriles, 5. However, two of these protocols are two step protocols and involve the oxidation of 3,5-dicyanodihydropyridines, 4, prepared in the first step to corresponding pyridines, 5, using TCBQ/DDQ or MnO2 as oxidants.3a,10b,11b On the other hand, Mishraet et al. have reported a sequential one-pot synthesis of pyridine-3,5-dicarbonitriles using Na2CO3–KMnO4 as the base–oxidant combination.13 All these protocols are associated with the limitations of elevated temperature and mainly the generation of undesired waste as well as difficulties in the isolation of the product from the reaction mixture. These limitations clearly highlight the need as well as scope for the development of an easily adaptable and high yielding protocol for the synthesis of this difficult to access 2-amino-3,5-dicarbonitrile-6-sulfanylpyridines, 5. Due to our continuous interest in the development of practical and problem solving synthetic methodologies,14a–e we planned to undertake the development of an easily adaptable protocol for the synthesis of pyridine-3,5-dicarbonitriles (5, R1 ≠ H).
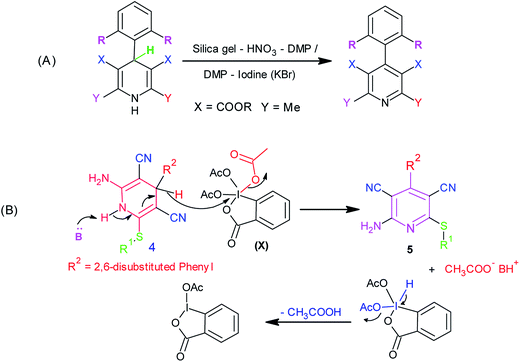
In recent years, there has been considerable growth in the exploration of applications of hypervalent iodine reagents in organic transformations.15 Amongst various hypervalent iodine reagents, Dess–Martin periodinane [1,1,1-tris (acetyloxy)-1,1-dihydro-1,2-benziodoxol-3-(1H)-one, DMP] (X, Scheme 2) is a highly useful hypervalent iodine reagent customarily used in the oxidation of alcohols.16 A literature survey revealed two earlier reports on the use of DMP in the oxidation of Hantzsch dihydropyridines to pyridines. Heravi et al. reported the use of a DMP-silica gel-HNO3 combination,17a whereas Karade et al. demonstrated the use of a DMP-iodine (or KBr) combination17b for this oxidation (Scheme 2A). However, to the best of our knowledge, there are no reports on the use of DMP in the oxidation of 3,5-dicyanodihydropyridines, 4, to pyridine-3,5-dicarbonitriles, 5.
From a mechanistic viewpoint, we surmised that instead of using a DMP-silica gel-HNO3 or DMP-iodine (or KBr) combination, the use of a base-Dess–Martin periodinane combination would be more appropriate for the oxidation of dihydropyridines (Scheme 2B). It was speculated that during the oxidation of dihydropyridines to pyridines using the aforementioned combination, the added base catalyst would abstract the acidic proton attached to nitrogen to generate an anion, which upon rearrangement followed by elimination of hydride ion would yield the corresponding pyridine derivative. This hydride ion on attack over iodine in Dess–Martin periodinane would cause the release of acetate ion. It was also surmised that the synthesis of intermediate dihydropyridines, 4, typically being a base-catalyzed reaction (Scheme 1a), success to the speculated base-catalyzed oxidation of dihydropyridines, 4, to pyridines, 5, would provide us an opportunity to achieve sequential, one-pot synthesis of pyridine-3,5-dicarbonitriles, 5 (Scheme 1B). Based upon this speculation, we initially tested the feasibility of a base-Dess–Martin periodinane combination in the oxidation of 3,5-dicyanodihydropyridines, 4, to pyridine-3,5-dicarbonitriles, 5 (Scheme 2B).
Results and discussion
For preliminary studies, representative 3,5-dicyanodihydropyridine, 4a, was prepared employing 2,6-dichlorobenzaldehyde, malononitrile, and thiophenol as substrates and diethylamine as the catalyst.12f Subsequently, a set of model reactions was carried out using Dess–Martin periodinane as an oxidant and Na2CO3, K3PO4, DBU, DABCO, piperidine, triethylamine, and diethylamine as representative base catalysts. Thus, to a well stirred solution of 4a (1 mmol) in dimethylformamide (2 mL) and chosen base catalyst (20 mol%), Dess–Martin periodinane (1 mmol) was added. Stirring was continued at ambient temperature, and the reactions were monitored by TLC. Upon completion of each reaction (TLC), water (10 mL) was added, and stirring was continued for further half an hour. The resultant free flowing solid was filtered, washed with water, dried, washed with chloroform, and dried again. Spectral analysis of the resultant product confirmed it as the desired pyridine-3,5-dicarbonitrile 5a. To confirm the role of the base as a catalyst, the model reaction was then carried out in the absence of the catalyst. However, the oxidation reaction took a very long time (4 h) and furnished the desired pyridine derivative in an unacceptable yield (Table 1, entry 9). In addition, we have most recently reported diethylamine-Oxone as an efficient catalyst–oxidant combination in the oxidation of sulfides to sulfones.18 Hence, the same combination was also screened in the oxidation of dihydropyridine, 4a, to pyridine, 5a. However, the reaction furnished the desired pyridine derivative in very poor yield (Table 1, entry 10). From the results summarized in Table 1, it is evident that as compared to inorganic bases such as potassium phosphate and sodium carbonate, with organobasic catalysts, the oxidation proceeded well. Furthermore, the organic bases, (e.g., triethylamine, diethylamine, DBU, and piperidine), with very close pKb values, were found to be equally effective (Table 1) as catalysts in this oxidation. From the results, it is also evident that from economic as well as environmental viewpoints, diethylamine is the best suited basic catalyst in Dess–Martin periodinane-mediated oxidation of dihydropyridine, 4a, to pyridine, 5a.| No. | Catalyst (20 mol%) | Time (min) | Yield (%) |
|---|---|---|---|
| a Reaction conditions: dihydropyridine, 4a (1 mmol), DMF (2 mL), Dess–Martin periodinane (1 mmol), base, RT.b Using 15 mol% diethylamine.c In the absence of base.d Using Oxone as an oxidant. | |||
| 1 | Na2CO3 | 20 | 84 |
| 2 | K3PO4 | 20 | 86 |
| 3 | DBU | 10 | 87 |
| 4 | DABCO | 10 | 89 |
| 5 | Piperidine | 10 | 90 |
| 6 | Triethylamine | 15 | 92 |
| 7 | Diethylamine | 5 | 93 |
| 8 | Diethylamineb | 10 | 91 |
| 9 | —c | 240 | 65 |
| 10 | Diethylamined | 240 | 20 |
The primary objective of this study was not limited to the oxidation of 3,5-dicyanodihydropyridines, 4, to the corresponding pyridine derivatives 5, but was mainly the development of a sequential, one-pot protocol for the synthesis of pyridine-3,5-dicarbonitriles, 5. In this regard, another model reaction was carried out wherein, to a well stirred solution of 2,6-dichlorobenzaldehyde, malononitrile, and thiophenol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
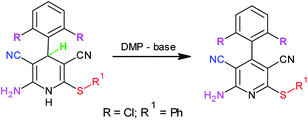
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 equiv.) in ethanol (3 mL), diethylamine (20 mol%) was added, and stirring was continued at ambient temperature. Upon completion of the reaction, the resultant dihydropyridine 4a was directly dissolved in dimethylformamide (2 mL), and Dess–Martin periodinane (1 mmol) was added to it. Stirring was continued, and the reaction was monitored by TLC. Upon completion of the reaction (TLC), followed by workup as described earlier, the desired product 5a was obtained in excellent yield (93%). Based on this result, it can be concluded that diethylamine Dess–Martin periodinane can serve as an efficient catalyst–oxidant combination in a sequential, one-pot synthesis of easily non-accessible 2-amino-3,5-dicarbonitrile-6-sulfanylpyridines, 5 (Scheme 2).
1 equiv.) in ethanol (3 mL), diethylamine (20 mol%) was added, and stirring was continued at ambient temperature. Upon completion of the reaction, the resultant dihydropyridine 4a was directly dissolved in dimethylformamide (2 mL), and Dess–Martin periodinane (1 mmol) was added to it. Stirring was continued, and the reaction was monitored by TLC. Upon completion of the reaction (TLC), followed by workup as described earlier, the desired product 5a was obtained in excellent yield (93%). Based on this result, it can be concluded that diethylamine Dess–Martin periodinane can serve as an efficient catalyst–oxidant combination in a sequential, one-pot synthesis of easily non-accessible 2-amino-3,5-dicarbonitrile-6-sulfanylpyridines, 5 (Scheme 2).
Next, we explored the scope and generality of the protocol. Accordingly, 2,6-dichloro, 2,6-dimethyl, 2,6-dimethoxy, and 2,6-difluoro benzaldehyde were chosen as aldehyde components while keeping malononitrile fixed as the second component. Thiols such as thiophenol, 4-bromothiophenol, 4-methylthiophenol, 4-chlorothiophenol, 4-methoxy-thiophenol, and cyclohexyl, n-butyl, and ethane thiol were allowed to react under the established reaction conditions. In each case, the corresponding 2-amino-3,5-dicarbonitrile-6-sulfanyl pyridine 5b–r was obtained in excellent yields (Table 2). It was noticed that yield of the desired pyridine derivative did not depend upon the nature of the substituent, either an aldehyde or thiol, on the aromatic ring. Even the aliphatic thiols equally worked well. The key advantages of this sequential one-pot protocol were associated with the facts that (i) each reaction could be arrested at the stage of the formation of the intermediate dihydrodicyanopyridine (4a–r) or may be directed towards the synthesis of the corresponding pyridine derivative (5a–r) and (ii) after isolation of the product at any of these two stages, the resultant product did not require any further purification.
| No. | Aldehyde (1) | Thiol (3) (R1=) | Product (4)b | Product (5) | Product (5)c | Melting point (°C) |
|---|---|---|---|---|---|---|
| Time (h) | Time (min) | Yield (%) | ||||
| a Reaction conditions: aldehyde (1 mmol), malononitrile (2 mmol), thiol (1 mmol), diethylamine (20 mol%), RT; after completion of reaction (step 1, product 4), add dimethylformamide (2 mL) and Dess–Martin periodinane (1 mmol), rt (step 2, product 5).b Reaction can be arrested at step 1 to isolate dihydropyridine, 4 or may be continued to obtain the corresponding pyridine derivative, 5.; for detailed characterization of product 4, see ESI.c Total time of the reaction is the sum of the time in columns 4 and 5. | ||||||
| a | 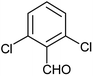 |
Phenyl | 2.5 | 10 | 93 | 190–192 |
| b | 4-Methylphenyl | 1.5 | 5 | 94 | 206–208 | |
| c | 4-Chlorophenyl | 1.5 | 5 | 95 | 174–178 | |
| d | 4-Bromophenyl | 2 | 5 | 96 | 184–186 | |
| e | Cyclohexyl | 2 | 10 | 96 | 192–194 | |
| f | n-Butyl | 2 | 10 | 95 | 142–144 | |
| g | Ethyl | 2 | 10 | 90 | 164–166 | |
| h | 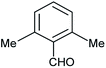 |
Phenyl | 2 | 5 | 95 | 210–212 |
| i | 4-Bromophenyl | 1.5 | 5 | 93 | 260–264 | |
| j | 4-Chlorophenyl | 1.5 | 5 | 96 | 216–218 | |
| k | 4-Methoxyphenyl | 2 | 10 | 95 | 243–245 | |
| l | 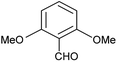 |
Phenyl | 2 | 5 | 94 | 196–198 |
| m | 4-Chlorophenyl | 1.5 | 5 | 96 | 208–210 | |
| n | 4-Methylphenyl | 1.5 | 5 | 96 | 194–196 | |
| o |  |
Phenyl | 2 | 10 | 92 | 274–276 |
| p | 4-Chlorophenyl | 1.5 | 5 | 95 | 280–282 | |
| q | 4-Methylphenyl | 1.5 | 5 | 94 | 196–198 | |
| r | Cyclohexyl | 2 | 5 | 96 | 164–166 | |
Conclusion
2-Amino-3,5-dicarbonitrile-6-sulfanylpyridines have been reported to possess a very wide range of medicinal and biological properties. However, the main issues involved in their syntheses are associated with the choice of an aldehyde component as well as the oxidation of intermediate dihydropyridines. In the present study, we have demonstrated for the first time that diethylamine Dess–Martin periodinane served as an extremely useful catalyst–oxidant combination in a sequential, one-pot synthesis of difficult to access 2-amino-3,5-dicarbonitrile-6-sulfanylpyridines. Possible arrest of the reaction at an intermediate stage to isolate dihydropyridines, obtain excellent yields, and avoid conventional isolation as well as a purification procedure are the noteworthy merits of this approach. Employing the results obtained in this study, the development of another sequential one-pot protocol for the synthesis of medicinally privileged compounds is being carried out by our group.Experimental
General
All the chemicals were commercially available and used as received. Melting points were obtained using a Kumar melting point apparatus and were uncorrected. IR spectra were obtained using a Thermo Scientific Nicolet iS10 FT-IR Spectrometer. 1H NMR (300 MHz) and 13C NMR (75 MHz) spectra were obtained using a Bruker Avance II spectrometer. High resolution mass spectra (HRMS) were obtained using a Thermo Scientific Q-Exactive, Accela 1250 pump, instrument.Representative procedure for the sequential, one-pot synthesis of 2-amino-3,5-dicarbonitrile-6-sulfanylpyridines
To a well-stirred solution of 2,6-disubstituted benzalldehyde, malononitrile and thiol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mmol) in ethanol (3 mL), diethylamine (20 mol%) was added, and stirring was continued at ambient temperature. Upon completion of the reaction (TLC), resultant dihydropyridine, 4, was (may be isolated and identified) dissolved in dimethylformamide (2 mL). To the resulting solution, Dess–Martin periodinane (1 mmol) was added, and stirring was continued. Upon completion of oxidation (TLC), water (10 mL) was added, and stirring was continued till free flowing solid separated out. Resultant solid was filtered, washed with water, dried, washed again with chloroform, and dried. Resultant 2-amino-3,5-dicarbonitrile-6-sulfanylpyridine, 5, was found to be pure and did not require any further purification.
1 mmol) in ethanol (3 mL), diethylamine (20 mol%) was added, and stirring was continued at ambient temperature. Upon completion of the reaction (TLC), resultant dihydropyridine, 4, was (may be isolated and identified) dissolved in dimethylformamide (2 mL). To the resulting solution, Dess–Martin periodinane (1 mmol) was added, and stirring was continued. Upon completion of oxidation (TLC), water (10 mL) was added, and stirring was continued till free flowing solid separated out. Resultant solid was filtered, washed with water, dried, washed again with chloroform, and dried. Resultant 2-amino-3,5-dicarbonitrile-6-sulfanylpyridine, 5, was found to be pure and did not require any further purification.
Spectral data of pyridine 3,5-dicarbonitriles, 5, is summarized below. For the original spectra of all the products (step 1 and step 2), please see the ESI†
Acknowledgements
Authors S. S. K. and U. V. D. are thankful to UGC, New Delhi, for the junior research fellowship and financial assistance [F. No. 43-221/2014 (SR)], respectively.References
- (a) J. Zhu and H. Bienayme, Multicomponent Reactions, Wiley-VCH, Wienheim, Germany, 2005 Search PubMed; (b) Synthesis of Heterocycles via Multicomponent Reactions: Topics in Heterocyclic Chemistry, ed. R. V. A. Orru and E. Ruijter, Springer, 1st edn, 2010, vol. 15, p. 280 Search PubMed; (c) H. Yi-Fang and M. Xia, Curr. Org. Chem., 2010, 14, 379 CrossRef; (d) C. De Graaff, R. V. A. Orru and E. Ruijter, Chem. Soc. Rev., 2012, 41, 3969 RSC.
- (a) B. E. Evans, et al., J. Med. Chem., 1988, 31, 2235 CrossRef CAS PubMed; (b) A. A. Patchett and R. P. Nargund, Annu. Rep. Med. Chem., 2000, 35, 289 CAS.
- (a) T. R. K. Reddy, R. Mutter, W. Heal, K. Guo, V. J. Gillet, S. Pratt and B. Chen, J. Med. Chem., 2006, 46, 607 CrossRef PubMed; (b) B. C. H. May, J. A. Zorn, J. Witkop, J. Sherrill, A. C. Wallace, G. Legname, S. B. Prusiner and F. E. Cohen, J. Med. Chem., 2007, 50, 65 CrossRef CAS PubMed.
- H. Chen, W. Zhang, R. Tam and A. K. Raney, PCT Int. Appl. WO 2005058315 A1 20050630, 2005.
- (a) S. B. Levy, M. N. Alekshun, B. L. Podlogar, K. Ohemeng, A. K. Verma, T. Warchol, B. Bhatia, T. Bowser and M. Grier, U.S. Patent Appl., 2005124678 A1 20050609, 2005; (b) S. K. Srivastava, R. P. Tripathi and R. Ramachandran, J. Biol. Chem., 2005, 280, 30273 CrossRef CAS PubMed.
- (a) M. T. Cocco, C. Congiu, V. Lilliu and V. Onnis, Eur. J. Med. Chem., 2005, 40, 1365 CrossRef CAS PubMed; (b) D. R. Anderson, N. W. Stehle, S. A. Kolodziej and E. J. Reinhard, PCT Int. Appl. WO 2004055015 A1 20040701, 2004; (c) M. A. Azuine, H. Tokuda, J. Takayasu, F. Enjyo, T. Mukainaka, T. Konoshima, H. Nishino and G. Kapadia, J. Pharmacol. Res., 2004, 49, 161 CrossRef CAS.
- H. Harada, S. Watanuki, T. Takuwa, K. Kawaguchi, T. Okazaki, Y. Hirano and C. Saitoh, PCT Int. Appl. WO 2002006237 A1 20020124, 2002.
- (a) U. Rosentreter, T. Kraemer, M. Shimada, W. Huebsch, N. Diedrichs, T. Krahn, K. Henninger and J. P. Stasch, DE 10238113 A1 20030618, 2003; (b) L. C. W. Chang, J. K. von Frijtag Drabbe Künzel, T. Mulder Krieger, R. F. Spanjersberg, S. F. Roerink, G. van den Hout, M. W. Beukers, J. Brussee and A. P. Ijzerman, J. Med. Chem., 2005, 48, 2045 CrossRef CAS PubMed and references therein.
- (a) B. B. Fredholm, A. P. Ijzerman, K. A. Jacobson, K. N. Klotz and J. Linden, Pharmacol. Rev., 2001, 53, 527 CAS; (b) V. Perrier, A. C. Wallace, K. Kaneko, J. Safar, S. B. Prusiner and F. E. Cohen, Proc. Natl. Acad. Sci. U. S. A., 2000, 97, 6073 CrossRef CAS PubMed.
- (a) N. M. Evdokimov, I. V. Magedov, A. S. Kireev and A. Kornienko, Org. Lett., 2006, 8, 899 CrossRef CAS PubMed; (b) N. M. Evdokimov, A. S. Kireev, A. A. Yakovenko, M. Y. Antipin, I. V. Magedov and A. Kornienko, J. Org. Chem., 2007, 72, 3443 CrossRef CAS PubMed.
- (a) K. Guo, M. J. Thompson, T. R. K. Reddy, R. Mutter and B. Chen, Tetrahedron, 2007, 63, 5300 CrossRef CAS; (b) K. Guo, M. J. Thompson and B. Chen, J. Org. Chem., 2009, 74, 6999 CrossRef CAS PubMed.
- (a) R. Mamgain, R. Sing and D. S. Rawat, J. Heterocycl. Chem., 2009, 46, 69 CrossRef CAS; (b) B. C. Ranu, R. Jana and S. Sowmiah, J. Org. Chem., 2007, 72, 3152 CrossRef CAS PubMed; (c) K. N. Singh and S. K. Singh, ARKIVOC, 2009, 13, 153 Search PubMed; (d) M. Lakshmikantam, K. Mahendar and S. Bhargava, J. Chem. Sci., 2010, 122(1), 63 CrossRef; (e) M. N. Khan, S. Pal, T. Parvinb and L. H. Choudhury, RSC Adv., 2012, 2, 12305 RSC; (f) U. V. Desai, M. A. Kulkarni, K. S. Pandit, A. M. Kulkarni and P. P. Wadgaonkar, Green Chem. Lett. Rev., 2014, 7, 228 CrossRef.
- S. Mishra and R. Ghosh, Synth. Commun., 2012, 42, 2229 CrossRef CAS.
- (a) K. S. Pandit, R. V. Kupwade, P. V. Chavan, U. V. Desai, P. P. Wadgaonkar and K. M. Kodam, ACS Sustainable Chem. Eng., 2016, 4, 3450 CrossRef CAS; (b) K. S. Pandit, P. V. Chavan, U. V. Desai, M. A. Kulkarni and P. P. Wadgaonkar, New J. Chem., 2015, 39, 4452 RSC; (c) R. V. Kupwade, K. S. Pandit, U. V. Desai, M. A. Kulkarni and P. P. Wadgaonkar, Res. Chem. Intermed., 2016, 42, 6313 CrossRef CAS; (d) M. A. Kulkarni, U. V. Desai, V. R. Pandurangi and P. P. Wadgaonkar, C. R. Chim., 2012, 15, 745 CrossRef CAS; (e) M. A. Kulkarni, K. S. Pandit, U. V. Desai, U. P. Lad and P. P. Wadgaonkar, C. R. Chim., 2013, 16, 689 CrossRef CAS.
- (a) T. Wurth, Hypervalent iodine chemistry, Springer publishing company, Switzerland, 2016, vol. 185, p. 208 Search PubMed; (b) A. Vargolis, Hypervalent iodine in organic synthesis, Academic Press, London, 1997 Search PubMed; (c) V. V. Zhdankin, Hypervalent Iodine Chemistry: Preparation, Structure, and Synthetic Applications of Polyvalent Iodine Compounds, Wiley, 2013. Search PubMed.
- (a) U. Ladziata and V. Zhdankin, ARKIVOC, 2006, 9, 26 Search PubMed; (b) A. Speicher, V. Bomm and T. Eicher, J. Prakt. Chem., 1996, 338, 588 CrossRef CAS.
- (a) M. M. Heravi, F. Dirkwand, H. A. Oskooie and M. Ghassemzadeh, Heterocycl. Commun., 2005, 11(1), 75 CAS; (b) N. N. Karade, S. V. Gampawar, J. M. Kondre and S. V. Shinde, ARKIVOC, 2008, 12, 9 Search PubMed.
- R. V. Kupwade, S. S. Khot, U. P. Lad, U. V. Desai and P. P. Wadgaonkar, Res. Chem. Intermed., 2017 DOI:10.1007/s11164-017-3026-0.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7ra07738f |
| This journal is © The Royal Society of Chemistry 2017 |