Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Photo-isomerization and light-modulated aggregation behavior of azobenzene-based ionic liquids in aqueous solutions†
Zhiyong Liab,
Huiyong Wangab,
Mengen Chuab,
Pengxin Guanab,
Yang Zhaoab,
Yuling Zhaoab and
Jianji Wang *ab
*ab
aCollaborative Innovation Center of Henan Province for Green Manufacturing of Fine Chemicals, Key Laboratory of Green Chemical Media and Reactions, Ministry of Education, School of Chemistry and Chemical Engineering, Henan Normal University, Xinxiang, Henan 453007, P. R. China. E-mail: jwang@htu.cn
bHenan Key Laboratory of Green Chemistry, Henan Normal University, Xinxiang, Henan 453007, P. R. China
First published on 18th September 2017
Abstract
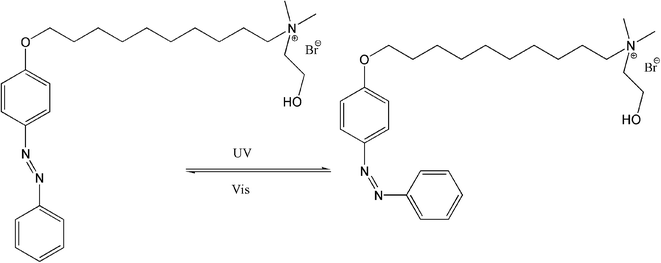
Light modulation of the isomerization and aggregation behavior of ionic liquids in aqueous solution is of great importance from a fundamental and technical point of view. In this work, 4 kinds of azobenzene-based photoresponsive ionic liquids 2-hydroxyethyl-dimethyl-[10-(4-phenylazo-phenoxy)-decyl]-ammonium bromide (ChoC10Azo), 2-hydroxyethyl-dimethyl-[6-(4-phenylazo-phenoxy)-hexyl]-ammonium bromide (ChoC6Azo), 2-hydroxyethyl-dimethyl-[4-(4-phenylazo-phenoxy)-butyl]-ammonium bromide (ChoC4Azo) and 2-hydroxyethyl-dimethyl-[2-(4-phenylazo-phenoxy)-ethyl]-ammonium bromide (ChoC2Azo) were designed, synthesized and characterized. The photo-isomerization and light modulation of the aggregation behavior of these ionic liquids were investigated in water using UV-vis spectroscopy, conductivity, dynamic light scattering and small-angle X-ray scattering measurements. The results showed that these ionic liquids had rapid photo-responsive performance, and their photoisomerization efficiencies were greater than 83% after 5 s of UV irradiation. The equilibrium time of the isomerization reaction was about 30 s for ChoC6Azo, ChoC4Azo and ChoC2Azo, and about 10 min for ChoC10Azo. However, there was no significant difference between the equilibrium isomerization efficiencies of these materials. In addition, only ChoC10Azo could form micelles in water among the studied ionic liquids, UV/visible light irradiation only changed the size of its aggregates, but could not change its structure. The results have been used to understand the effect of azobenzene group position and alkyl chain length in the ionic liquids on the photoisomerization and self-assembly of the azobenzene-based ionic liquids in aqueous solutions.
1. Introduction
Stimuli-responsive materials have attracted increasing attention in a variety of areas, such as drug delivery,1 sensing,2 coating,3 and catalysis.4 Up to now, pH,5 temperature,6 light,7 redox agents8 and CO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 have been used as external stimuli for stimuli-responsive materials. Among these external stimuli, the light trigger is of great importance and has many advantages,10 since no “invasion” is introduced into the system during the stimuli-responsive process, and the optical signal is easy to obtain with stability and reliability. Furthermore, light can be directed at a precise spot with a resolution of a few micrometers, which is of particular value in nanoscience and nanotechnology applications. Photoinduced isomerization of the molecules might trigger the rearrangement of the building blocks to bring about morphological or size transitions, and they are therefore considered as promising photoresponsive materials.
9 have been used as external stimuli for stimuli-responsive materials. Among these external stimuli, the light trigger is of great importance and has many advantages,10 since no “invasion” is introduced into the system during the stimuli-responsive process, and the optical signal is easy to obtain with stability and reliability. Furthermore, light can be directed at a precise spot with a resolution of a few micrometers, which is of particular value in nanoscience and nanotechnology applications. Photoinduced isomerization of the molecules might trigger the rearrangement of the building blocks to bring about morphological or size transitions, and they are therefore considered as promising photoresponsive materials.
Ionic liquids have found widespread applications in many areas of chemistry because of their unique physicochemical properties and highly tunable features by varying the chemical structures of the cations and anions comprised.11 It is thus possible to design a functionalized ionic liquid to have a photoresponsive property by introducing a photoresponsive group into an ionic liquid. The most common light-responsive groups include phenylazosulfonate, stilbene, cinnamate, and azobenzene in the general photoresponsive compounds.7 Indeed, some photoresponsive ionic liquids have been reported in the literatures in recent years.12–15 In this context, ionic liquids with a photoresponsive stilbene chromophore were prepared and their emission properties were investigated by Arai et al.12 Two ionic liquids with photoisomerizable p-hydroxycinnamic acid moieties were synthesized, and their physical properties upon irradiation were studied in solution and neat conditions.14 It was found that physical and chemical transformations of these ionic liquids in acetonitrile were completely reversible upon irradiation at 300 nm. Some azobenzene-13 and cinnamate-based15 photoresponsive ionic liquids were also prepared and showed reversible modulation of ionic conductivity in specific solvents under alternative UV/visible light irradiation.
Recently, some important studies have been reported on the aggregation behavior of photoresponsive ionic liquids.16,17 It is shown that aggregation behavior of ionic liquids composed of 1-alkyl-3-methylimidazolium cations, [Cnmim]+ (n = 8, 10, 12), and trans-cinnamic acid anion can be efficiently modulated by UV light in aqueous solution. After UV light irradiation, the values of critical aggregation concentration (CAC), degree of ionization (α) of the aggregates and the standard Gibbs energy of aggregation increase, while the size of the aggregates decreases.16 Due to the fact that the photoisomerization of cinnamic acid anion used in that work is not reversible,18 the authors cannot reversibly control the aggregation behavior of these ionic liquids.16 As one of the most common photoresponsive groups, azobenzene and its derivatives are often used as switching unit19 because of their high medium sensitivity and reversibility, simple synthetic procedure and high photo-stability, which allows a large number of switching cycles can be achieved. Therefore, ionic liquids containing azobenzene group have been also developed in recent years. Toward to this end, a surface active azobenzene-based ionic liquid 4-butylazobenzene-4′-hexyloxytrimethyl-ammonium trifluoro-acetate ([C4AzoC6TMA][TfO]) with azobenzene unit in the middle of the alkyl chain has been synthesized to achieve light-responsive and reversible micelle-vesicle transformation by UV- and visible-light irradiation.20 Similarly, photoresponsive ionic liquid 1-(4-methyl azobenzene)-3-tetradecylimidazolium bromide ([C14mimAzo]Br) with azobenzene directly linked to the headgroup has been designed by Zheng et al.,17 and reversible micelle-vesicle transformation can be controlled by photostimuli. In both of these investigations involving azobenzene-based ionic liquids, it seems that the position of azobenzene group in the alkyl chain of the ionic liquids does not affect the structure of aggregates. However, it is worth to note that the CAC values of [C4AzoC6TMA][TfO] are much higher than that of [C14mimAzo]Br, which means that photoresponsive group located in different positions of the alkyl chain has a great effect on the aggregation capacities of the azobenzene-based ionic liquids. In order to have a deeper understanding for the relationship between azobenzene position and photoresponsive characteristics/aggregation behavior of the azobenzene-based ionic liquids, investigation of such class of ionic liquids with azobenzene group in other positions of the alkyl chain is necessary.
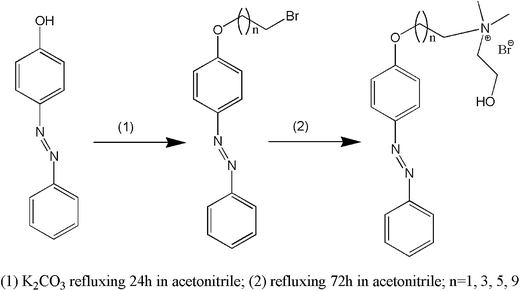
In this work, we have designed, synthesized and characterized 4 kinds of photoresponsive ionic liquids 2-hydroxyethyl-dimethyl-[10-(4-phenylazo-phenoxy)-decyl]-ammonium bromide (ChoC10Azo), 2-hydroxyethyl-dimethyl-[6-(4-phenylazo-phenoxy)-hexyl]-ammonium bromide (ChoC6Azo), 2-hydroxyethyl-dimethyl-[4-(4-phenylazo-phenoxy)-butyl]-ammonium bromide (ChoC4Azo) and 2-hydroxyethyl-dimethyl-[2-(4-phenylazo-phenoxy)-ethyl]-ammonium bromide (ChoC2Azo). In the cations of these ionic liquids, the azobenzene group is at the end of the alkyl chain (i.e. the azobenzene and headgroup are bridged by the alkyl chain, see Scheme 1). The photoisomerization and aggregation behavior modulation of these ionic liquids in water through UV light irradiation have been investigated by UV-vis spectroscopy, conductivity, dynamic light scattering and small-angle X-ray scattering techniques. Parameters of photo-responsive rate, photoisomerization efficiency, and size and structure of aggregates have been determined to provide useful information for the understanding of structure–property relationship of the azobenzene-based ionic liquids in aqueous solutions.
2. Experimental
2.1 Materials
N,N-Dimethylethanolamine (99%), 4-(phenylazo)phenol (98%), 18-crown-6 (99%), 1,10-dibromodecane (99%), 1,6-dibromohexane (99%), 1,4-dibromobutane (99%) and 1,2-dibromoethane (99%) were purchased from Shanghai Aladdin company and used without further purification. Petroleum ether (b.p. 60–90 °C), acetonitrile (99.9%), dichloromethane (99.9%) and potassium carbonate (99%) were commercial products from Shanghai Adamas-beta.2.2 Synthesis of the azobenzene-based ionic liquids
Synthesis route of the azobenzene-based ionic liquids was shown in Scheme 1. Here, ChoC10Azo was selected as an example to show the synthesis procedures briefly. First, 4-(phenylazo)phenol (2.3 g, 0.011 mol) was added to 1,10-dibromodecane (16.5 g, 0.055 mol, 5 equiv.) in acetonitrile with magnetic stirring for the synthesis of [4-(10-bromo-decyloxy)-phenyl]-diazene (C10Azo). Next, K2CO3 (3.1 g, 0.022 mol, 2 equiv.) and 0.05 g of 18-crown-6 were added into the mixture. The solution was refluxing for 24 h under N2 protection. Then, the solvent was removed and the combined mixture was purified by silica gel column chromatography eluted with 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 dichloromethane: petroleum ether. The evaporation of the solvent gave a yellow powder compound. After this process, excessive N,N-dimethylethanolamine was added to C10Azo in acetonitrile for the synthesis of ChoC10Azo. The mixture was kept stirring for 72 h at 75 °C under N2 protection. After evaporation of acetonitrile and N,N-dimethylethanolamine, the yellow solid was dissolved in chloroform. The solution was added stepwise with magnetic stirring to plenty of petroleum ether, and the yellow precipitate was filtered and dried in a vacuum oven. The synthesis procedure for the other ionic liquids was similar with ChoC10Azo.
5 dichloromethane: petroleum ether. The evaporation of the solvent gave a yellow powder compound. After this process, excessive N,N-dimethylethanolamine was added to C10Azo in acetonitrile for the synthesis of ChoC10Azo. The mixture was kept stirring for 72 h at 75 °C under N2 protection. After evaporation of acetonitrile and N,N-dimethylethanolamine, the yellow solid was dissolved in chloroform. The solution was added stepwise with magnetic stirring to plenty of petroleum ether, and the yellow precipitate was filtered and dried in a vacuum oven. The synthesis procedure for the other ionic liquids was similar with ChoC10Azo.
The chemical structures of these ionic liquids were confirmed by 1H NMR (Bruker Avance-400 spectroscopy), and their purity was found to be greater than 0.97 in mass fraction. The detailed data were given in ESI.†
2.3 UV-vis spectroscopy measurements
UV-vis spectra of the azobenzene-based ionic liquids in aqueous solutions before and after UV irradiation were determined using a UV-4100 spectrophotometer with 1.0 cm quartz cell at 25.0 °C. The spectra were recorded in a wavelength range from 250 nm to 600 nm with a step of 1 nm, and deionized water was used as the blank. The changes in UV absorbance of 0.055 mM of azobenzene-based ionic liquids aqueous solution were measured after UV irradiation at 365 nm (with intensity of 100 mW cm−2) and then vis-light irradiation with incandescent lamp as light source.2.4 Critical aggregate concentration measurements of ChoC10Azo in water
Conductivity titrations were performed to determine the critical aggregate concentration of the ionic liquid in aqueous solution with a Mettler-Toledo InLab 741 ISM electrode at 25 °C by using a Mettler-Toledo Seven compact conductivity with a resolution of 1 × 10−3 μS cm−1. The conductance cell was equipped with a water circulating jacket, and the temperature was controlled within 25.00 ± 0.01 °C with a HAAKE V26 thermostat (Thermo Electron, Germany). The cell was calibrated with aqueous KCl solutions at different concentrations, and a cell constant of 1.0129 cm−1 was determined.2.5 Dynamic light scattering measurements
Dynamic light scattering (DLS) measurements were carried out at 25.0 °C by using a laser light scattering photometer (Nano-ZS90, Malvern, U. K). Light of λ = 633 nm from a solid-state He–Ne laser (4.0 mW) was used as the incident beam. All sample solutions were filtered through a 0.22 μm hydrophilic PVDF membrane filter to remove the dust. All measurements were made at 90° scattering angle. At least three measurements were taken for each solution, and the reproducibility of aggregate sizes from DLS data was found to be within ±3%.2.6 Small angle X-ray scattering measurements
Small-angle X-ray scattering (SAXS) experiments were performed on a SAXS Space small-angle X-ray scattering instrument (Anton Paar, Austria) equipped with a Kratky block-collimation system at 25.0 °C. The samples were held in a quartz capillary placed in a stainless steel tank. The X-ray was generated using sealed-tube X-ray generator with Cu target and the wavelength was 0.1542 nm.3. Results and discussion
3.1 Photo-isomerization behavior of the azobenzene-based ionic liquids
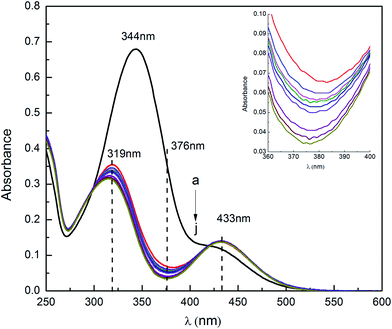
It is well known that azobenzene is the most efficient and extensively investigated photosensitive unit, and the azobenzene derivatives generally present in trans-isomer in water because it is more thermodynamically stable than the cis-isomer. They show reversible light-induced switching between trans- and cis-isomers in aqueous solutions. Light-induced isomerization of the azobenzene-based ionic liquids was investigated by UV-vis spectroscopy. As an example, Fig. 1 shows the isomerization process of ChoC10Azo in aqueous solutions, and Fig. 2 shows the UV-vis spectrum of 0.055 mM ChoC10Azo aqueous solution as a function of UV irradiation time. The UV-vis spectra for other ionic liquids (ChoC6Azo, ChoC4Azo and ChoC2Azo) were given in Fig. S5–S7.†As shown in Fig. 2, the typical maximum absorption wavelength at 344 nm was resulted from the π → π* transition of trans-isomer. After the UV irradiation, the absorption peak at 344 nm disappeared while two new peaks at 319 nm and 433 nm appeared due to the n → π* transition of cis-isomer.21 Similar results could be observed from Fig. S5–S7.† Here, the absorption at 376 nm exactly between the peaks at 319 nm and 433 nm of the cis-form was minimal after UV irradiation. According to the approach of Zakrevskyy et al.,22 the absorbance at 376 nm of the original spectrum (before UV irradiation) was used to estimate the amount of trans-isomer in solution after the isomerization process with the assumption that the cis-isomer at 376 nm is negligible and the initial state before irradiation exists only in the pure trans-isomer.20,22 Thus isomer composition of ChoC10Azo after different irradiation times, together with ChoC6Azo, ChoC4Azo and ChoC2Azo can be calculated, and the results were shown in Table 1. It can be seen that after 5 s irradiation, photoisomerization efficiencies of all the ionic liquids investigated in this work were greater than 83%, indicating that these ionic liquids had rapid photo-responsive performance. Continuous irradiation of the samples by UV for 30 s, the isomerization efficiencies of ChoC6Azo, ChoC4Azo and ChoC2Azo were increased until the equilibrium of the isomerization reaction was achieved. However, for ChoC10Azo, the equilibrium time of the isomerization reaction was about 10 min, this implies that a longer alkyl chain was not beneficial for the photoisomerization reaction rate of these ionic liquids. The possible reason is that the head group of ionic liquids is an electron withdrawing group, which could reduce the isomerization energy barrier and accelerate the isomerization process of azobenzene.23 For ChoC10Azo, the alkyl chain between head group and azobenzene group is longer, therefore, the acceleration of the azobenzene isomerization process by electron withdrawing head group is negligible. However, there was no significant difference between the equilibrium isomerization efficiencies of all of them.
| Irradiation time/s | ChoC10Azo | ChoC6Azo | ChoC4Azo | ChoC2Azo | ||||
|---|---|---|---|---|---|---|---|---|
| trans% | cis% | trans% | cis% | trans% | cis% | trans% | cis% | |
| 0 | 100 | 0 | 100 | 0 | 100 | 0 | 100 | 0 |
| 1 | 19.5 | 80.5 | 26.4 | 73.6 | 42.3 | 57.7 | 52.4 | 47.6 |
| 2 | 17.2 | 82.8 | 11.2 | 88.8 | 16.1 | 83.9 | 34.4 | 65.6 |
| 5 | 16.1 | 83.9 | 10.1 | 89.9 | 10.9 | 89.1 | 15.6 | 84.4 |
| 10 | 15.5 | 84.5 | 9.7 | 90.3 | 10.5 | 89.5 | 10.2 | 89.8 |
| 30 | 15.0 | 85.0 | 9.1 | 90.9 | 10.3 | 89.7 | 9.5 | 90.5 |
| 60 | 14.1 | 85.9 | 8.1 | 91.9 | 10.7 | 89.3 | 9.0 | 91.0 |
| 300 | 11.6 | 88.4 | 7.9 | 92.1 | 10.7 | 89.3 | 9.2 | 90.8 |
| 600 | 10.2 | 89.8 | 9.1 | 90.9 | 10.5 | 89.5 | 9.5 | 90.5 |
| 1800 | 9.6 | 90.4 | 9.7 | 90.3 | 10.9 | 89.1 | 9.5 | 90.5 |
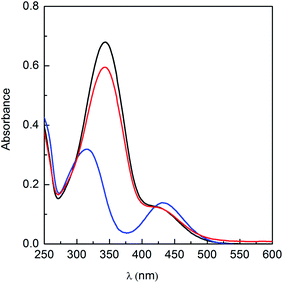
Furthermore, reversibility of photo-isomerism of the azobenzene-based ionic liquids was investigated in detail. It was found from Fig. 3 that after UV irradiation, the isomer mixture of ChoC10Azo did not result in a complete transition to the initial state by further irradiation of vis-light, because both isomers absorbed vis-light and the steady state depended on the ratio of the corresponding absorption of both isomers at the given excitation wavelength. Therefore, about 12% of ChoC10Azo molecules still remained in the cis state, which can be used to explain why the absorption peak at 344 nm could not be fully regenerated. Changes of the absorbance value at 344 nm after several vis and UV irradiation cycles (up to 8 cycles) were shown in Fig. S8 (ESI).† Clearly, a good reversibility was indicated in the cis–trans transformation.
3.2 Photo-induced change in aggregation behavior of azobenzene-based ionic liquids
The aggregation behavior of the azobenzene-based ionic liquids was investigated by conductivity measurements of their aqueous solutions at 25.0 °C. The results showed that only ChoC10Azo could form aggregates in water and the concentration dependent conductivity of aqueous ChoC10Azo before and after UV irradiation were shown in Fig. 4. These curves exhibited typical behavior with two linear fragments, and the concentration at which the two linear fragments intersect was assigned to critical aggregation concentration (CAC). The degree of ionization (α) of the aggregates was taken to be the ratio of the values of dκ/dC before and after the CAC, and could be calculated from the slope of the two straight lines in these regions. Actually, α value indicates the ability of anion bound on the aggregates, an increase of α value suggests a decrease in the ability of the anions bound to the aggregates.24,25 The values of α and CAC for aqueous ChoC10Azo solutions before and after UV irradiation were included in Table 2. It is clear that there is no significant difference in the CAC value of ChoC10Azo before and after UV irradiation.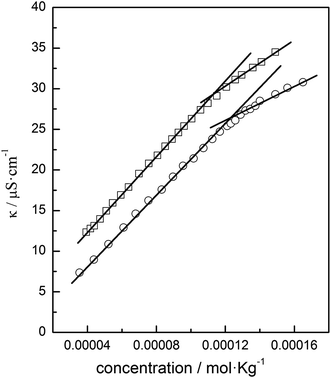 | ||
| Fig. 4 Plots of the conductivity of ChoC10Azo in aqueous solutions as a function of ChoC10Azo concentration at 25.0 °C before (□) and after (○) UV irradiation. | ||
| UV irradiation | CAC/mol kg−1 | α |
|---|---|---|
| Before | 1.1 × 10−4 | 0.64 |
| After | 1.3 × 10−4 | 0.52 |
It is known that hydrophobic interaction of the alkyl chains of ionic liquids is the main driving force of ionic liquids to form the aggregates.25,26 Usually, ionic liquids with carbon atom number equal to or greater than eight in the alkyl chain are able to form micelles in water.25 This explains why only ChoC10Azo could form micelles among the studied ionic liquids. We tried to investigate the aggregation of ionic liquid ChoC12Azo, but its solubility in water was too low to perform such an experiment.
3.3 Photo-induced modulation of the aggregates size of ChoC10Azo
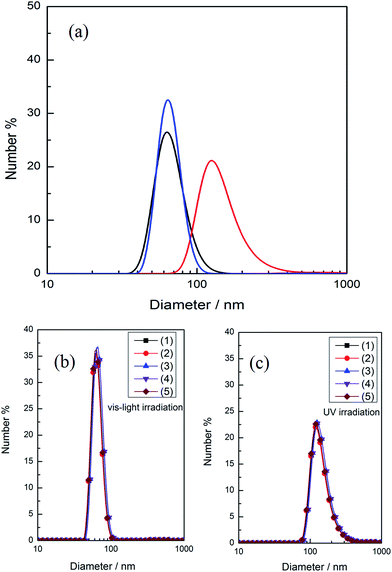
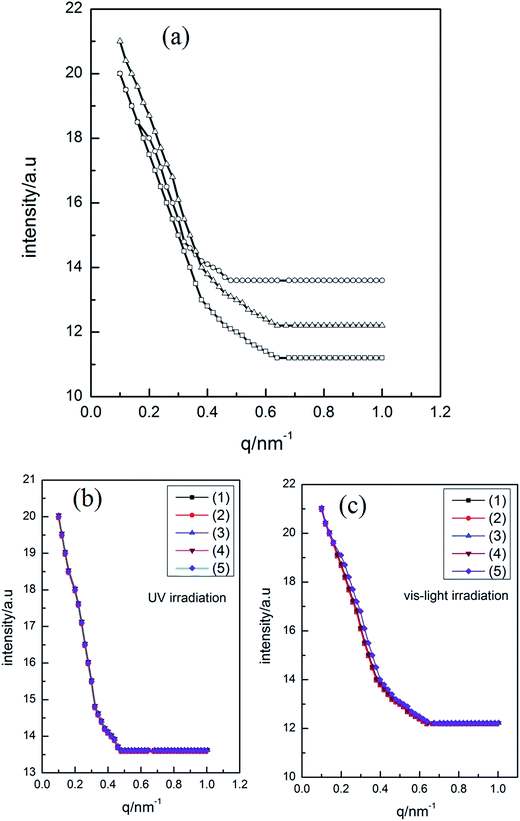
In order to investigate the influence of UV light irradiation on the aggregates size, DLS measurement was employed to detect the changes of aggregates size of ChoC10Azo before and after light irradiation in aqueous solution. The size distribution of 0.26 mM ChoC10Azo aqueous solution for different irradiation stages at 25.0 °C were shown in Fig. 5a. It can be clearly seen that the small sized aggregates of 63 nm formed from trans-isomer grown up into large sized aggregates of 125 nm formed mainly by cis-isomer after UV irradiation; then it returned to the small sized aggregates after further irradiation by visible light. However, the average size was slightly different from that before UV irradiation, which was attributed to the fact that the aggregates were composed of trans/cis mixture after further irradiation with vis-light, while the aggregates formed before UV irradiation were constructed by almost pure trans-isomer. Moreover, this result indicates that the aggregate size of ChoC10Azo became larger upon UV irradiation, proving that UV irradiation was beneficial to the aggregates growth of ChoC10Azo. The size distributions of the aggregate by alternative UV/vis light irradiation for five circles were shown in Fig. 5b and c, which suggest a good reversibility.Small-angle X-ray scattering (SAXS) measurements were performed to confirm the size change of the aggregates by alternative UV/vis light irradiation. SAXS patterns and the size change in the aggregates were shown in Fig. 6a and Table 3, respectively. Several fitting models were attempted to reproduce the experimental data, and the best fits for the curves in Fig. 6a were completed by using a spherical micelle model. As shown in Table 3, the smaller sized aggregates of trans-isomer grown up into larger sized aggregates formed mainly by cis-isomer after UV irradiation. However, after further irradiation by visible light, they returned to the smaller sized aggregates, which is in accordance with the result obtained from DLS measurements. Similarly, SAXS data of the aggregate by alternative UV/vis light irradiation for five circles were shown in Fig. 6b and c, a good reversibility was also suggested.
| State | DLS results/nm | SAXS results/nm |
|---|---|---|
| Initial | 63 | 62 |
| After UV irradiation | 125 | 91 |
| After vis-light irradiation | 63 | 65 |
As mentioned above, α value decreased after UV irradiation, the decrease in α value means that the stronger association of anion with the aggregates, which resulted in the formation of larger sized aggregates. Furthermore, the aggregates formed before UV irradiation were spherical micelles, this is similar to the results of [C4AzoC6TMA][TfO]20 and [C14mimAzo]Br17 reported in literatures. However, it is worth to note that after UV irradiation, the structure of ChoC10Azo aggregates remained spherical micelles, this was different from the structure of [C4AzoC6TMA][TfO]20 and [C14mimAzo]Br,17 which changed from micelles into vesicles in aqueous solutions. Combined with the chemical structure of ChoC10Azo, [C4AzoC6TMA][TfO] and [C14mimAzo]Br, this result suggests that for the azobenzene ionic liquids with a similar anion, azobenzene group in different positions of the alkyl chain had great effect on the aggregation behaviors of the ionic liquids after UV irradiation. Azobenzene at the end of the alkyl chain was not beneficial for the formation of vesicles due to the hindering of azobenzene group for the hydrophobic interaction of the alkyl chain.
4. Conclusion
In summary, the reported photoresponsive ionic liquids ChoC10Azo, ChoC6Azo, ChoC4Azo and ChoC2Azo with azobenzene located at the end of the alkyl chain showed fast and effectively reversible transition between trans- and cis-forms in water under UV/vis light irradiation. For example, after 5 s of UV irradiation, their photoisomerization efficiencies were greater than 83%. The photoisomerization reaction rate was affect by the length of alkyl chain, but there was no significant difference between the equilibrium isomerization efficiency of them. It was also found that among the investigated ionic liquids, only ChoC10Azo could form micelle in water. Unlike other azobenzene-based ionic liquids reported in literatures, UV and visible light irradiation only changed the size of its aggregates, but could not change the micelle into vesicle. This result suggests that azobenzene at the end of the alkyl chain of ionic liquids was not beneficial for the formation of vesicles after UV irradiation. These findings show some new insights into the relationship between structure and photoresponsive characteristics/aggregation behavior of the azobenzene-based ionic liquids, and may be useful for the design of microscale photo-control devices and sensors.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 21673068, 21573060), the Youth Science Foundation of Henan Normal University (2014QK14) and the Program for Innovative Research Team in Science and Technology in University of Henan Province (No. 16IRTSTHN002).References
- M. Stuart, W. Huck, J. Genzer, M. Müller, C. Ober, M. Stamm, G. Sukhorukov, I. Szleifer, V. Tsukruk and M. Urban, Nat. Mater., 2010, 9, 101–113 CrossRef PubMed.
- M. Ikeda, R. Ochi, A. Wada and I. Hamachi, Chem. Sci., 2010, 1, 491–498 RSC.
- X. Chen, J. Gao, B. Song, M. Smet and X. Zhang, Langmuir, 2009, 26, 104–108 CrossRef PubMed.
- D. Díaz, D. Kühbeck and R. Koopmans, Chem. Soc. Rev., 2011, 40, 427–448 RSC.
- Y. Zhang, M. Jiang, J. Zhao, Z. Wang, H. Dou and D. Chen, Langmuir, 2005, 21, 1531–1538 CrossRef CAS PubMed.
- W. Yao, H. Wang, G. Cui, Z. Li, A. Zhu, S. Zhang and J. Wang, Angew. Chem., Int. Ed., 2016, 55, 7934–7938 CrossRef CAS PubMed.
- J. Eastoe and A. Vesperinas, Soft Matter, 2005, 1, 338–347 RSC.
- J. Ryu, R. Roy, J. Ventura and S. Thayumanavan, Langmuir, 2010, 26, 7086–7092 CrossRef CAS PubMed.
- Y. Shi, D. Xiong, H. Wang, Y. Zhao and J. Wang, Langmuir, 2016, 32, 6895–6901 CrossRef CAS PubMed.
- Z. Chu, C. Dreiss and Y. Feng, Chem. Soc. Rev., 2013, 42, 7174–7203 RSC.
- K. Dong, X. Liu, H. Dong, X. Zhang and S. Zhang, Chem. Rev., 2017, 117, 6636–6695 CrossRef CAS PubMed.
- H. Tamura, Y. Shinohara and T. Arai, Chem. Lett., 2010, 39, 240–241 CrossRef CAS.
- S. Zhang, S. Liu, Q. Zhang and Y. Deng, Chem. Commun., 2011, 47, 6641–6643 RSC.
- J. Avó, L. Cunha-Silva, J. Lima and A. Jorge Parola, Org. Lett., 2014, 16, 2582–2585 CrossRef PubMed.
- J. Yang, H. Wang, J. Wang, Y. Zhang and Z. Guo, Chem. Commun., 2014, 50, 14979–14982 RSC.
- J. Yang, H. Wang, J. Wang, X. Guo and Y. Zhang, RSC Adv., 2015, 5, 96305–96312 RSC.
- A. Wu, F. Lu, P. Sun, X. Gao, L. Shi and L. Zheng, Langmuir, 2016, 32, 8163–8170 CrossRef CAS PubMed.
- H. Sakai, Y. Orihara, H. Kodashima, A. Matsumura, T. Ohkubo, K. Tsuchiya and M. Abe, J. Am. Chem. Soc., 2005, 127, 13454–13455 CrossRef CAS PubMed.
- A. Fihey, A. Perrier, W. Browne and D. Jacquemin, Chem. Soc. Rev., 2015, 44, 3719–3759 RSC.
- S. Shi, T. Yin, X. Tao and W. Shen, RSC Adv., 2015, 5, 75806–75809 RSC.
- Y. Wang, N. Ma, Z. Wang and X. Zhang, Angew. Chem., Int. Ed., 2007, 46, 2823–2826 CrossRef CAS PubMed.
- Y. Zakrevskyy, M. Richter, S. Zakrevska, N. Lomadze, R. von Klitzing and S. Santer, Adv. Funct. Mater., 2012, 22, 5000–5009 CrossRef CAS.
- C. Crecca and A. Roitberg, J. Phys. Chem. A, 2006, 110, 8188–8203 CrossRef CAS PubMed.
- A. González-Pérez, J. del Castillo, J. Czapkiewicz and J. Rodríguez, J. Phys. Chem. B, 2001, 105, 1720–1724 CrossRef.
- H. Wang, H. Li, G. Cui, Z. Li and J. Wang, Acta Phys.–Chim. Sin., 2016, 32, 249–260 CAS.
- H. Wang, L. Zhang, J. Wang, Z. Li and S. Zhang, Chem. Commun., 2013, 49, 5222–5224 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7ra08419f |
| This journal is © The Royal Society of Chemistry 2017 |