Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Synergistic action of TiO2 particles and surfactants on the foamability and stabilization of aqueous foams
Huiying Cao *ab,
Xuan Zhangc,
Baiyong Dingb,
Long Wanga and
Naiyan Lu*c
*ab,
Xuan Zhangc,
Baiyong Dingb,
Long Wanga and
Naiyan Lu*c
aSchool of Information and Engineering, Guangdong Medical University, Zhanjiang 524023, China. E-mail: hycao@gdmu.edu.cn
bNational Laboratory of Solid State Microstructures, Nanjing University, Nanjing 210093, China
cState Key Laboratory of Food Science and Technology, Jiangnan University, Wuxi 214122, China. E-mail: lunaiyan@jiangnan.edu.cn
First published on 20th September 2017
Abstract
Small particles can be activated via a synergistic effect with surfactants and adsorbed to the air–water interface to generate and stabilize foams, which has been applied extensively to develop new materials and techniques. Here, we studied the synergistic effects of TiO2 particles with ionic surfactant SDS (sodium dodecyl sulfate), ionic AOT (sodium di-2-ethylhexylsulfosuccinate), and cationic CTAB (cetyltrimethylammonium bromide) in aqueous solution, and their impacts on the foamability and foam stability were assessed by measuring the foam volume and observing the number of particles adsorbed onto the foam surface. The results showed the interactions of TiO2 particles with SDS and CTAB surfactants were synergistic in both foamability and foam stability. The degree of synergy of the CTAB–TiO2 mixed system was stronger than that of the SDS–TiO2 mixed system as a whole. However, the interaction of TiO2 with AOT (a double carbochain anionic surfactant) in the TiO2–AOT mixed system was generally not synergistic. Unexpectedly, a maximum synergistic effect among these mixed systems occurred in the TiO2–AOT system at an AOT concentration of approximately 5 mM. This study provides further understanding for the mechanism of foaming and stability of foam modulated by surfactant and colloidal particles and provides a useful reference for future applications.
Introduction
Foams stabilized with particles in aqueous surfactant solutions have brought significant technological impacts, including particles assembling at the air–liquid interface to form dry water (water in air)1–3 and liquid marbles (large liquid droplets encapsulated in air),4–6 as well as colloidosomes7 and anisotropic particles.8 Furthermore, this technology has been applied in industrial processes extensively. Oil recovery is enhanced using foam behavior mediated by the interaction between nanoparticles and mixed surfactant in brine solution.9 Wastewater is cleared by removing hydrophobic contaminations that attach to air bubbles.10 Porous ceramics and porous metals are fabricated using particle-rich foam precursors.11 Therefore, a comprehensive understanding to the mechanism of foamability and foam stability mediated by surfactant and particles in aqueous solution would be beneficial to further practical applications.Small particles (nanometres to several micrometres), with a suitable wettability at their surfaces, can be strongly attached to liquid–vapor or liquid–liquid interfaces, and thus behave as the foam or the macroemulsion stabilizers, respectively.12–14 This process results in irreversible adsorption,1,15 unlike that of surfactant molecules with adsorption and desorption on a rapid timescale. And this adsorption, namely, small particles accumulated at the two-phase interface need much higher adsorption free energies than the thermal energy kT.15 Usually, particles promote their adsorption free energy via the activation of surface or the modification of surface wettability, which can be characterized by the contact angle of the particle at the aqueous side of the interface. There are three approaches to obtain higher surface activation for small particles or change their surface wettabillity in general. The first is to prepare “Janus” particles with asymmetric surface chemistry, i.e., regionally hydrophilic on one side and hydrophobic on the other side.16,17 The “Janus” particles, however, involve complicated preparation processes and are difficult to produce in large quantities. Another solution is to modify the wettability of the non-surface-active or low-surface-active small particles using a homogeneous surface coating, such as, modifying SiO2 from extremely hydrophilic to very hydrophobic,18 but the high cost of activating particles makes this method less commercially viable. The third is to use the interaction of amphiphilic compounds with particle to make surface active.19,20 Surfactant, as typical amphiphilic compound, is usually added to solutions to change the activation of particles by the adsorption of surfactant molecules onto particle surface. This method is comparatively less complicated and less expensive, and is therefore practically significant.
Recently, Binks et al.21 used surfactant SDS (sodium dodecyl sulfate) to successfully achieve the inversion of dry water to aqueous foam by changing the activation of silica surface particles in aqueous system, whereby the hydrophobic surface of the particle was converted into hydrophilic surface by adsorbing SDS molecules. AOT, an ionic surfactant with double carbon chain, was used as a usual charge control agent for particles charging in apolar dispersions22,23 is considered for the activation of CaCO3 nanoparticles and investigated their ability to stabilize aqueous foam.24 Mineral oxide nanoparticles and cationic CTAB were investigated for their synergistic effect on stabilization of CO2 foam for the application in enhanced oil recovery.25 In addition, the mechanism of particle stabilizing aqueous foams was revealed by the synergy between SiO2 particles and surfactants at different length scale.26 Langevin and co-workers also studied the mechanism of foam stabilization based on the interaction of SiO2 particles and surfactant in the mixed aqueous solution.27 Nevertheless, although these works have described the surface of particle activated by the adsorption of surfactant to stabilize aqueous foams at the interface, the investigated objects mainly focused on anionic surfactant (SDS in particular), and SiO2 particles but less involved for other surfactants and particles. As a common material, TiO2 particles have very important applications in food, cosmetics, medicine and functional materials,28–30 while the studies of TiO2 particles in stabilizing aqueous foam are relatively inadequate. Therefore, the systematic investigation of the surface activation of TiO2 particles by interaction with different surfactants in aqueous solution is advantageous to further understand the mechanism of particle stabilizing aqueous foams.
In this paper, we investigated the surface activation of TiO2 by the interaction with anionic surfactants SDS, AOT (sodium di-2-ethylhexylsulfosuccinate), and cationic surfactant CTAB (cetyltrimethylammonium bromide) in aqueous solution. The synergistic interactions between TiO2 particles and SDS, AOT, and CTAB in both foamability and foam stabilization were analyzed by measuring volume of foam and observing the adsorption of particle on the surface of foam. Possible reasons for the discrepancy of these synergistic effects of particles with these surfactants were suggested. The aim of this paper was to obtain a further insight into the activation of the particle via surface active agent to stabilize aqueous foam at water–air interface. Meantime, the study would also provide the references for potential applications of TiO2-surfactant aqueous systems in relevant industries.
Methods
Materials
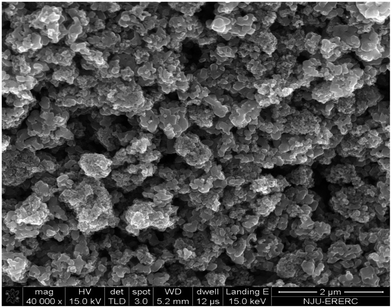
TiO2 particles with a primary particle diameter of 110 nm were supplied by Sigma-Aldrich. The particles were originally delivered as a dispersion in deionized water. Obtaining dry particles needed a dehydration process through a multistage solvent swap using ethanol as an intermediate solvent, followed by vacuum drying at 30 °C. Fig. 1 shows a scanning electron microscopy (SEM, Quanta 200, FEI) image of a dried aqueous dispersion of the particles, where the particles fused with each other and formed micron-sized agglomerates. SDS and CTAB (each 99% of purity) were from Shanghai Geneland Biotech Co., Ltd., China, and AOT of 99% purity was the commercial production of Sigma-Aldrich. All surfactants were used as received. Deionized water with a resistance of 18.2 MΩ cm at 25 °C was obtained using a deionized water system (LDD-01, Ludao, Shanghai, China).Preparation of aqueous surfactant dispersions without and with TiO2 particles
A series of different concentrations of surfactants (0.01–50 mM) were dispersed in deionized water. The dried TiO2 particles were weighed and added into these aqueous surfactant solutions. The mixed solutions without and with particles were respectively sealed into 50 ml beaker and then dispersed using constant temperature magnetic stirrer (85-2, Changzhou, China) at 2400 rpm for 2–5 min.Preparation and measurement of aqueous foams
The aqueous surfactant dispersion without TiO2 particles (10 ml) was transferred to a 100 ml cylindrical graduated flask. The flask was stoppered and then shaken up and down vigorously about 30 times. The foam volume immediately after shaking and 30 min after shaking were recorded as V0 and V30, respectively. Similarly, a 10 ml dispersion of aqueous surfactant solution with TiO2 particles was placed into a 100 ml cylindrical graduated flask with a stopper, and the same procedure was repeated, except shaking was performed 50 times to facilitate full interaction between particles and surfactants. And foam volume immediately after shaking was recorded as V0P, and that 30 min after shaking was as V30P. V0 and V0P were taken as the measure of foamability of aqueous surfactant solution without and with particles, respectively. V30 and V30P were the measure of foam stability of aqueous surfactant solution without and with particles, respectively. These experiments were repeated three times.Characterization of aqueous foams with optical microscopy
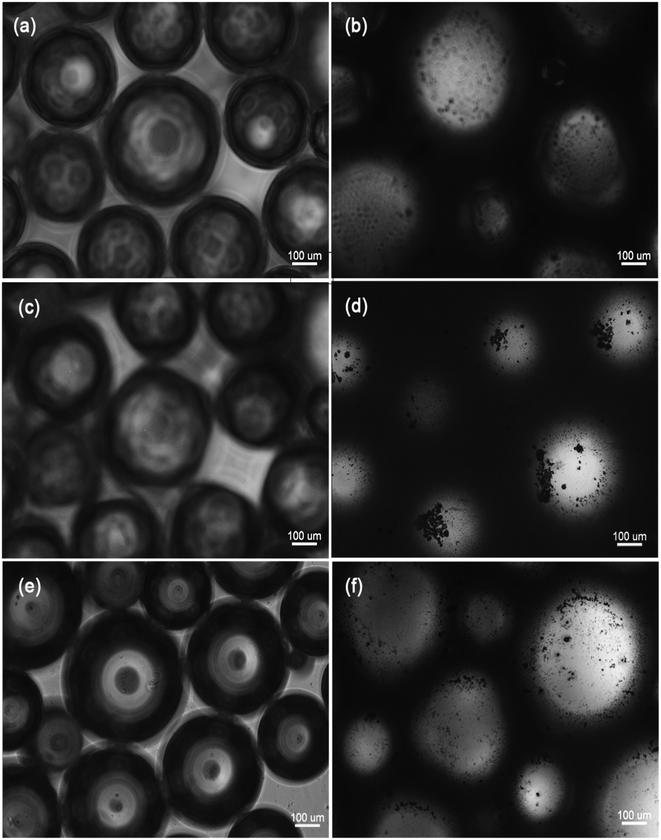
To observe foams stabilized by a surfactant alone and by a combination of TiO2 particles and surfactants, each 5 ml dispersion was transferred to a 10 ml bottle and aerated at 5000 rpm for 5 min using a YSDF-400 centrifuge (Yuesheng, Shanghai). The foam was removed and placed on a microscope slide (without coverslip), and micrographs were taken (before moisture evaporated) using a Motic BA400 microscope system (Motic, Xiamen).Results
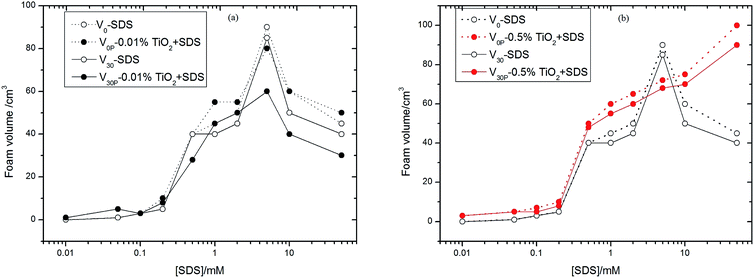
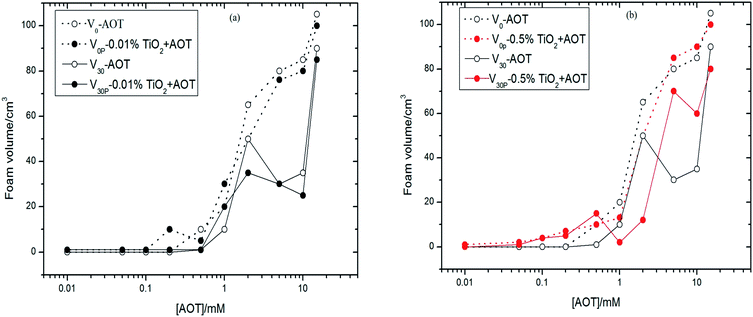
Fig. 2 showed foam volumes, as the function of surfactant concentration, in aqueous solution with SDS alone immediately after shaking (V0), SDS alone 30 minutes after shaking (V30), SDS plus TiO2 immediately after shaking (V0P), and SDS plus TiO2 30 minutes after shaking (V30P), the particle concentrations of which were 0.01% (Fig. 2a) and 0.5% (Fig. 2b). Foam volumes were all small at SDS concentrations below 0.5 mM with and without TiO2 particles in Fig. 2. Beyond this concentration, the foam volumes with SDS alone (V0 and V30) increased quickly at first, and reached a maximum peak value near the concentration of 8 mM, which is the critical micelle concentration (CMC) of SDS in pure water,31 then decreased with further increase of SDS concentration. For the TiO2 particle concentration of 0.01%, foam volumes of V0P and V0, as well as V30P and V30, showed similar trends. Beyond 8 mM, the foam volumes of V30P were less than those of V30. However, for higher particle concentration of 0.5% (see Fig. 2b), the curves of V0P and V30P monotonously increased with the increase of SDS concentration, which was obviously different from the corresponding curves of 0.01% particle concentration. The V0P and V30P values were larger than V0 and V30 values in the entire range of surfactant concentrations, except near the CMC of SDS, where the values of V0 and V30 were the highest. Thus, we initially inferred that TiO2 particles can affect foamability and foam stability in SDS aqueous solutions and this influence was strengthened as particle concentration increased. The curves of V0 and V30, as well as those of V0P and V30P, almost overlapped in Fig. 2b, which denoted the systems of SDS-aqueous solution without and with TiO2 particles both had strong foam stabilities in water.The four foam volumes were also measured with 0.01% and 0.5% of TiO2 particles dispersed in AOT-aqueous solution at different AOT concentrations compared with AOT alone, as shown in Fig. 3. The value of AOT alone (V0) increased with the increase of AOT concentration, while the value of AOT alone (V30) showed three phases, i.e., going up with the increase of AOT concentration from 1 mM to 2 mM, rapidly falling from 2 mM to 5 mM, then quickly rising again above 5 mM. And the CMC of AOT in pure water is just near 2 mM.32 With 0.01% of TiO2 particles dispersed AOT-aqueous solutions, the values of V0P and V30P showed similar changes as those observed for the corresponding V0 and V30 values, and the values of V30 and V30P were generally smaller than those of V0 and V30, which was similar to that observed for the TiO2–SDS mixed aqueous solutions. When particle concentration was increased to 0.5%, the values of V0P and V30P were still no larger than those of V0 and V30, except from 5 mM to 10 mM where V30P was beyond V30, which were obviously different from those of TiO2–SDS solution at same particle concentration of 0.5%. Therefore, it was indicated that TiO2 particles did not provide a strong active effect on foamablity and foam stability as a whole in AOT aqueous solutions.
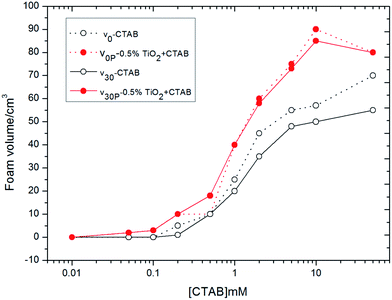
Considering the influence of the low particle concentration of 0.01% on the foam volumes above, V0P and V30P were measured only with 0.5% of TiO2 particles dispersed in aqueous solutions of CTAB, and the results were given in Fig. 4. The values of V0 steadily increased with increasing CTAB concentrations, where the minimum concentration of CTAB is required to stabilize foams approached 0.9 mM, which is its CMC in water.33 When TiO2 particles were added into CTAB aqueous solutions, the values of V0P and V30P presented remarkable increases beyond the CMC, which were much higher than the values of V0 and V30 in the whole range of CTAB concentration, although the values slightly decreased above 10 mM. Similar to the foam volumes of the mixed system of SDS plus TiO2 in Fig. 2b (0.5% of TiO2), the curves of V0P and V30P almost overlapped, implying that TiO2 particles, with the participation of CTAB, provided an excellent stabilizing effect on the foam at the air–water interface.
Using Sf% = (V0P − V0) × 100%/V0 to denote the degrees of foamability (shaken immediately with surfactant alone (V0) and surfactant plus TiO2 particles (V0P)) and Ssf% = (V30P − V30) × 100% to denote foam stability (shaken after 30 min with surfactant alone (V30) and surfactant plus TiO2 particles (V30P)), we further studied the effect of interaction of TiO2 particles with surfactants on the foamability and foam stability in aqueous solutions. The values of Sf and Ssf above zero indicated the interaction of particles with surfactants was synergistic, otherwise the interaction was antagonistic. The relations of Sf and Ssf with surfactant concentration were shown in Fig. 5, where the data used to calculate Sf and Ssf were from Fig. 2b, 3b and 4. The Sf and Ssf values for TiO2–CTAB were higher than those for TiO2–SDS from 0.5 mM to 10 mM; however, this was reversed when surfactant concentration was beyond 10 mM. Therefore, the synergistic effects of TiO2 and CTAB were stronger than those of TiO2 and SDS at moderately higher surfactant concentrations.
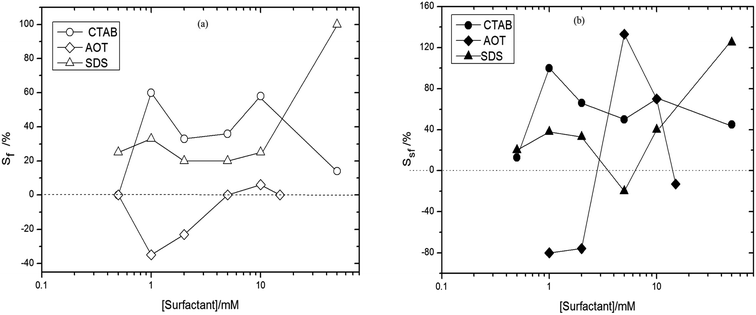 | ||
| Fig. 5 Effect of surfactant concentration on (a) Sf (foamability) and (b) Ssf (foam stability), calculated using the data in Fig. 2b, 3b and 4, for TiO2 particle plus SDS, AOT and CTAB systems, respectively. | ||
Discussion
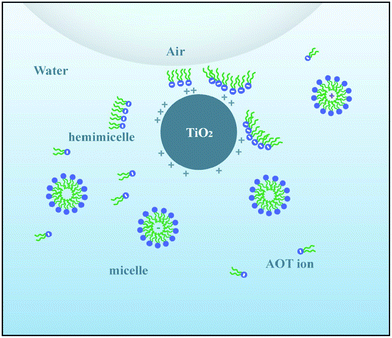
For the SDS–TiO2 mixed aqueous solution (Fig. 2b), foam volumes for SDS alone were small at surfactant concentrations below 0.2 mM, the cause of which may be fewer free surfactant molecules that would be unable to form a sufficiently dense network at the air–water interface surrounding the bubbles to prevent coalescence. With the increase of surfactant molecules, the curves of V0 and V30 presented para-curve with the maximum values near the CMC of 8 mM. The ascending part of the curves was due to the presence of more surfactant molecules that spread a dense layer at air–water interface to prevent the drainage between bubbles, and the descending of the curves was linked to the increase in the lifetime of the micelles with concentration, leading to the reduction of the supply of monomeric SDS molecules required for the stabilization of the fresh bubble at the interfaces.34,35 As was observed, at low concentrations, i.e., from 0.2 mM to 0.5 mM, the foam stabilization mainly depended on SDS molecules and TiO2 particles only had a slight effect on foams. Beyond 0.5 mM, the values of V0P and V30P were obviously larger than those of V0 and V30 (excluding those values near the CMC of SDS) at the same concentration, the improvement of which just came from the synergistic effect between SDS molecules and TiO2 particles. The hydrophilic headgroups of SDS molecules were adsorbed onto the surfaces of TiO2 particles and hydrophobic carbochains were directed toward air during the interaction, rendering the surfaces more hydrophobic, and thus they were adsorbed easily onto the surfaces of foams as shown in Fig. 6b. Therefore, the V0P and V30P curves nearly monotonously ascended with the increase of SDS concentration in Fig. 2b.As for the AOT–TiO2 mixed system (Fig. 3b), the values of V0 monotonously increased from 1 mM and were higher than the corresponding values for SDS in aqueous solution, which accounted for a double chain anion surfactant being more easier to aggregate at interface than its single chain analogues. Unlike the curves of SDS alone and CTAB alone (see Fig. 2b and 4), the foam curves of V0 and V30 of AOT showed an abnormal fall of V30 in the range of 2 mM to 5 mM where was just the initial formation of AOT micelles,32 which might be the cause for the decrease of V30. However, at this concentration region, adding TiO2 particles into AOT aqueous solution, it caused the value of V30P to quickly increase, even exceeding that of V30. Additionally, the value of Ssf near 5 mM reached a maximum value among the three mixed systems, which was certified in Fig. 6d where the number of particles adsorbed onto the surface of foam was significantly higher than that in Fig. 6b and f. Why did such a strong synergistic effect between TiO2 and AOT in foam stability occur in the range of 2 mM to 5 mM? In relation to the abnormal fall in foam stability of AOT alone, we guessed that TiO2 particles became so hydrophobic in this phase because of the electrostatic interaction with AOT hemimicelles or quasimicelles. Because these hemimicelles or quasimicelles were not closed, their charged hydrophilic head groups would adsorb onto TiO2 particles by electrostatic interaction with counter charges of the surface of TiO2 particle, and bulky hydrophobic tails would extend to the interface, causing TiO2 particles easily to adsorb onto the surface of the foam to prevent coalescence, which was described in Fig. 7. After AOT formed stable-closed micelles at AOT concentration above 5 mM, the hydrophilic head groups of the outer micelles lead to particles desorbing from the air–water interface.
Different from the interaction of CaCO3 and CTAB, which was no synergistic effects in aqueous solution,24 a strongly synergistic action occurred between TiO2 and CTAB in both foamability and foam stability, as showed in Fig. 4 and 6f. The foam volume plus TiO2 particles was much larger than that of CTAB alone above CMC (Fig. 4). And, the values of Sf and Ssf were all larger than zero in Fig. 5. Moreover, the foamability and foam stability of the TiO2–CTAB mixed system were superior to those of the TiO2–SDS mixed system in a wide range of surfactant concentration, i.e., the surfaces of TiO2 particles, due to the interaction with cationic CTAB surfactant, were more hydrophobic than those with anionic SDS surfactant. Besides, the surface of SiO2 was also activated by CTAB in aqueous system, where the hydrophobicity of particles were enhanced by the adsorption of the hydrophilic headgroup of the molecule onto the particle surface while hydrophobic chain is directed towards interface.21 When the concentration was above 10 mM, a second layer of surfactant gradually formed on the surface of TiO2 particles by chain–chain interactions with the first layer of molecules, which reduced the hydrophobicity of TiO2. Therefore, the values of V0P and V30P for the TiO2–CTAB mixed system began to decline with the increase of surfactant concentration from 10 mM. Contrastively, the foam values of V0P and V30P of the TiO2–SDS mixed system monotonously increased with the increase of SDS concentration, implying that only a single layer of surfactant molecules was adsorbed onto the surface of TiO2 particles, even at higher SDS surfactant concentrations.
Conclusion
The synergistic effects of TiO2 particles with surfactants SDS, AOT, and CTAB were studied by measuring foam volumes in aqueous solutions with surfactant alone and surfactant-TiO2 mixed systems, calculating the degrees of the foamability Sf and foam stability Ssf, and observing the adsorption of activated TiO2 particles on the surface of foams using optical micrographs. The results indicated the interaction between TiO2 and SDS, as well as that between TiO2 and CTAB, was significantly synergistic above the surfactant concentration of 0.5 mM; the effect of TiO2 and AOT was only synergistic in foam stability near the CMC of AOT, and asynergistic in both foamability and foam stability at other concentrations. The system of TiO2 and CTAB generally provided more synergistic effects than that of TiO2 and SDS. However, the strongest synergistic effect occurred between TiO2 and AOT near the concentration of 5 mM. AOT hemimicelles or quasimicelles at the CMC likely accounted for this case. Though SDS and CTAB surfactants were all advantageous to enhance the hydrophobicity of the surfaces of TiO2 particles during the interaction, SDS molecules may be adsorbed as a single layer onto the particle surfaces, and CTAB molecules maybe formed a bilayer to reduce the hydrophobicity of particle at higher concentrations.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors would like to acknowledge the financial support of the Natural Science Foundation of Guangdong Province (2015A030310178), the National Natural Science Foundation of China (31401589), Zhanjiang Science and Technology Plan (2014B01068) and Guangdong Medical College Research Foundation (XB1355).References
- B. P. Binks and R. Y. O. Murakami, Nat. Mater., 2006, 5, 865 CrossRef CAS PubMed.
- S. Hasenzahl, A. Gray, E. Walzer and A. Braunagel, SOFW J., 2005, 131, 2 Search PubMed.
- L. Forny, et al., Powder Technol., 2007, 171, 15 CrossRef CAS.
- P. Aussillous and D. Quere, Nature, 2001, 411, 924 CrossRef CAS PubMed.
- G. McHale and M. I. Newton, Soft Matter, 2011, 7, 5473 RSC.
- S. Ogawa, et al., Langmuir, 2014, 30, 9071 CrossRef CAS PubMed.
- A. D. Dinsmore, et al., Science, 2002, 298, 1006 CrossRef CAS PubMed.
- O. D. Velev, A. M. Lenhoff and E. W. Kaler, Science, 2000, 287, 2240 CrossRef CAS PubMed.
- A. Bera, K. Ojha and A. Mandal, J. Surfactants Deterg., 2013, 16, 621 CrossRef CAS.
- R. Zhang and P. Somasundaran, Adv. Colloid Interface Sci., 2006, 123, 213 CrossRef PubMed.
- U. T. Gonzenbach, et al., Angew. Chem., Int. Ed., 2006, 45, 3526 CrossRef CAS PubMed.
- S. Fujii and R. Murakami, KONA Powder Part. J., 2008, 26, 153 CrossRef CAS.
- S. Melle, M. Lask and G. G. Fuller, Langmuir, 2005, 21, 2158 CrossRef CAS PubMed.
- R. G. Alargova, D. S. Warhadpande, V. N. Paunov and O. D. Velev, Langmuir, 2004, 20, 10371 CrossRef CAS PubMed.
- B. P. Binks, Curr. Opin. Colloid Interface Sci., 2002, 7, 21 CrossRef CAS.
- A. Perro, et al., J. Mater. Chem., 2005, 15, 3745 RSC.
- V. N. Paunov and O. Cayre, Adv. Mater., 2004, 16, 788 CrossRef CAS.
- B. R. Midmore, J. Colloid Interface Sci., 1999, 213, 352 CrossRef CAS PubMed.
- I. Grosse and K. Estel, Colloid Polym. Sci., 2000, 278, 1000 CAS.
- P. Somasundaran and L. Huang, Adv. Colloid Interface Sci., 2000, 88, 179 CrossRef CAS PubMed.
- B. P. Binks, A. J. Johnson and J. A. Rodrigues, Soft Matter, 2010, 6, 126 RSC.
- H. Y. Cao, et al., Phys. Chem. Chem. Phys., 2013, 15, 12227 RSC.
- H. Y. Cao, et al., Nanotechnology, 2011, 22, 445709 CrossRef PubMed.
- Z. G. Cui, et al., Langmuir, 2010, 26(15), 12567 CrossRef CAS PubMed.
- S. Kumar and A. Mandal, Appl. Surf. Sci., 2017, 420, 9 CrossRef CAS.
- A. Carl, A. Bannuscher and R. von Klitzing, Langmuir, 2015, 31, 1615 CrossRef CAS PubMed.
- L. R. Arriaga, et al., Soft Matter, 2012, 8, 11085 RSC.
- A. Weir, et al., Environ. Sci. Technol., 2012, 46, 2242 CrossRef CAS PubMed.
- R. Singh and H. S. Nalwa, J. Biomed. Nanotechnol., 2011, 7, 489 CrossRef CAS PubMed.
- R. Poulomi, B. Steffen and S. Patrik, Angew. Chem., Int. Ed., 2011, 50, 2904 CrossRef PubMed.
- M. Motin, et al., J. Saudi Chem. Soc., 2015, 19(2), 172 CrossRef.
- K. Mukherjee and S. P. Moulik, Langmuir, 1993, 91, 1727 CrossRef.
- Z. G. Cui, L. L. Yang, Y. Z. Cui and B. P. Binks, Langmuir, 2010, 26, 4717 CrossRef CAS PubMed.
- S. G. Oh and D. O. Shah, Langmuir, 1991, 7, 1316 CrossRef CAS.
- B. P. Binks, M. Kirkland and J. A. Rodrigues, Soft Matter, 2008, 4, 2373 RSC.
| This journal is © The Royal Society of Chemistry 2017 |