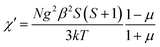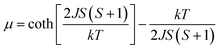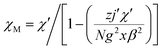Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
The diversities of magnetic behaviors appearing simultaneously in three isomorphs based on 1H-tetrazolyl-acetic acid ligand†
Tong Yan‡
a,
Lin Du‡*a,
Lin Sunb,
Xiao-Feng Zhanga,
Tao Wanga,
Jing Fenga,
Jie Zhoua and
Qi-Hua Zhao *a
*a
aKey Laboratory of Medicinal Chemistry for Natural Resource Education Ministry, School of Chemical Science and Technology Pharmacy, Yunnan University, Kunming, 650091 P. R. China. E-mail: lindu@ynu.edu.cn; qhzhao@ynu.edu.cn; Fax: +86-871-5032929; Tel: +86-871-5032929
bDepartment of Chemistry, Northwest University, Xi'an, Shaanxi, 710069 P. R. China
First published on 27th October 2017
Abstract
Three isomorphous metal–organic complexes with 1H-tetrazolyl-acetic acid (TAA), namely [M(TAA)2]n (M = MnII, 1, CoII, 2 and NiII, 3), have been synthesized under hydrothermal conditions. All the compounds were fully characterized by X-ray crystallography, elemental analysis, IR spectroscopy, thermal analysis and magnetic studies. The single crystal X-ray analyses show that the 1D chains of [M(COO−)2]n (M = MnII, 1, CoII, 2 and NiII, 3) are further joined by tetrazolyl spacers to form a 3D network. Based on the topological perspective, the complexes can be simplified into a (3,6)-connected 2-nodal net with (42.6)2(44.62.88.10) “ant” topology. The magnetic measurements reveal different magnetic exchange-coupling interactions: antiferromagnetic for 1 and ferromagnetic for 2 and 3. Furthermore, the magnetic measurements confirm that spin glass behaviour occurs in 3 but not in 2.
1. Introduction
Metal–organic complexes have been attracting considerable attention in the field of crystal engineering owing to their intriguing architectures and topologies and potential applications.1–8 Molecular magnetic materials are an important part of the research concerning the application of metal–organic complexes and to date, we have attained impressive advancement in their synthesis, design and characterization. These materials are of great theoretical interest in fields such as quantum tunnelling of magnetization (QTM) and potential technological applications such as qubits for quantum computing etc.9–12 However, the design and preparation of magnetic materials such as single molecular magnets (SMMs) or single-chain magnets (SCMs) with bulk magnetic behaviors or special magnetic phenomena are still a challenge. So far, researchers have placed particular emphasis not only on designing new SMMs or SCMs, but also on exploiting other unique magnetic phenomena and behaviors. The selection of metals and ligands for the magnetic materials is extremely important. Usually, the preparation of magnetic materials is focused on the use of paramagnetic metal and bridging ligands because of their abilities in efficient transfer of magnetic exchange interactions.13,14 Therefore, the proper choice of ligands is considerably important and it may provide us with the new structures and types as desired. 1H-Tetrazolyl-acetic acid (TAA) is a good candidate as it exhibits a variety of coordination abilities and diverse coordination modes.15–21 Bai and colleagues reported a 3D mixed-valence iron complex based on the TAA ligand, which shows spin-crossover behaviour.15 The Liu research group reported an Mn(II) compound {[Mn(TAA)2(HTAA)2]·2H2O} grounded on TAA, and the magnetic data displayed that a weak AF interaction exists between the two Mn centers.22In addition, 3D isomorphous organic–inorganic hybrid frameworks with diverse magnetic properties haven been relatively rarely reported in literature. The Kitagawa group23 reported two isomorphous 3D networks of Mn(II) and Co(II) based on naphthalene-1,4-dicarboxylic acid (1,4-napdcH2), showing metamagnetic behaviour (TC = 5.5 K) for Co(II) and a weak antiferromagnetic interaction for Mn(II). Two isomorphous 3D complexes based on CoII/NiII, with the CoII system exhibiting spin-canted antiferromagnetism were reported by Bu and co-workers.24 Other examples based on the lanthanide(III) state with diverse magnetic properties were reported.25,26 Herein, we report three isostructural coordination compounds of the type [M(TAA)2]n based on TAA ligand and three different metal ions: MnII for 1, CoII for 2 and NiII for 3, in which the metal ions are bridged via the carboxyl groups to give an eight-membered ring infinite chain with pillared μ-1-tetrazolyl groups generating a 4,6-connected 3D structure. These compounds were synthesized under hydrothermal conditions. The magnetic susceptibility properties of all complexes were measured, and the alternating current (ac) magnetic susceptibility measurements of 2 and 3 were carried out. In addition, thermal stabilities and the IR spectra of the complexes were studied.
2. Experimental
2.1 Materials and methods
All chemicals were commercially available and used without further purification. Elemental analysis (C, H, N) was performed using an ElementarVario ELIII analyzer. Magnetic susceptibility data of the crystalline samples were obtained using a Quantum Design MPMS-XLSQUID magnetometer. The variable-temperature susceptibility measurements were performed in the temperature range of 2–300 K under an applied field of 1000 Oe. Alternating current (ac) susceptibility measurements were performed with a 2.5 Oe ac oscillating field in an operating frequency range of 10–997 Hz under a dc field of 0 or 1000 Oe. Thermogravimetric analysis (TGA) data were collected using a simultaneous SDT thermal analyzer at a heating rate of 10 °C min−1 under a N2 atmosphere (N2 flow rate = 0.06 L min−1). Fourier transform infrared (FT-IR) spectra were recorded with a Nicolet iS10 Near/Mid-IR Std spectrophotometer using the pressed KBr pellet in the range of 400–4000 cm−1.2.2 Synthesis of complex 1
A mixture of MnCl2·4H2O (0.2 mmol) and TAA (0.4 mmol) was dissolved in 10 mL of acetonitrile, and the mixture was sealed in a Teflon-lined autoclave and heated at 130 °C for 3 days. Moccasin-block crystals were collected and washed sequentially by acetonitrile. Yield: ∼55% based on TAA. Anal. calcd (%) for C6H6MnN8O4 (309.13): C, 23.31; H, 1.96; N, 36.25. Found (%): C, 22.94; H, 2.32; N, 35.70.2.3 Synthesis of complex 2
The synthesis method used for compound 2 was similar to that of 1 except that MnCl2·4H2O was used instead of Co(NO3)2·6H2O. Orange red-block crystals were collected and washed sequentially with acetonitrile. Yield: ∼75% based on TAA. Anal. calcd (%) for C6H6CoN8O4 (313.12): C, 23.02; H, 1.93; N, 35.79. Found (%): C, 22.81; H, 2.23; N, 35.02.2.4 Synthesis of complex 3
The synthesis method for compound 3 is similar to that of 1 except that MnCl2·4H2O was used instead of Ni(NO3)2·6H2O. Spring green-block crystals were collected and washed sequentially with acetonitrile. Yield: ∼79% based on TAA. Anal. calcd (%) for C6H6NiN8O4 (312.90): C, 23.03; H, 1.93; N, 35.82. Found (%): C, 22.97; H, 2.19; N, 35.06.2.5 Crystallographic data collection and refinement
The selected suitable single-crystal was used for the X-ray diffraction analyses. Crystallographic data were collected with graphite-monochromated Mo-Kα radiation (λ = 0.71073 Å) on Bruker Smart APEX II CCD at 293 K. Empirical absorption corrections were carried out using the SADABS program.27 The structures were solved using direct methods and then refined on F2 by the full-matrix least-squares technique using SHELXL-97 software.28,29 All of the non-hydrogen atoms were located using difference Fourier syntheses and were then refined with anisotropic displacement parameters. All the hydrogen atoms of the organic ligands were located using geometrical considerations and isotropically refined with fixed U values using a riding model. Crystallographic data and refinement parameters for complexes 1–3 are summarized in Table 1. The main bond lengths and angles are presented in Tables S1–S3.†| a R1 = Σ||Fo| − |Fc||/Σ|Fo|.b wR2 = [Σw(Fo2 − Fc2)2/Σw(Fo2)2]1/2. | |||
|---|---|---|---|
| Compound | 1 | 2 | 3 |
| Empirical formula | C6H6MnN8O4 | C6H6CoN8O4 | C6H6NiN8O4 |
| Fw | 309.13 | 313.12 | 312.90 |
| Crystal system | Monoclinic | Monoclinic | Monoclinic |
| Space group | C2/c | C2/c | C2/c |
| a/Å | 14.7220(15) | 14.4184(16) | 14.285(2) |
| b/Å | 8.4723(9) | 8.3825(8) | 8.3073(14) |
| c/Å | 9.6326(10) | 9.4836(9) | 9.4700(16) |
| β (°) | 119.0620(10) | 118.5310(10) | 118.178(2) |
| V/Å3 | 1050.19(19) | 1007.01(18) | 990.6(3) |
| Z | 4 | 4 | 4 |
| Dc/g cm−3 | 1.955 | 2.065 | 1.991 |
| 2θ range for data collection | 5.76 to 56.52 | 5.82 to 56.58 | 5.88 to 56.66 |
| μ/mm−1 | 1.287 | 1.736 | 1.287 |
| Reflections collected | 3286 | 4191 | 2986 |
| Unique reflections | 1174 | 1174 | 1150 |
| F(000) | 620.0 | 628.0 | 632.0 |
| Rint | 0.0165 | 0.0165 | 0.0165 |
| GOF on F2 | 1.001 | 1.002 | 0.997 |
| R1a, wR2b [I > 2σ(I)] | 0.0234, 0.0634 | 0.0230, 0.0617 | 0.0271, 0.0694 |
| R1a, wR2b [all data] | 0.0243, 0.0641 | 0.0236, 0.0623 | 0.0319, 0.0727 |
3. Results and discussion
3.1 Crystal structure analysis
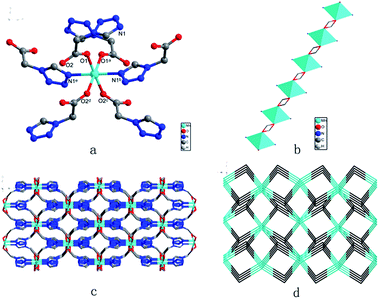
X-ray diffraction analysis indicates that the three compounds are isomorphous with the reported Zn/Cd compounds.19,29 They crystallized in the monoclinic space group C2/c. Herein, we only describe the crystal structure of 1 in detail as an example.The asymmetric unit of 1 is crystallographically independent with one Mn(II) and one TAA ligand. As shown in Fig. 1a, the MnII ions localize the two fold axis and the crossing point of the mirror plane, which exhibits a distorted octahedral geometry, completed by the four O atoms from the carboxyl groups in the equatorial plane and two tetrazolyl N atoms at axial positions with Mn–O distances slightly shorter than the Mn–N distances. Each TAA ligand acts as a tridentate-N, O, O′ mode ligand coordinated to three MnII ions through two 1, 1 − μ2 carboxyl oxygen atoms bridging the intrachain MnII ions to form a 1D chain (Fig. 1b) and the tetrazolyl nitrogen atom linking the third MnII ions in the adjacent chain. The MnII ions are spanned via two syn–anti carboxylate oxygen atoms simultaneously composed of [Mn2(COO−)2] 8-membered ring chain structure with Mn⋯Mn distances of 4.865 Å (4.786 Å for 2 and 4.785 Å for 3, respectively). The dihedral angle between the two tetrazolyl rings of the TAA ligands coordinated to the same MnII ions is 79.3° (80.67° for 2 and 80.41° for 3), while the two tetrazolyl rings coordinated to the adjacent bridged MnII ions are parallel with each other. Such [Mn2(COO−)2] 8-member ring chains are further pillared via tetrazolyl spacers to form a 3D network, where the shortest Mn⋯Mn interchain separation is 7.451 Å (7.371 Å for 2 and 7.314 Å for 3) (Fig. 1c). To better understand the structure, topological analysis was undertaken. The MnII ions can be simplified as a 6-connected node and the TAA ligand can be regarded as a 3-connected vertex, which link three nodes to form a (3,6)-connected “ant” network with the point symbol of (42.6)2(44.62.88.10) (Fig. 1d). It is a seldom reported topological net and the reported center material (Zn/Cd) is not the same.20,22
It is worth nothing that the three are isomers reported for complexes 1 and 2 by Dong.20 Comparing complex 1/2 and iso-1/2 indicates remarkable differences, which results in visible structures and different space groups. The difference between the complexes can be attributed to the discrimination of the N–M–O angles (θ1), the N–M–N angles (θ2), the C–C–N angles (θ3), the dihedral angle (θ4) between the plane A and B, the dihedral angle (θ5) between the two tetrazolyl planes and the modes of the two TAA ligands linked by the central metal (Scheme 1). The angles in Table S4† indicate that the atoms N, M, N of the iso-1/2 are almost on a line; the N–M–N angles θ2 in complex 1/2 are 179.45(6)° and 179.11(6)°. The N–M–N angles θ2 are nearly the same as the difference values are about 0.3° for complex 1 and 0.2° for complex 2. The difference is very apparent when the dihedral angles θ4 and θ5 are contrasted with the angles θ2 and θ3. The dihedral angles θ4 between the plane A and B are 84.486(32)° for 1 and 86.176(34)° for 2, and the angles of θ4 are 73.202(29)° in iso-1 and 76.814(46)° in iso-2. The dihedral angles (θ5) between the two tetrazolyl planes are 79.307(53)° in complex 1 and 80.667(45)° in complex 2, and the two tetrazolyl planes are parallel in iso-1 and 2. The metal atoms are linked by the TAA ligand in a similar syn–anti tridentate coordination mode; in contrast, the modes of the two TAA ligands linked by the central metal are different, which is mode I: syn–syn coordination in complexes 1 and 2, and mode II: syn–anti coordination in iso-1 and 2.
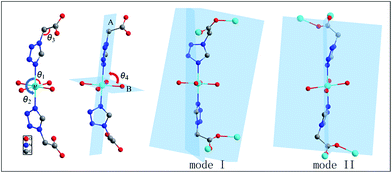 | ||
| Scheme 1 The four N–M–O angles θ1, N–M–N angles θ2, C–C–N angles θ3, dihedral angle between planes (A) and (B) and modes of two TAA ligands linked by central metal. | ||
3.2 Magnetic properties
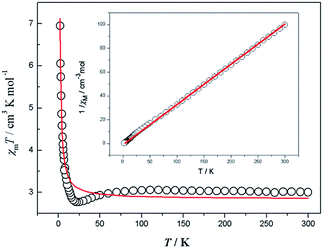
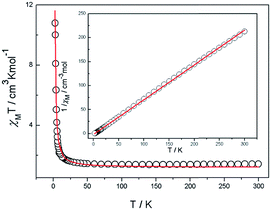
Magnetic susceptibility data were collected on powdered crystalline samples of the complexes 1, 2 and 3 at an applied magnetic field of 1 kOe in the temperature range of 2–300 K, which are shown as χMT and 1/χM versus T plots in Fig. 2 for 1, Fig. 3 for 2 and Fig. 4 for 3.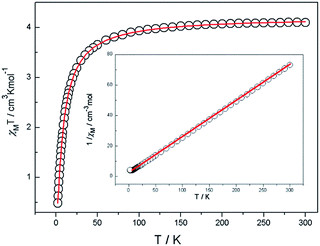 | ||
| Fig. 2 Temperature dependence of the magnetic susceptibility for complex 1 and 1/χM vs. T curve for complex 1. The red line represents the best-fit curves. | ||
 | ||
| Fig. 3 Temperature dependence of the magnetic susceptibility for complex 2 and 1/χM vs. T curve for complex 2. The red line represents the best-fit curves. | ||
 | ||
| Fig. 4 Temperature dependence of the magnetic susceptibility for complex 3 and 1/χM vs. T curve for complex 3. The red line represents the best-fit curves. | ||
For 1, the χMT value at 300 K is equal to 4.10 cm3 mol−1 K, which is compatible with the expected spin MnII (S = 5/2, g = 2, 4.37 cm3 kmol−1) anions. With the decrease in temperature, the χMT decreases slowly to 45 K and then decreases sharply to 2 K. In the temperature range of 5–300 K, the data can be fitted to the Curie–Weiss law χM = C/(T − θ) expression with a Curie constant C = 4.311 cm3 mol−1 K and the Weiss constant θ = −9.47 K, which further suggests the existence of an overall antiferromagnetic exchange interaction between MnII ions.
In the case of 2, the value of 4.56 cm3 mol−1 K χMT at room temperature exceeds the spin only value of 3.75 cm3 mol−1 K as expected for two uncoupled high-spin CoII (S = 3/2) ions with g = 2. Thus, it indicates that an important orbital contribution exists due to the distorted octahedral ions of CoII. The χMT first decreases smoothly to a rounded minimum of 2.76 cm3 mol−1 K at 25 K along with the possible antiferromagnetic interactions between the CoII ions. At lower temperatures the χMT plot rises rapidly to a maximum of 6.94 cm3 mol−1 K at 2 K. The magnetic data above 25 K obey the Curie–Weiss law with C = 3.097 and a high Weiss constant θ of −1.97 K, which further suggest the existence of an overall ferromagnetic exchange interaction between CoII ions.
For complex 3, the χMT value is equal to 1.40 cm3 mol−1 K at room temperature. Overall, the χMT value slowly increases with decreasing temperature from 300 K to 20 K. However, a dramatic increase in the value to 10.79 cm3 mol−1 K at 2 K was observed. The magnetic behavior of the complex 3 follows the Curie–Weiss law in the entire range of temperature with Curie constants and Weiss temperatures of 1.38 cm3 mol−1 K and 2.57 K, respectively. It suggests ferromagnetic exchange interactions.
According to the structural features of the complexes, the magnetic interaction between metal(II) ions can be estimated by simple chain models, and the mean-field, zj′, is also introduced. The simplified equation30 is as follows:
where the values of S are 5/2 for complex 1, 3/2 for complex 2, and 1 for complex 3. The best fits to the experimental data give g = 1.96, J = −0.79 cm−1, zj′ = −0.025 cm−1, and R = 1.3 × 10−5 for complex 1, g = 2.46, J = 0.57 cm−1, zj′ = −0.025 cm−1, and R = 1.3 × 10−5 for complex 2, and g = 2.52, J = 4.97 cm−1, zj′ = 0.15 cm−1, and R = 1.3 × 10−5 for complex 3 (R value is defined as Σ[(χM)obs − (χM)calcd]2/Σ(χM)obs2). The fitting results confirm that weak antiferromagnetic coupling exists in 1, and weak ferromagnetic coupling exists in 2 and 3.
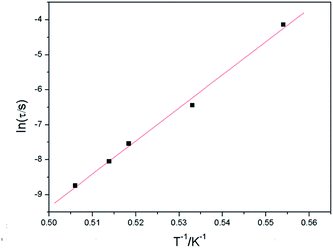
In order to confirm the existence of special magnetic behavior in complexes 2 and 3, alternating current (ac) magnetic susceptibility measurements were carried out (Fig. S5 for 2 and S6† for 3). However, there was no frequency dependence for both the in-phase (χ′M) and out-of phase (χ′′M) susceptibility signals of complex 2. Furthermore, the ac susceptibility measurements of 3 were performed in the temperature range of 1.80–2.95 K under a zero static field oscillating at various frequencies (10–997 Hz). As revealed in Fig. 5, both in-phase (χ′M) and out-of phase (χ′′M) susceptibility signals were obviously frequency dependent with a maximum between 2.0–2.2 K and 1.8–2.0 K, respectively. This was accompanied by an increase in peak temperature as well as a decrease in peak height of χ′M with increasing temperature, while the maximum of the χ′′M peaks shifted to the lower temperature region with decreasing frequencies. Such a shift in temperature of the maximum of χ′M and χ′′M with frequency is the positive signature of single molecule magnets (SMMs), single chain magnets (SCMs), and spin glass or a superparamagnetic behavior.31–33 The frequency dependence is measured by a parameter ϕ = (ΔTp/Tp)/Δ(log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) f) = 0.06, in which Tp is the temperature at which χ′ reaches a maximum. This is the typical value for spin-glass magnets (ϕ < 0.1).33 In order to gain insight into the real magnetic behavior of 3, we first tried to determine the relaxation time. The relaxation time τ(T) data obtained from the χ′′M peaks follow the Arrhenius equation τ = τo
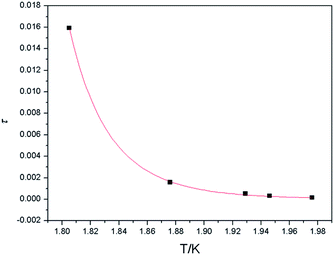
f) = 0.06, in which Tp is the temperature at which χ′ reaches a maximum. This is the typical value for spin-glass magnets (ϕ < 0.1).33 In order to gain insight into the real magnetic behavior of 3, we first tried to determine the relaxation time. The relaxation time τ(T) data obtained from the χ′′M peaks follow the Arrhenius equation τ = τo![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp(Δτ/T), resulting in the relaxation time τo = 2.3 × 10−25 s and the energy barrier Δτ = 94.7 K (Fig. 6). The rather small value of τo is obtained beyond the normal range of typical SMMs or SCMs (10−7–10−12 s),34 locating in the usual range for spin-glass dynamics in case of 3.33 Moreover, another quantitative measurement in a spin-glass system is also determined by fitting the frequency dependence maxima in χ′′M using the conventional critical scaling law of the spin dynamics, τ = τo[(Tp − Tf)/Tf]−zv (Tf and zv represent the freezing temperature and the critical exponent, respectively),35,36 with τo = 5.1 × 10−8 s, zν = 9.4, and Tf = 1.5 K (Fig. 7). The obtained zν value just falls in the typical range (4–12) of the conventional spin glasses.32,33 As shown above, all ac measurements and the deduced results are indicative of the canonical spin glass characteristic for 3, which is ascribed to be a ferromagnetic-like layer with spin-glass dynamic relaxation. Although slow relaxation has been observed in SMMs, SCMs and some magnetic frustration systems, three-dimensional compounds comprising ferromagnetic chains/layers and showing slow magnetic relaxation are very scarce.36–39
exp(Δτ/T), resulting in the relaxation time τo = 2.3 × 10−25 s and the energy barrier Δτ = 94.7 K (Fig. 6). The rather small value of τo is obtained beyond the normal range of typical SMMs or SCMs (10−7–10−12 s),34 locating in the usual range for spin-glass dynamics in case of 3.33 Moreover, another quantitative measurement in a spin-glass system is also determined by fitting the frequency dependence maxima in χ′′M using the conventional critical scaling law of the spin dynamics, τ = τo[(Tp − Tf)/Tf]−zv (Tf and zv represent the freezing temperature and the critical exponent, respectively),35,36 with τo = 5.1 × 10−8 s, zν = 9.4, and Tf = 1.5 K (Fig. 7). The obtained zν value just falls in the typical range (4–12) of the conventional spin glasses.32,33 As shown above, all ac measurements and the deduced results are indicative of the canonical spin glass characteristic for 3, which is ascribed to be a ferromagnetic-like layer with spin-glass dynamic relaxation. Although slow relaxation has been observed in SMMs, SCMs and some magnetic frustration systems, three-dimensional compounds comprising ferromagnetic chains/layers and showing slow magnetic relaxation are very scarce.36–39
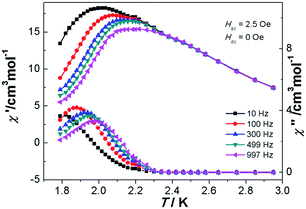 | ||
| Fig. 5 Temperature dependence of the in-phase (χ′) and out-of-phase (χ′′) ac susceptibilities in the range of 10–997 Hz at Hac = 2.5 Oe for complex 3 measured without an applied dc field. | ||
3.3 FT-IR spectra and thermal stability
The IR spectra of the complexes are shown in Fig. S7.† The thermogravimetric analysis (TGA), as shown in Fig. S8,† was performed for complexes 1–3 to examine their stability. Caution: the complexes are easy to explode when heated. Complex 1 remains stable up to ca. 285 °C, finally leading to the formation of the stoichiometric amount of MnO2 as a residue. For complexes 2 and 3, the framework structure began to decompose form 270 °C and 200 °C, respectively.4. Conclusions
We used a newly developed tetrazolate carboxylic acid ligand as the bridging ligand in three isomorphous 3D MnII, CoII and NiII coordination polymers and they were observed to possess totally different magnetic properties. Framework 1 shows simple antiferromagnetic interactions. Framework 2 combines canted antiferromagnetic exchange, whereas framework 3 shows spin glass behavior between the NiII ions bridged by the syn–anti carboxyl. Herein, we provide evidence for the existence of diversity in magnetic properties of three isomorphous frameworks with a simple difference of metal ions and thereby demonstrate the importance of engineering metal–organic coordination polymers in the field of molecular-based magnetic materials.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was supported financially by the National Natural Science Foundation of China (No. 21461029, 21561033) and the Natural Science Foundation of YunNan province (Project 2016FB023).Notes and references
- X. Y. Dong, B. Li, B. B. Ma, S. J. Li, M. M. Dong, Y. Y. Zhu, S. Q. Zang, Y. Song, H. W. Hou and T. C. Mak, J. Am. Chem. Soc., 2013, 135, 10214–10217 CrossRef CAS PubMed.
- D. N. Dybtsev, H. Chun, S. H. Yoon, D. Kim and K. Kim, J. Am. Chem. Soc., 2004, 126, 32–33 CrossRef CAS PubMed.
- O. M. Yaghi, M. O'Keeffe, N. W. Ockwig, H. K. Chae, M. Eddaoudi and J. Kim, Nature, 2003, 423, 705–714 CrossRef CAS PubMed.
- Y. Kondo, K. Kon-i, A. Iwasaki, T. Ooi and K. Maruoka, Angew. Chem., Int. Ed., 2000, 39, 414–416 CrossRef CAS PubMed.
- Y. W. Zhao, F. Q. Zhang and X. M. Zhang, ACS Appl. Mater. Interfaces, 2016, 8, 24123–24130 CAS.
- P. Verma, K. D. Vogiatzis, N. Planas, J. Borycz, D. J. Xiao, J. R. Long, L. Gagliardi and D. G. Truhlar, J. Am. Chem. Soc., 2015, 137, 5770–5781 CrossRef CAS PubMed.
- K.-J. Chen, R.-B. Lin, P.-Q. Liao, C.-T. He, J.-B. Lin, W. Xue, Y.-B. Zhang, J.-P. Zhang and X.-M. Chen, Cryst. Growth Des., 2013, 13, 2118–2123 CAS.
- Z. Z. Lu, R. Zhang, Y. Z. Li, Z. J. Guo and H. G. Zheng, J. Am. Chem. Soc., 2011, 133, 4172–4174 CrossRef CAS PubMed.
- Y. Tian, W. Wang, Y. Chai, J. Cong, S. Shen, L. Yan, S. Wang, X. Han and Y. Sun, Phys. Rev. Lett., 2014, 112, 017202 CrossRef PubMed.
- Y. Dong, P. Yan, X. Zou, T. Liu and G. Li, J. Mater. Chem. C, 2015, 3, 4407–4415 RSC.
- T. N. Nguyen, W. Wernsdorfer, M. Shiddiq, K. A. Abboud, S. Hill and G. Christou, Chem. Sci., 2016, 7, 1156–1173 RSC.
- K. R. Vignesh, S. K. Langley, K. S. Murray and G. Rajaraman, Chem.–Eur. J., 2017, 23, 1654–1666 CrossRef CAS PubMed.
- A. K. Boudalis, B. Donnadieu, V. Nastopoulos, J. M. Clemente-Juan, A. Mari, Y. Sanakis, J.-P. Tuchagues and S. P. Perlepes, Angew. Chem., Int. Ed., 2004, 43, 2266–2270 CrossRef CAS PubMed.
- T. Gong, X. Lou, J.-J. Fang, E.-Q. Gao and B. Hu, Dalton Trans., 2016, 45, 19109–19116 RSC.
- Y. L. Bai, J. Tao, R. B. Huang, L. S. Zheng, S. L. Zheng, K. Oshida and Y. Einaga, Chem. Commun., 2008, 1753–1755 RSC.
- Y.-B. Lu, S. Jin, F.-M. Jian, Y.-R. Xie and G.-T. Luo, J. Mol. Struct., 2014, 1061, 14–18 CrossRef CAS.
- X.-Q. Zhang, Q. Yu, H.-D. Bian, X.-G. Bao and H. Liang, J. Coord. Chem., 2009, 62, 2108–2117 CrossRef CAS.
- D. Ding, M. Zhang, L. Li, Y. Fan and H. Hou, J. Coord. Chem., 2009, 62, 2675–2681 CrossRef CAS.
- Q. Yu, X. Zhang, H. Bian, H. Liang, B. Zhao, S. Yan and D. Liao, Cryst. Growth Des., 2008, 8, 1140–1146 CAS.
- W.-W. Dong, J. Zhao and L. Xu, Cryst. Growth Des., 2008, 8, 2882–2886 CAS.
- W.-W. Dong, J. Zhao and L. Xu, J. Solid State Chem., 2008, 181, 1149–1154 CrossRef CAS.
- D.-S. Liu, W.-T. Chen, Y.-P. Xu, P. Shen, S.-J. Hu and Y. Sui, J. Solid State Chem., 2015, 226, 186–191 CrossRef CAS.
- T. K. Maji, W. Kaneko, M. Ohba and S. Kitagawa, Chem. Commun., 2005, 4613–4615 RSC.
- J. R. Li, Q. Yu, Y. Tao, X. H. Bu, J. Ribas and S. R. Batten, Chem. Commun., 2007, 2290–2292 RSC.
- Y. L. Wang, Y. L. Jiang, Z. J. Xiahou, J. H. Fu and Q. Y. Liu, Dalton Trans., 2012, 41, 11428–11437 RSC.
- X.-W. Liu, R. Guo, H. Liu, Y.-Q. Yu, X.-W. Qi, J.-Y. Xu and C.-Z. Xie, RSC Adv., 2015, 5, 15059–15068 RSC.
- G. M. Sheldrick, Program for Empirical Absorption Correction of Area Detector Data, Germany, 1996 Search PubMed.
- G. M. Sheldrick, SHELX-97, University of Göttingen, Germany, 1997 Search PubMed.
- G. M. Sheldrick, SHELX-97, University of Göttingen, Germany, 1997 Search PubMed.
- A. Das, G. M. Rosair, M. S. El Fallah, J. Ribas and S. Mitra, Inorg. Chem., 2006, 45, 3301–3306 CrossRef CAS PubMed.
- Z. X. Wang, X. L. Li, T. W. Wang, Y. Z. Li, S. Ohkoshi, K. Hashimoto, Y. Song and X. Z. You, Inorg. Chem., 2007, 46, 10990–10995 CrossRef CAS PubMed.
- D. Li, L. Zheng, Y. Zhang, J. Huang, S. Gao and W. Tang, Inorg. Chem., 2003, 42, 6123–6129 CrossRef CAS PubMed.
- J. A. Mydosh, Nurs. Times, 1993, 78, 654 Search PubMed.
- R. Clérac, H. Miyasaka, M. Yamashita and C. Coulon, J. Am. Chem. Soc., 2002, 124, 12837–12844 CrossRef.
- Y. Z. Zheng, M. L. Tong, W. X. Zhang and X. M. Chen, Angew. Chem., Int. Ed., 2006, 45, 6310–6314 CrossRef CAS PubMed.
- X. J. Li, X. Y. Wang, S. Gao and R. Cao, Inorg. Chem., 2006, 45, 1508–1516 CrossRef CAS PubMed.
- X. N. Cheng, W. X. Zhang, Y. Z. Zheng and X. M. Chen, Chem. Commun., 2006, 3603–3605, 10.1039/b606765d.
- X. M. Zhang, P. Li, W. Gao, J. P. Liu and E. Q. Gao, Dalton Trans., 2015, 44, 511–514 RSC.
- J. Liu, J. Luo, Q. Han, J. Cao, L. Chen, Y. Song and J. Zhao, J. Mater. Chem. C, 2017, 5, 2043–2055 RSC.
Footnotes |
| † Electronic supplementary information (ESI) available: TGA and IR spectra. CCDC: 725886 for 1, 725885 for 2, 725887 for 3. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c7ra07997d |
| ‡ These authors contributed equally to this paper. |
| This journal is © The Royal Society of Chemistry 2017 |