Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Effects of nano-grooved gelatin films on neural induction of human adipose-derived stem cells
Chen-Yu Tsaia,
Chih-Ling Lina,
Nai-Chen Cheng*b and
Jiashing Yu *a
*a
aDepartment of Chemical Engineering, National Taiwan University, No. 1, Sec. 4, Roosevelt Rd., Da'an Dist., Taipei City 106, Taiwan. E-mail: jiayu@ntu.edu.tw
bDepartment of Surgery, National Taiwan University Hospital, No. 1, Changde St., Zhongzheng Dist., Taipei City 10048, Taiwan, Republic of China
First published on 21st November 2017
Abstract
The extra cellular matrix (ECM) and cell–cell interactions facilitate the survival, self-renewing and differentiation capabilities of stem cells. Biomaterials with specific structures such as grooves, ridges, pits, or pillars can mimic the topographic landscape of the niche. Cells can “sense” the mechanical properties and surface patterns, ranging from the micro- to nano-scale, of the substrate; hence, different sizes of nano-grooves on a gelatin surface were designed. The design of grooves can be systematically modified and those structures can reflect the organization of the ECM. In previous studies, polystyrene (PS) was often used because it is easy to fabricate topographic structures. For better biocompatibility, gelatin was chosen and fabricated into ideal nano-groove films. On the other hand, gelatin can be crosslinked by using several crosslinking agents, which leads to a higher mechanical strength and better flexibility. It was known that stem cells can serve as a source of neurons in transplantation therapies. The differentiation of neurons is associated with directionality of stem cell. To investigate the effect of topographic cues on stem cells, groove pattern arrays were constructed onto gelatin surfaces. Human adipose-derived stem cells (hASCs) were seeded onto the patterned gelatin films to observe cell proliferation and differentiation.
Introduction
According to a neurological disorders report from the World Health Organization (WHO) in 2006,1 the number of people who die from neurological disorders increases year by year. It is estimated that around 9 million people will die from these disorders in 2030. In general, injured nerves can regenerate by themselves.2 However, surgery is required if the injuries are too severe. Autologous nerve grafting is the most common surgery traditionally,3 but nerves cannot recover fully. Therefore, the aim of neural tissue engineering is to support traditional surgeries, even replace them. Recently, stem cell therapy has drawn a lot of attention.4 Stem cell therapy uses neural cells differentiated from stem cells to enhance nerve repair. There are many kinds of stem cells that can differentiate into neural cells, but some of them are hard to harvest.To investigate the behavior of stem cells in human tissues, scientists have constructed substrates with both physical and chemical features. These stimuli can mimic stem cell niches to affect cell proliferation, morphology and differentiation.5 Stiffness and topographic cues are commonly used to imitate physical features of the ECM. Matrix stiffness has a large effect on cell function. It regulates cell adhesion, growth, survival and motility, and even the cell phenotype.6 The stiffness of tissues can be measured by the elastic modulus E of a solid, which ranges from 0.5 kPa (fat tissue) to 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 kPa (bone tissue). Comparing these values with a tissue culture on plastic or glass, where the elastic modulus is in the GPa range, it clearly shows that cells are not usually cultured in a non-physiological environment. As a result, many researchers have studied cells in vitro under a more physiological environment. For example, McDaniel et al. revealed that the stiffness of collagen fibrils can influence the phenotype of muscle cells.7 Synthetic substrates with controllable stiffness showed the differences in cell motility and adhesion.8 Besides, stiffness can further regulate cell lineage commitment and differentiation state, such as the differentiation of precursor cells into osteoblasts, neurites outgrowth and the striation of muscle cells.9
000 kPa (bone tissue). Comparing these values with a tissue culture on plastic or glass, where the elastic modulus is in the GPa range, it clearly shows that cells are not usually cultured in a non-physiological environment. As a result, many researchers have studied cells in vitro under a more physiological environment. For example, McDaniel et al. revealed that the stiffness of collagen fibrils can influence the phenotype of muscle cells.7 Synthetic substrates with controllable stiffness showed the differences in cell motility and adhesion.8 Besides, stiffness can further regulate cell lineage commitment and differentiation state, such as the differentiation of precursor cells into osteoblasts, neurites outgrowth and the striation of muscle cells.9
On the other hand, the effect of topography on cell behavior has been investigated since 1911.10 Cells can respond to topographic cues as small as 5 nm. Therefore, topographic features from the micro- to the nano-scale have been widely developed. In general, surface topography is affected by roughness and patterns on the surface.11 Many types of features, for instance, grooves, dots and pillars, are developed, because these structures can be systematically modified and reflect the fibrillar organization of the ECM.
Among these structures, ridges and grooves have been investigated extensively. Micro- or nano-grooves can guide cells to align along the patterns. However, a previous study showed that fibroblasts do not align with groove depths below 35 nm or ridge widths smaller than 100 nm.12 Yang et al. investigated the influence of nano-grooves on the morphology of osteoblast-like cells with a groove-to-ridge ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (90–500 nm in width and 300 nm in depth). It showed an increased cell-spreading area compared to flat surfaces and elongated nuclei.13 Sciancalepore et al. fabricated fibronectin (FN) micro-patterns to drive the differentiation of adult renal progenitor cells (ARPCs) in the absence of an exogenous chemical or cellular reprogramming.14
1 (90–500 nm in width and 300 nm in depth). It showed an increased cell-spreading area compared to flat surfaces and elongated nuclei.13 Sciancalepore et al. fabricated fibronectin (FN) micro-patterns to drive the differentiation of adult renal progenitor cells (ARPCs) in the absence of an exogenous chemical or cellular reprogramming.14
As mentioned above, there are lots of stem cells that can differentiate into neural cells including embryonic stem cells (ESCs),15–17 neural stem cells (NSCs)18–20 and adipose-derived stem cells (ASCs).21–24 Among these stem cells, ASCs are commonly used because they can be harvested easily and are able to differentiate into multiple lineages including adipocytes,25 myocytes,26 osteoblasts,27 chondrocytes28 and neural cells.29 Many studies have investigated how topography affect differentiation of human adipose-derived stem cells (hASCs). Deiwick et al. fabricated a “Lotus” structure using titanium to investigate how it affected the osteogenic differentiation of hASCs.30 In addition, Mobasseri et al. used polymers to fabricate grooves and studied neural differentiation of hASCs.31 However, materials used to fabricate topography are too rigid for cells.
In this study, to mimic the physiological environment of normal tissue, nano-grooves were fabricated onto a gelatin substrate, which is a natural and soft biomaterial. The mechanical properties of gelatin can be adjusted through crosslinking. Grooves were chosen because they can be fabricated into different sizes easily. To study the relationship between neural differentiation of stem cells and surface topography, hASCs were cultured on different groove sizes on gelatin films.
Experimental section
Materials and methods
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (groove width/depth (nm): 400/100, 400/400, 800/100 and 800/400) were used to fabricate topography onto polydimethylsiloxane (PDMS, Dow Corning, USA) molds and gelatin films (Fig. 1). At first, the silicone rubber molds were fabricated by mixing the elastomer with a curing agent with a ratio of 10
1 (groove width/depth (nm): 400/100, 400/400, 800/100 and 800/400) were used to fabricate topography onto polydimethylsiloxane (PDMS, Dow Corning, USA) molds and gelatin films (Fig. 1). At first, the silicone rubber molds were fabricated by mixing the elastomer with a curing agent with a ratio of 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The mixture was cast onto silicon substrates and degassed to remove entrapped bubbles. After polymerizing at 80 °C for 2 h, PDMS molds with negative replicas of topography on substrates could be easily removed from the silicon substrates.
1. The mixture was cast onto silicon substrates and degassed to remove entrapped bubbles. After polymerizing at 80 °C for 2 h, PDMS molds with negative replicas of topography on substrates could be easily removed from the silicon substrates.
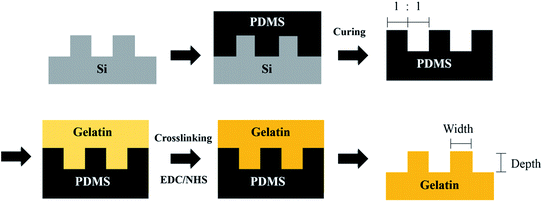 | ||
| Fig. 1 Schematic illustration of the fabrication of nano-grooved gelatin films from silicon substrates. | ||
To fabricate the nano-grooved gelatin films, PDMS molds were placed and fixed in a Petri dish with the patterned side upwards. An aqueous solution of 5 wt% gelatin (Sigma-Aldrich, Germany) and 1% PSA (Sigma-Aldrich, Germany) was cast into the Petri dish, and air-dried overnight at 25 °C. The gelatin samples were subsequently immersed in binary solvent mixtures containing N-(3-dimethylaminopropyl)-N′-ethylcarbodiimide hydrochloride (EDC, Sigma-Aldrich, Germany) and N-hydroxysuccinimide (NHS, Sigma-Aldrich, Germany). The reaction was allowed to proceed at 25 °C for 96 h.32 Crosslinked gelatin films were cut down and thoroughly rinsed with deionized (DI) water to remove excess EDC and the urea by-product. Before cell culturing, gelatin films were immersed in cell culture medium for 24 h to ensure the removal of excess crosslinking agent.
A 5 mL aliquot of the aqueous phase was removed from each sample and heated for 15 min in a hot water bath. After cooling to room temperature, samples were diluted again with 15 mL of water. The absorbance was measured at 346 nm in a UV/Vis spectrophotometer (Cary 100, Agilent, USA) against a blank. Four replicates were used in each determination. Blanks were prepared in triplicate by the same procedure as above, except that HCl was added before TNBS to prohibit the reaction of amino groups with TNBS.
To induce neural induction, hASCs were cultured in an induction medium (DMEM/HG (Hyclone, USA) containing 1% FBS and supplementary 100 ng mL−1 bFGF) for 7 days. Then cells were cultured in the presence of 10 μM forskolin (Sigma-Aldrich, USA) for another 7 days. The expression of neural differentiation markers was analyzed by immunofluorescence and qPCR.
To test cell proliferation of hASCs on gelatin films, we cultured 5000 cells on nano-grooved and flat gelatin films for 1, 4 and 7 days. CytoScan™ WST-1 Cell Cytotoxicity Assay (G-Biosciences, USA) was used. With a final volume of 200 μL per well culture medium, blank wells with culture medium only were prepared. 20 μL WST-1/CEC assay dye solution was added to each well and shook gently to mix the chemicals with the medium. Then, the plates were incubated for 2 h in the cell culture incubator. After that, the plates were shaken for 1 min on a shaker and the absorbance at 450 nm was measured using a microplate reader. The wavelength of 630 nm was set as a reference.
Then, cDNA, F/R primers (Yuanying, Taiwan), Power SYBR Green PCR Master Mix (Thermo Fisher scientific, USA) and DNase/RNase Free Water (Yuanying, Taiwan) were mixed in Eppendorf PCR tubes (8-tube strips). The sequence of primers is shown in Table 1. After sealing using clear adhesive film, a PCR was run using a real-time PCR instrument (StepOnePlus, Applied Biosystems, USA). The expression level was analyzed and normalized to glyceraldehyde 3-phosphate dehydrogenase (GAPDH) for each sample. The relative quantity (RQ) of gene expression was calculated using the comparative CT method. The sequence of primers is shown in Table 1.
| Target gene | Primer sequences | |
|---|---|---|
| Tuj-1 | Forward | 5′-CATGGATGCCGCTCAG-3′ |
| Reverse | 5′-CAGGCAGTCGCAGTTTTCAC-3′ | |
| Nestin | Forward | 5′-CTCTGACCTGTCAGAAGAAT-3′ |
| Reverse | 5′-GACGATGACACTTACAGAAT-3′ | |
| GAPDH | Forward | 5′-CAAGGCTGAGAACGGGAAGC-3′ |
| Reverse | 5′-AGGGGGCAGAGATGATGACC-3′ | |
Results and discussion
Topography measurement
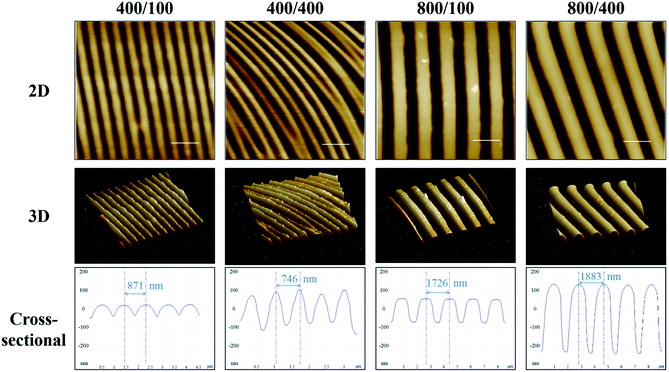
AFM was used to measure nano-grooves on gelatin films (Fig. 2). The distance between adjacent peaks was around 800 nm on 400/100 and 400/400, and 1800 nm on 800/100 and 800/400. The vertical distance between the peak and valley was around 150 nm on 400/100 and 800/100, and 200 to 300 nm on 400/400 and 800/400. The groove depth of 400/400 was not as deep as 800/400, which may result from the groove with a 400 nm width being too narrow for gelatin to permeate. Besides, the softness of gelatin and soaring grooves led to a curvature in 2D AFM image of 400/400.Crosslinking extent measurement
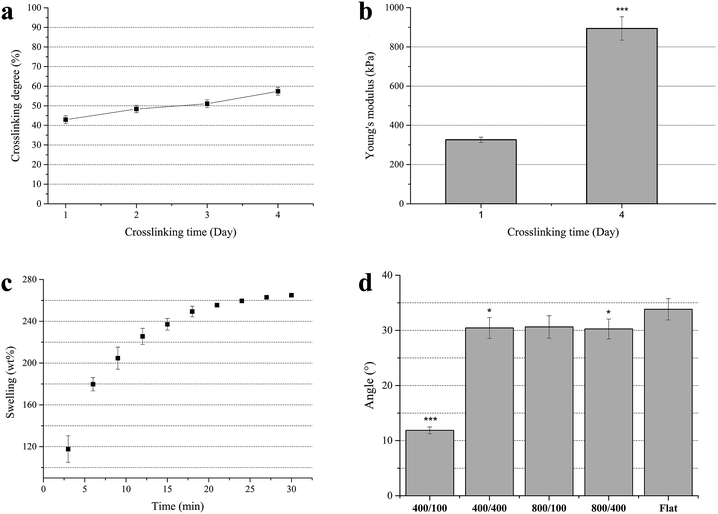
To determine the crosslinking degree of the gelatin films, TNBS was used to bind with the ε-amino groups in gelatin. It showed yellow and had an absorbance at 346 nm. Less ε-amino groups refers to a higher extent of crosslinking. The result showed that the crosslinking extent of the gelatin films after crosslinking by EDC and NHS for 1 to 4 days rose from 42% to 57% (Fig. 3a).The softness of the gelatin films after crosslinking for 4 days was more suitable for experimental operations. Although the crosslinking extent of gelatin crosslinked by EDC and NHS was not as high as glutaraldehyde (∼90%), lower cytotoxicity and proper mechanical strength made it a better choice.
Mechanical test
The Young's modulus of crosslinked gelatin films was used to determine whether the softness of gelatin is close to natural organs. The Young's modulus of gelatin films after crosslinking for 1 and 4 days was calculated from the linear region of the stress–stain curve (initial slope: 0–10%). It was found that the Young's modulus rose from 326 to 894 kPa (Fig. 3b), and was similar to human organs.34 Hence, cells were cultured on soft substrates whose stiffness was close to natural organs instead of stiff substrates such as polystyrene and polyurethane.Swelling test
Crosslinked gelatin films were bendable and would absorb a lot of water. To investigate when gelatin films stop swelling, the swelling ratio of gelatin films was measured. The results showed that weight of gelatin films crosslinked for 4 days reached 260% of dry weight after immersing in DI water for 30 min (Fig. 3c), which means gelatin films had a high water content. On the other hand, it was found that gelatin films can be dehydrated–hydrated repeatedly. In this study, all of the gelatin films were immersed in DI water or culture medium for at least 30 min until use to prevent topography change.Contact angle measurement
To investigate the hydrophilicity of nano-grooved gelatin films, the contact angle was measured (Fig. 3d). As gelatin films were immersed in culture medium during cell culture, air bubble contact angle was measured rather than water contact angle. In general, gelatin is hydrophilic and has high air bubble contact angle. The results showed that the contact angle of the air bubble on the 400/100 surface was significantly lower than the other groups, which means the surface of 400/100 was more hydrophobic. A previous study revealed that wettability affects cell adhesion, as cells adhered well on hydrophilic surfaces.35Cytotoxicity/cell viability
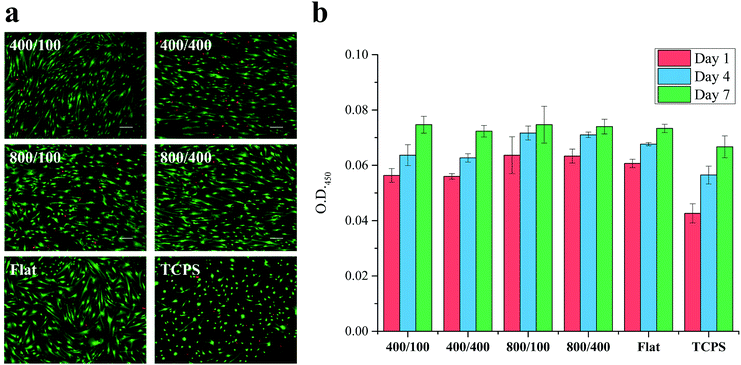
To test the cytotoxicity of gelatin films, the live/dead assay was used for hASCs proliferated on nano-grooved and flat gelatin films for 7 days, and TCPS was used as the control (Fig. 4a). The image showed a massive green fluorescence, which indicates that most of the cells are alive. The ratios of living cells in each group were higher than 90%. This proved that unreacted crosslinking agent had been removed successfully or has only little toxicity to cells.In addition to cytotoxicity, cell viability was tested using the WST-1 assay (Fig. 4b). hASCs proliferated on nano-grooved and flat gelatin film for 1, 4 and 7 days were tested, and TCPS was used as the control. The results showed cell amounts increase steadily from day 1 to 7. There is no significant difference between each group except TCPS, which may result from the difference of surface area between the 24 well and gelatin films (1 × 1 cm2).
Cell alignment analysis
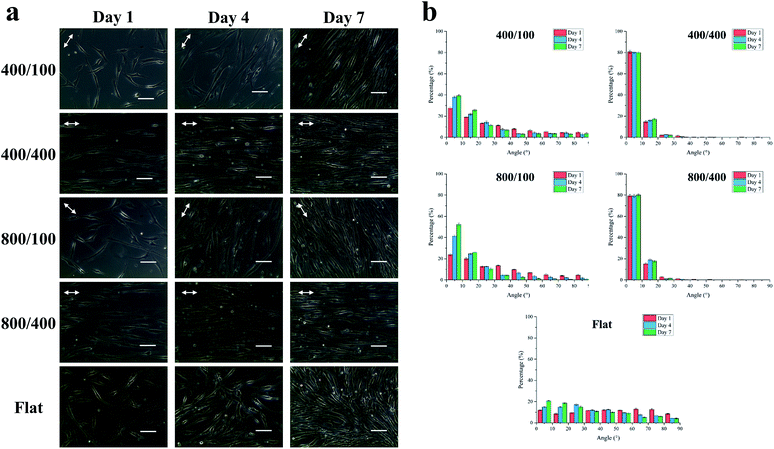
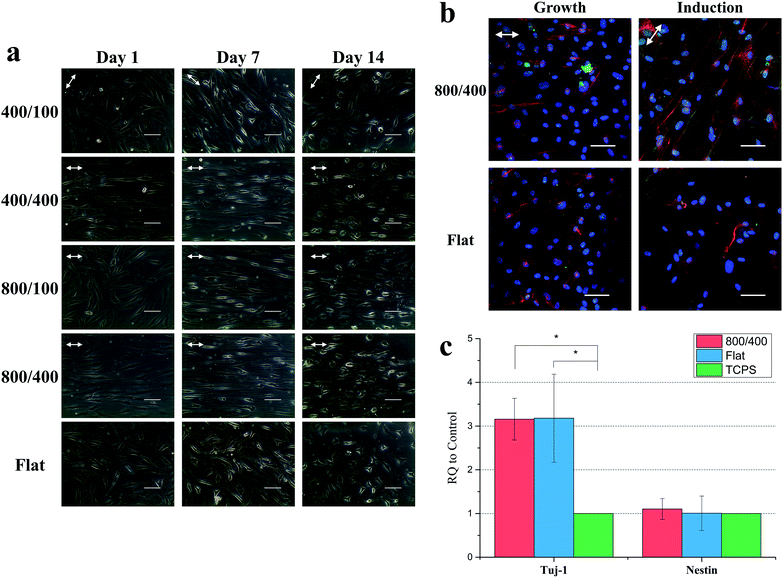
To observe the cell morphology of hASCs on nano-grooved and flat gelatin films for 1, 4 and 7 days, phase contrast microscope images were taken (Fig. 5a). On day 1, cells were aligned with the grooves on 400/400 and 800/400, whereas part of cells were aligned with grooves on 400/100 and 800/100 until day 7. It was assumed that groove depth has a greater influence on cell alignment than width.To quantify cell alignment, the angle between the cell and groove (Fig. 5b) was measured. As mentioned above, about 80% of cells aligned with grooves within 10° on 400/400 and 800/400 in the beginning. However, 40 to 55% of cells on 400/100 and 800/100 seemed to align with grooves within 10° until day 7, whereas cells on flat gelatin films still remained in random alignment.
hASCs morphology after neural induction
Compared to cells proliferated on gelatin films for 14 days, cells differentiated on gelatin films for 14 days showed contraction and neurites outgrowth (Fig. 6a). At the first stage of differentiation, cells were still elongating on day 7. Then, the cell body gradually contracted and neurites projected. There was no significant difference observed for cell morphology between each group from the phase contrast microscope images.The expression of neural markers
To study whether the cells successfully differentiate into neural cells and the effect of grooves on differentiation, we stained Tuj-1 and nestin for cells proliferated in growth medium and differentiated in the induction medium on 800/400 and flat gelatin films for 14 days (Fig. 6b). Tuj-1 is a neural marker expressed in immature neurons and nestin is an intermediate filament protein expressed during the early stage of neural differentiation. Confocal images showed a higher ratio of neural marker expression after neural induction, nestin especially. However, there is no difference between 800/400 and flat gelatin films in these images.To quantify the expression of Tuj-1 and nestin, we ran qPCR for cells differentiated on 800/400 and flat gelatin films for 14 days with Tuj-1 and nestin (Fig. 6c). The results showed that Tuj-1 expression of hASCs on 800/400 was three times higher than TCPS, whereas nestin expression was similar between each group. The increase of the Tuj-1 and nestin expression also correlated with our results from previous studies.36,37 Li et al. demonstrated adult neural stem cells showed a significant increase in neuronal differentiation on engineered anisotropic substrates (Si wafer) compared to the control. In their western blot analysis, upregulated Tuj-1 expression was also seen.36 Béduer et al. demonstrated that pre-coated micropatterned PDMS surfaces can serve as effective neurite guidance surfaces for human NSCs. Immunocytochemistry analysis showed that the channel width can strongly impact development and differentiation.37
Conclusions
Topography has shown a great influence on cell behavior in previous studies. Cells were cultured on patterned materials to investigate how topography affects cells. However, most of the materials such as polystyrene (Young's modulus > 3 GPa) are not as soft as natural tissues. In this study, nano-grooves on gelatin, whose Young's modulus is close to natural organs, was successfully fabricated. Gelatin films are believed to benefit cell adhesion due to their hydrophilic properties and high water content. In addition, gelatin crosslinked by EDC and NHS showed low cytotoxicity to hASCs. Cells adhered well on gelatin films and aligned with grooves on 400/400 and 800/400. As for neural differentiation, hASCs contracted and neurites projected after 14 days of induction and immunochemistry and qPCR results revealed that expression of neural markers (Tuj-1 and nestin) was higher after neural induction on gelatin substrates, which are more physical and chemically relevant to real tissue, especially the nano-groove patterned gelatin. Engineered anisotropic topographical cues could improve neurite outgrowth and promote neural differentiation.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the Ministry of Science and Technology, Taiwan (104-2221-E-002-124-MY3).Notes and references
- B. Saraceno, T. Dua, A. Janca, A. Muscetta, R. Kale, F. Montero and M. Peden, Neurological disorders: public health challenges, WHO, 2006 Search PubMed.
- D. Grinsell and C. P. Keating, BioMed Res. Int., 2014, 2014, 13 CrossRef PubMed.
- M. R. Raffe, Principles of Peripheral Nerve Repair, 1985 Search PubMed.
- S. Walsh and R. Midha, Neurosurg. Focus, 2009, 26, E2 CrossRef PubMed.
- Y. Zhang, A. Gordon, W. Qian and W. Chen, Adv. Healthcare Mater., 2015, 4, 1900–1914 CrossRef CAS PubMed.
- B. N. Mason, J. P. Califano and C. A. Reinhart-King, in Engineering Biomaterials for Regenerative Medicine, ed. S. K. Bhatia, Springer-Verlag, New York, 1st edn, 2012, ch. 2, DOI:10.1007/978-1-4614-1080-5.
- D. P. McDaniel, G. A. Shaw, J. T. Elliott, K. Bhadriraju, C. Meuse, K.-H. Chung and A. L. Plant, Biophys. J., 2007, 92, 1759–1769 CrossRef CAS PubMed.
- R. J. Pelham and Y.-L. Wang, Cell Biol., 1997, 94, 13661–13665 CAS.
- A. J. Engler, M. A. Griffin, S. Sen, C. G. Bönnemann, H. L. Sweeney and D. E. Discher, Myotubes differentiate optimally on substrates with tissue-like stiffness: pathological implications for soft or stiff microenvironments, J. Cell Biol., 2004, 166(6), 877–887 CrossRef CAS PubMed.
- R. G. Harrison, Science, 1911, 34, 279–281 CAS.
- K. Metavarayuth, P. Sitasuwan, X. Zhao, Y. Lin and Q. Wang, ACS Biomater. Sci. Eng., 2016, 2, 142–151 CrossRef CAS.
- W. A. Loesberg, J. te Riet, F. C. M. J. M. van Delft, P. Schön, C. G. Figdor, S. Speller, J. J. W. A. van Loon, X. F. Walboomers and J. A. Jansen, Biomaterials, 2007, 28, 3944–3951 CrossRef CAS PubMed.
- J.-Y. Yang, Y.-C. Ting, J.-Y. Lai, H.-L. Liu, H.-W. Fang and W.-B. Tsai, J. Biomed. Mater. Res., Part A, 2009, 90, 629–640 CrossRef PubMed.
- A. G. Sciancalepore, A. Portone, M. Moffa, L. Persano, M. De Luca, A. Paiano, F. Sallustio, F. P. Schena, C. Bucci and D. Pisignano, Biomaterials, 2016, 94, 57–69 CrossRef CAS PubMed.
- M. Kim, A. Habiba, J. M. Doherty, J. C. Mills, R. W. Mercer and J. E. Huettner, Dev. Biol., 2009, 328, 456–471 CrossRef CAS PubMed.
- W. Wongpaiboonwattana and M. P. Stavridis, J. Visualized Exp., 2015, 52823, DOI:10.3791/52823.
- J.-H. Chuang, L.-C. Tung and Y. Lin, World J. Stem Cell., 2015, 7, 437–447 CrossRef PubMed.
- Y.-Q. Liu, L.-B. Zhan, T.-T. Bi, L.-N. Liang, X.-X. Sun and H. Sui, RSC Adv., 2016, 6, 34959–34969 RSC.
- H. W. Choi, Y. J. Hong, J. S. Kim, H. Song, S. G. Cho, H. Bae, C. Kim, S. J. Byun and J. T. Do, PLos One, 2017, 12, e0170735 Search PubMed.
- B.-S. Moon, J.-Y. Yoon, M.-Y. Kim, S.-H. Lee, T. Choi and K.-Y. Choi, Exp. Mol. Med., 2009, 41, 116–125 CrossRef CAS PubMed.
- A. Abdanipour, T. Tiraihi and A. Delshad, Iran. Biomed. J., 2011, 15, 113–121 CAS.
- J. Dong, G. Zhu, T.-c. Wang and F.-s. Shi, J. Zhejiang Univ., Sci., B, 2017, 18, 445–448 CrossRef CAS PubMed.
- H. Ning, G. Lin, T. Fandel, L. Banie, T. F. Lue and C.-S. Lin, Differentiation, 2008, 76, 488–494 CrossRef CAS PubMed.
- P. J. Kingham, D. F. Kalbermatten, D. Mahay, S. J. Armstrong, M. Wiberg and G. Terenghi, Exp. Neurol., 2007, 207, 267–274 CrossRef CAS PubMed.
- J. H. Choi, E. Bellas, G. Vunjak-Novakovic and D. L. Kaplan, Meth. Mol. Biol., 2011, 702, 319–330 CAS.
- Y. S. Choi, G. J. Dusting, S. Stubbs, S. Arunothayaraj, X. L. Han, P. Collas, W. A. Morrison and R. J. Dilley, J. Cell Mol. Med., 2010, 14, 878–889 CrossRef CAS PubMed.
- L. de Girolamo, M. F. Sartori, W. Albisetti and A. T. Brini, J. Tissue Eng. Regener. Med., 2007, 1, 154–157 CrossRef CAS PubMed.
- A. A. Hamid, R. B. H. Idrus, A. B. Saim, S. Sathappan and K.-H. Chua, Clinics, 2012, 67, 99–106 CrossRef PubMed.
- S. Jang, H.-H. Cho, Y.-B. Cho, J.-S. Park and H.-S. Jeong, BMC Cell Biol., 2010, 11, 25 CrossRef PubMed.
- A. Deiwick, E. Fadeeva, L. Koch, R. Gebauer, B. Chichkov and S. Schlie-Wolter, J. Nanomed. Nanotechnol., 2014, 5, 1000239 Search PubMed.
- A. Mobasseri, A. Faroni, B. M. Minogue, S. Downes, G. Terenghi and A. J. Reid, Tissue Eng., Part A, 2015, 21, 1152–1162 CrossRef CAS PubMed.
- J.-Y. Lai, Y.-T. Li, C.-H. Cho and T.-C. Yu, Int. J. Nanomed., 2012, 7, 1101–1114 CrossRef CAS PubMed.
- I. Clyde, M. Ofner and W. A. Bubnis, Pharm. Res., 1996, 13(12), 1821–1827 CrossRef.
- T. R. Cox and J. T. Erler, Disease Models & Mechanisms, 2011, 4, 165–178 CAS.
- H. H. Ahn, I. W. Lee, H. B. Lee and M. S. Kim, Int. J. Mol. Sci., 2014, 15, 2075–2086 CrossRef PubMed.
- L. Qi, N. Li, R. Huang, Q. Song, L. Wang, Q. Zhang, R. Su, T. Kong, M. Tang and G. Cheng, PLoS One, 2013, 8, e59022 CAS.
- A. Béduer, C. Vieu, F. Arnauduc, J.-C. Sol, I. Loubinoux and L. Vaysse, Biomaterials, 2012, 33, 504–514 CrossRef PubMed.
| This journal is © The Royal Society of Chemistry 2017 |