Open Access Article
Open Access ArticleHierarchical flower-like NiCo2O4@TiO2 hetero-nanosheets as anodes for lithium ion batteries
Wei Chenabc,
Luya Weia,
Zhiya Lina,
Qian Liua,
Yue Chena,
Yingbin Lin *abc and
Zhigao Huang
*abc and
Zhigao Huang abc
abc
aCollege of Physics and Energy, Fujian Normal University, Fujian Provincial Key Laboratory of Quantum Manipulation and New Energy Materials, Fuzhou, 350117, China. E-mail: yblin@fjnu.edu.cn; Fax: +86-591-2286-8132; Tel: +86-591-2286-8132
bFujian Provincial Engineering Technology Research Center of Solar Energy Conversion and Energy Storage, Fuzhou, 350117, China
cFujian Provincial Collaborative Innovation Center for Optoelectronic Semiconductors and Efficient Devices, Xiamen, 361005, China
First published on 11th October 2017
Abstract
Flower-like NiCo2O4 consisting of nanosheets are synthesized by hydrothermal technique and subsequently surface-modified with a TiO2 ultrathin layer by a hydrolysis process at low temperature. It is found that NiCo2O4@TiO2 exhibits superior electrochemical performances over NiCo2O4 in terms of rate capability and cyclability. After 60 cycles at 100 mA g−1, NiCo2O4@TiO2 showed 78% capacity retention compared with 57% for bare NiCo2O4. Analysis from the electrochemical measurements indicates that the improved electrochemical performances of NiCo2O4@TiO2 might be attributed to a higher lithium diffusion rate, smaller charge-transfer resistance and more structural stability. Kelvin probe force microscopy measurements reveal that NiCo2O4@TiO2 has a lower work function than those of the pristine one, which help to facilitate electron transfer in composites. In addition, the electric field between NiCo2O4 and TiO2 resulting from the difference in work functions is also expected to enhance the electrochemical performances.
1. Introduction
Owing to their advantages on lifespan and energy density, rechargeable lithium-ion batteries have attracted increasing interest due to their wide application in energy storage systems (ESSs) and electric vehicles (EV/HEV/PHEV).1–5 The increasing demands for high-energy or high-power batteries are driving the research interest in electrode materials with a large specific energy.6–8 Unfortunately, graphite or carbon-based materials with low theoretical specific capacity (ca. 372 mA h g−1) are not highly desirable for the high energy-density batteries.9,10 In contrast, binary metal oxides, such as ZnFe2O4, NiFe2O4, ZnMn2O4 and NiCo2O4, seem to be a more promising alternative because of their high theoretical capacity and high redox activity.11–14 Among the numerous investigated binary metal oxides, spinel nickel cobaltite (NiCo2O4) has been regarded as a promising electrode composite due to the high specific capacity (890 mA h g−1), environmental friendliness and low cost.15,16 However, NiCo2O4 also suffers from sluggish reaction kinetics and drastic volume change during lithium insertion/extraction processes, resulting in the structure deterioration (pulverization or aggregation) and consequent severe decay in capacity.17,18 To address above significant drawbacks, lots of effective strategies have been implemented and surface-modification has been proved to be an effective way to improve electrochemical performances, which not only suppresses the formation of excessive amounts of SEI but also stabilizes structure of the active materials.19 Kou et al.19 reported that Al2O3-coated NiCo2O4 exhibits improved cyclability with a reversible capacity of 395 mA h g−1 after 50 cycles. Titanium oxide (TiO2) has been investigated extensively as an anode material, whose volume expansion is less than 4% during the lithium insertion processes.20,21 The low volume expansion would be desirable for adhesion of the coating to the matrix materials, resulting in the enhanced structural stability and a excellent cycle life. On the other hand, TiO2-coating layer acting as an interfacial barrier can also significantly enhance cyclic performances by suppressing the exothermic reaction between the active material and the electrolyte.In view of all the above, we employ hydrolysis technique to coat TiO2 on flower-like NiCo2O4 consisting of nanosheets at low temperature, and the effect of TiO2-coating on the kinetics of Li+ insertion/extraction is systematically investigated. It is found that the high capacity of NiCo2O4 and the excellent stability of TiO2 as well as the hierarchical structure make the designed composite demonstrate improved rate capability and cycling stability.
2. Experimental
2.1 Preparation and characterization of anode materials
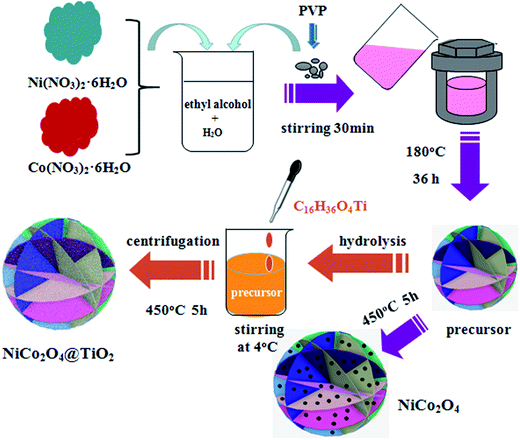
Flower-like NiCo2O4 consisting of nanosheets are prepared by hydrothermal technique. All chemicals are purchased from Aladin and used without further purification. In a typical synthesis, 6 mmol of Ni(NO3)2·6H2O and 12 mmol of Co(NO3)2·6H2O are thoroughly dissolved in 30 ml of deionized water and 30 ml of absolute ethanol, followed by stirring until a light pink solution is formed. Then, 0.1 g of polyvinyl pyrrolidone (PVP) is added to the above aqueous solution under continuous stirring. After vigorous stirring for another 60 min, the resulted mixture is transferred into a 100 ml Teflon-lined autoclave, sealed and maintained at 180 °C for 36 h. After being cooled to room temperature, the precipitates are collected through centrifugation, washed several times with de-ionized water and ethanol, dried at 100 °C overnight under vacuum. The obtained precursors (Ni–Co–O) are calcinated at 450 °C for 5 h in air to get flower-like NiCo2O4 powders. NiCo2O4@TiO2 composites are synthesized by a hydrolysis process at low temperature using tetrabutyl titanate (Ti(OC4H9)4) and Ni–Co–O powders as precursors. 0.23 g of the as-prepared Ni–Co–O precursors are dispersed in 20 ml of absolute ethanol and 1 ml of deionized water under vigorous stirring at 4 °C. Then, 10 ml 10−3 M Ti(OC4H9)4 ethanol solution is added dropwise into above solution. After stirring at 4 °C for another 24 h, the resulting precipitates are isolated by centrifugation, dried at 60 °C for 12 h and subsequently sintered at 450 °C for 5 h to obtain flower-like NiCo2O4 surface-modified with TiO2. The schematic illustration of the synthesis process for the NiCo2O4 and NiCo2O4@TiO2 characterized anode materials is shown in Fig. 1.The crystalline structure of the as-synthesized powders is characterized by X-ray diffraction (XRD, Rigaku MiniFlex II) using CuKα radiation (λ = 0.15405 nm). Thermo-gravimetric analysis (TGA) analysis are carried out using thermogravimetric analysis (TGA, Netzsch STA449F3) from 30 to 600 °C at a heating rate of 5 °C min−1 under an air atmosphere. Scanning electron microscope (SEM) images are obtained on a Hitachi SU8010 field-emission scanning electron microscope equipped with an energy-dispersive spectroscopy (EDS). The TiO2 content in the composite is determined by inductively coupled plasma OES spectrometer (ICP). Raman scattering is carried out on a Horiba/Jobin Yvon Raman instrument using a 532 nm emission line. Nitrogen sorption isotherms are measured at 77 K using a Micromeritics Tristar 3020 analyzer. Specific surface areas of the as-prepared powders are calculated according to the Brunauer–Emmett–Teller (BET) method. The pore size distribution is determinated according to the theory of Barrett, Joyner and Halenda (BJH).
The surface potentials of NiCo2O4 and NiCo2O4@TiO2 are measured by Kelvin probe atomic force microscopy (KPAFM) (Bruker dimension ICON, Germany).
2.2 Cell fabrication and characterization
The electrochemical performances of the as-fabricated samples are evaluated with CR2025-type coin cell and assembled in an argon-filled glove box (O2, H2O < 1 ppm). The working electrodes are prepared by coating anode slurries which are made up of 70 wt% active material (NiCo2O4 or NiCo2O4@TiO2) with 10 wt% polyvinylidene fluoride (PVDF) and 20 wt% super-P in N-methyl-2-pyrrolidone. The anode slurry is cast onto a copper current collector and dried in vacuum at 110 °C for 12 h to remove the residual solvent. A lithium foil is used as the reference and counter electrodes, Cellgard 2300 microporous polyethylene membrane as separator. The electrolyte consists of 1 M LiPF6 in a mixture of ethylene carbonate (EC) and dimethyl carbonate (DMC) (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 in volume). The cells are galvanostatically charged and discharged on a multichannel battery testing system (Land CT2001A, Wuhan, China) in the voltage range of 0.01–2.5 V. The cyclic voltammetry (CV) measurements are carried out using an Arbin instruments BT-2000 battery testing station, and the electrochemical impedance spectra of the electrodes are determined by an electrochemical workstation (Zahner-Zennium) in the frequency range of 100 kHz to 10 mHz with an amplitude of 5 mV.
1 in volume). The cells are galvanostatically charged and discharged on a multichannel battery testing system (Land CT2001A, Wuhan, China) in the voltage range of 0.01–2.5 V. The cyclic voltammetry (CV) measurements are carried out using an Arbin instruments BT-2000 battery testing station, and the electrochemical impedance spectra of the electrodes are determined by an electrochemical workstation (Zahner-Zennium) in the frequency range of 100 kHz to 10 mHz with an amplitude of 5 mV.
3. Results and discussion
3.1 Material characterization
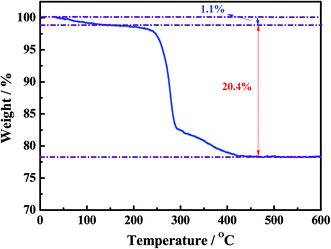
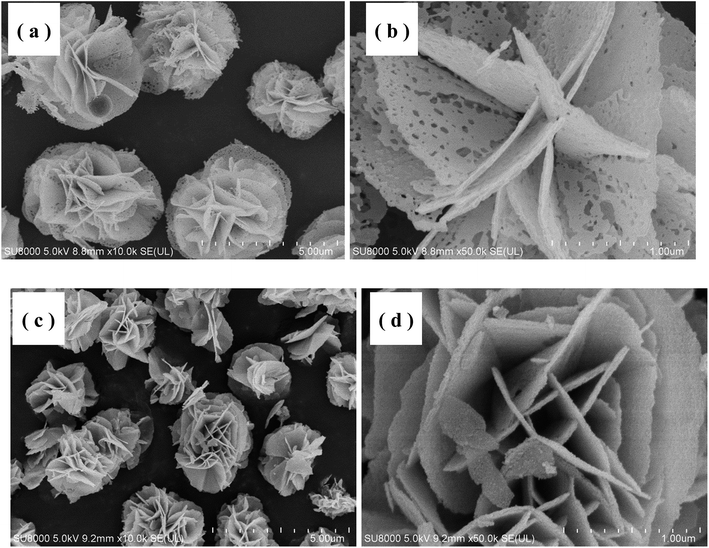
Fig. 2 shows the thermogravimetric (TGA) curve of the as-synthesized Ni–Co–O precursor, measured from 30 to 800 °C at a heating rate of 3 °C min−1 in air atmosphere. The initial 1.1% weight loss at the low temperature (30–250 °C) would result from the loss of the evaporation of moisture and the decomposition of crystal water in the precursor. The following 20.4% weight loss with a big step occurs between 250 and 450 °C, which might be attributed to the conversion of anhydrous precursors to spinel cubic crystals. Therefore, we reasonably chose 450 °C as calcination temperature in our experiment.The morphology and microstructure of the as-prepared NiCo2O4 and NiCo2O4@TiO2 powders are characterized by scanning electron microscopy as shown in Fig. 1. It is clear that NiCo2O4 powders are all rose flower-like morphology (Fig. 3(a)), which is composed of thin transparent nanosheets with a thickness of ∼20 nm. Fig. 3(b) reveals that the nanosheets of NiCo2O4 contains many micro-pores, which is mainly attributed to the organics loss accompanying removal of PVP and gases during the calcination process.22,23 Such hierarchical structure would be highly desirable for rapid Li-ion diffusion and electron transfer. Porosity structure of the NiCo2O4 nanosheet may be benefit for lithium-ion transportation from the electrolyte into the active sites with less resistance, and buffer efficiently large volume expansion during the Li-ion insertion/extraction processes.24 With TiO2-coating, NiCo2O4@TiO2 powders also maintain the nanosheet-built flower-like nanostructure as same to the bare one, shown in Fig. 3(c). In contrast, the nanosheets of NiCo2O4@TiO2 have a smooth and integrated surface morphology and the micro-pores on the “petals” disappears, indicating TiO2 layer is uniformly coated on the nanosheet surface.
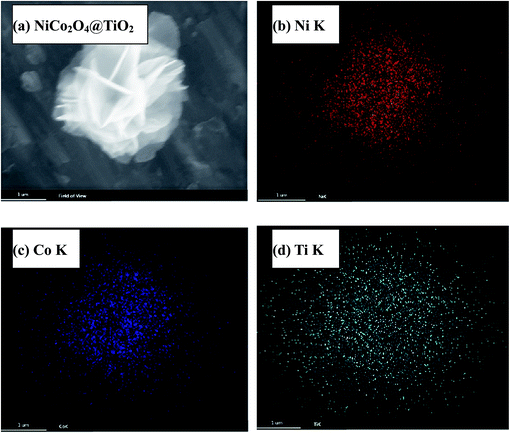
Fig. 4(a) presents nitrogen adsorption–desorption isotherms of NiCo2O4 and NiCo2O4@TiO2 powders, indicating a typical hysteresis mesoporous system.12,25 According to Brunauer–Emmett–Teller (BET) equation, the specific surface areas of NiCo2O4 and NiCo2O4@TiO2 are calculated to be 98.78 and 78.09 m2 g−1, respectively. Fig. 4(b) shows the corresponding pore-size distribution based on Barrett–Joyner–Halenda (BJH) method, indicating that NiCo2O4@TiO2 have larger average pore size (10.3 nm) that (5.6 nm) of NiCo2O4. An increase in average pore size and reduction in surface area could be reasonably explained by the disappearance of micro-pores (5.6 nm) because of TiO2-coating. The obtained results are consistent with the analysis from SEM images. The distribution of corresponding elements of NiCo2O4@TiO2 is investigated by EDS. Element mapping images for Ni, Co and Ti in NiCo2O4@TiO2 powders (Fig. 5) reveal that the corresponding elements uniformly distribute on the surface of the NiCo2O4@TiO2 particles.
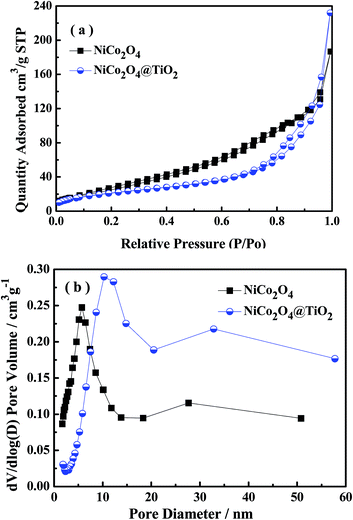 | ||
| Fig. 4 (a) Nitrogen sorption isotherms and (b) pore diameter distribution of NiCo2O4 and NiCo2O4@TiO2 powders. | ||
Fig. 6(a) shows the XRD patterns of NiCo2O4 and NiCo2O4@TiO2 powders. All of the diffraction peaks are characteristic of a spinel NiCo2O4 structure with space group Fd3m (JCPDS card no. 73–1702).22,26 It is found that no visible differences in XRD patterns between two composites, which is attributed to the low content of TiO2 phase. Fig. 6(b) presents Raman spectra of the as-prepared NiCo2O4@TiO2 samples. Five obvious peaks at 212, 313, 366, 536 and 671 cm−1 are found in the Raman spectrum of NiCo2O4@TiO2 composites, which can be assigned to the vibrational modes of spinel NiCo2O4.27,28 The peak at around 149 cm−1 is related to the Eg vibration modes of the TiO2 anatase structure.29 The TiO2 content in NiCo2O4@TiO2 composite is further determined to be ca. 3.53 wt% by inductively coupled plasma OES spectrometer (ICP).
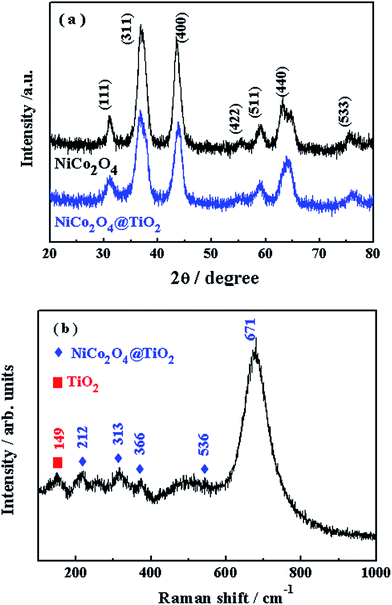 | ||
| Fig. 6 (a) XRD patterns of NiCo2O4 and NiCo2O4@TiO2 powders; (b) Raman spectra of the as-prepared NiCo2O4@TiO2 powders. | ||
The elemental composition and the oxidation state of the NiCo2O4@TiO2 powder is further characterized by X-ray photoelectron spectroscopy (XPS) measurements and the corresponding results are present in Fig. 7(a–e). The survey spectrum (Fig. 7(a)) reveals the presence of Ni, Co, Ti and O as well as C elements without any other impurities. By using a Gaussian fitting method, the Ni 2p core-level spectrum (Fig. 7(b)) has two spin–orbit doublets and two shake-up satellites, which are in good agreement with the characteristic of Ni2+ and Ni3+.30 Similarly, two spin–orbit doublets and shake-up satellites can also be observed in the Co 2p spectrum, corresponding to the characteristic of Co2+ and Co3+.30 The peaks located at 458.7 and 464.4 eV are attributed to the Ti 2p3/2 and Ti 2p1/2 spin–orbit doublets, indicating the predominant state of the Ti element in composite is Ti4+.31 The O 1s spectra can be divided into two main oxygen peaks at 529.6 and 531 eV. The peak located at 529.6 eV is typical characteristic of metal–oxygen bonds.32 The XPS results are in good agreement with the analysis from XRD and ED measurements.
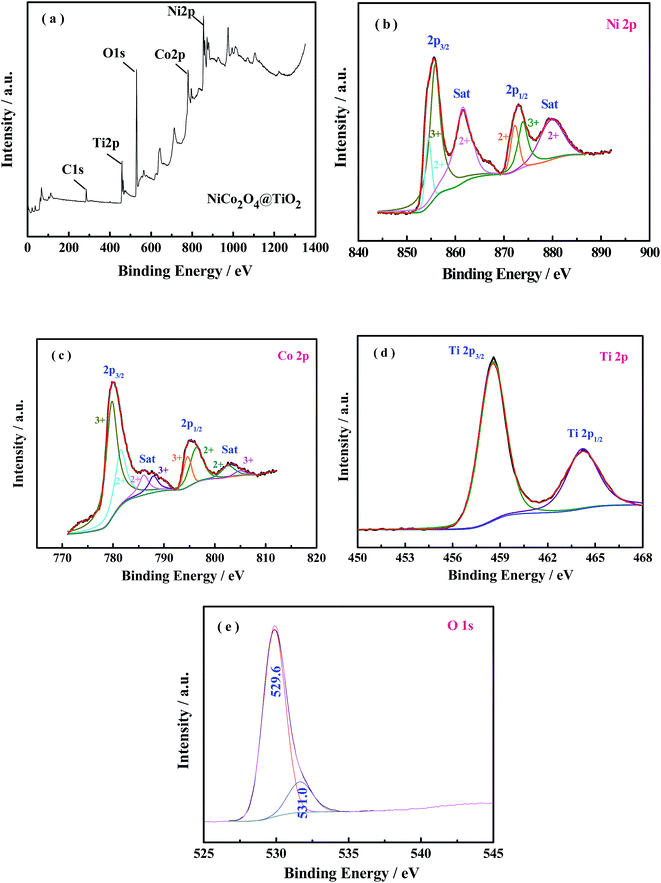 | ||
| Fig. 7 XPS spectra of (a) survey spectrum, (b) Ni 2p, (c) Co 2p, (d) Ti 2p and (e) O 1s for the NiCo2O4@TiO2 product. | ||
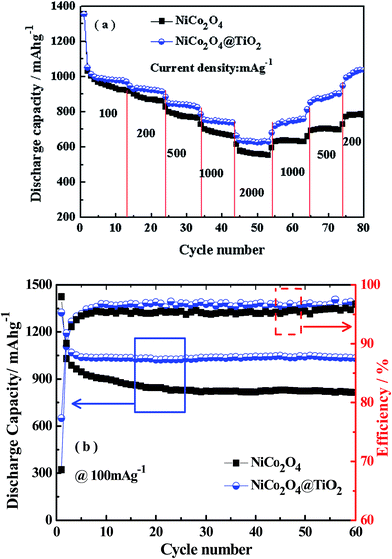
Fig. 8(a) shows the rate capabilities of NiCo2O4 and NiCo2O4@TiO2 electrodes at various current density, measured from 100 to 2000 mA g−1 in rising order and subsequently followed by returning 1000, 500 and 200 mA g−1. In comparison, NiCo2O4@TiO2 composite exhibits better rate performance than that of the bare one, especially at a higher rate. When the current density increases to 100, 200, 500, 1000 and 2000 mA g−1, the NiCo2O4@TiO2 electrode shows reversible discharge capacities of 988, 930, 840, 750 and 624 mA h g−1, respectively. Even at a high current density of 2000 mA g−1, the discharge capacity still retains 63.2%. When the current density returns back to 1000, 500 and 200 mA g−1, the NiCo2O4@TiO2 electrode still recovers 735, 837 and 1004 mA h g−1, indicating excellent structure stability of the nano-composite. In contrast, the NiCo2O4 electrode delivers a lower discharge capacity at current density. The discharge capacities of the NiCo2O4 electrode are measured to be 983, 878, 778, 683 and 562 mA g−1 at the same respective current density. It has been reported that small anatase TiO2 particles would be turning from an insulator into an electronic conductor during the Li+ insertion possess.33,34 Therefore, TiO2-coating on NiCo2O4 nanosheets is beneficial for both structural stability as well as the rate capability.
Fig. 8(b) presents the cycling performance of NiCo2O4 and NiCo2O4@TiO2 electrodes at a current density of 100 mA g−1. In comparison with NiCo2O4, the capacity loss is significantly suppressed after coating with TiO2. The initial discharge capacity at 100 mA g−1 of NiCo2O4 is 1424 mA h g−1 and found to decrease to 815 mA h g−1 after 60 cycles (i.e., only 57% of its initial discharge capacity). The discharge capacity of the NiCo2O4@TiO2 is found to decay gradually with continuous cycling, retaining 78% of its maximum discharge capacity after 60 cycles. In addition, the coulombic efficiency of NiCo2O4 is relatively low and unstable, which might result from the SEI formation repeatedly on NiCo2O4 nanosheet during the charge/discharge processes.19 Similar results are reported in Lotfabad's work.33 Here, we have made a comparison of the electrochemical performances between our NiCo2O4@TiO2 and other NiCo2O4 with different morphologies previously reported, as summarized in Table 1. It is found that NiCo2O4@TiO2 nanocomposites exhibit superior cycling stability, indicating its potential application in high-energy lithium-ion batteries.
| Materials | Current density (mA g−1) | Capacity (mA h g−1) | Ref. |
|---|---|---|---|
| Plum-like NiCo2O4 | 0.1 | 801 after 50 cycles | 22 |
| NiCo2O4/3DGN | 0.5 | 790 after 50 cycles | 35 |
| NiCo2O4/Ni | 0.1 | 413 after 50 cycles | 36 |
| NiCo2O4@SnO2@C-HSs | 0.1 | 720 after 100 cycles | 37 |
| NiCo2O4@G | 0.3 | 806 after 55 cycles | 38 |
| NiCo2O4 nanosheets | 0.1 | 767 after 50 cycles | 17 |
| NiCo2O4@RGO | 0.1 | 816 after 70 cycles | 39 |
| NiCo2O4@NiCo2O4 NCAs | 0.12 | 830 after 100 cycles | 40 |
| NiCo2O4@TiO2 | 0.1 | 1033 after 60 cycles | This work |
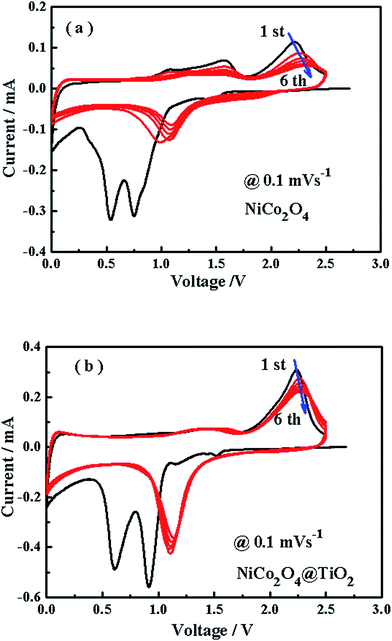
Fig. 9(a and b) shows cyclic voltammetry profiles of NiCo2O4 and NiCo2O4@TiO2 electrode for the first six cycles at a scan rate of 0.1 mV s−1 and from 0.01 to 2.5 V. Two peaks are observed at around 0.6 and 0.9 V in the initial cathodic sweep for both samples, which are assigned to the formation of the solid electrolyte interface layer and the reaction of Co3+ and Ni2+ to Co0 and Ni0, respectively.41 Two oxidation peaks at around 1.4 and 2.2 V are also observed in the initial anodic sweep, which are attributed to the oxidation of Co0 and Ni0 to Co3+ and Ni2+, respectively.42 According to the previous reports,43 the redox reactions can be expressed as follows:
| NiCo2O4 + 8Li+ + 8e− → Ni + 2Co + 4Li2O | (1) |
| Ni + Li2O ↔ NiO + 2Li+ + 2e− | (2) |
| Co + Li2O ↔ CoO + 2Li+ + 2e− | (3) |
| CoO + 1/3Li2O ↔ 1/3Co3O4 + 2/3Li+ + 2/3e− | (4) |
In comparison with NiCo2O4, the CV curves from 2nd to 6th cycles for NiCo2O4@TiO2 exhibit a better overlapping degree, indicating a better reversibility of the electrochemical reactions.
To further investigate the potential mechanism behind the improved performances with surface-modified of TiO2 layer, the cells after cycling are disassembled, washed, dried in vacuum and characterized by SEM. Fig. 10 presents the morphologies of NiCo2O4 and NiCo2O4@TiO2 powders characterized by SEM after 10 and 30 cycles, respectively. It is obvious that NiCo2O4 powders has serious structure-deterioration (pulverization or aggregation) and losses its flower-like structure with increasing cycles due to the repeated volume change between metals and metal oxides. The aggregation of the active materials tends to reduce the effective contact areas between active materials and the electrolyte. In contrast, NiCo2O4@TiO2 powders can remain in the flower-like structure well, which further confirms that TiO2-layer would stabilize structure of the active materials and consequently offer more active sites during the lithium-ion insertion/extraction process. Combined with the analysis of the SEM images after cycling, it is expected that stable hierarchical nanostructures are desirable for the improved electrochemical performances.
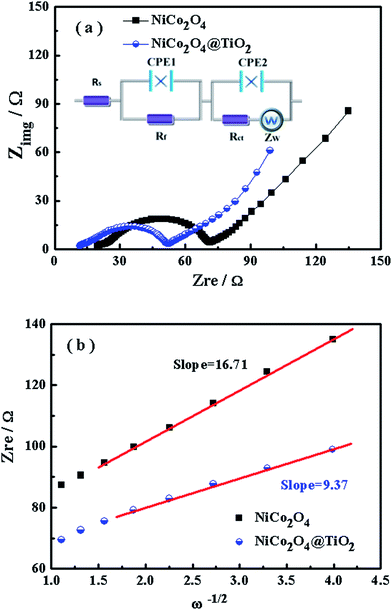
Electrochemical impedance spectra are carried out to get insight into the improved rate and cyclic performances of NiCo2O4@TiO2. Fig. 11(a) presents the typical EIS of NiCo2O4 and NiCo2O4@TiO2 electrode in the fully discharged state. Both EIS profiles consist of two depressed semicircles in the medium-to-high frequency range and a straight line in the low-frequency region. According to the equivalent circuit in the inset of Fig. 11(a), the charge-transfer resistance (Rct) are calculated as 36.6 Ω for NiCo2O4 and 20.4 Ω for NiCo2O4@TiO2, respectively. The decrease in Rct for NiCo2O4@TiO2 would derive from the more stable structure by TiO2-coating with continuous cycling.44 The TiO2-coating layer is expected to efficiently prevent the pulverization of NiCo2O4 during the Li+ intercalation/extraction process and mediate the increase in charge transfer resistance of the composites, which facilitates Li-ions transfer at the interface between the active material and electrolyte. As a result, the electrochemical performances are improved.
The diffusion coefficients (DLi) of the Li-ion kinetic of the cells can be also calculated according to the EIS profiles in the low frequency.
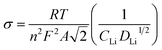 | (5) |
| Zre = R + σω−1/2 | (6) |
To evaluate the effect of TiO2-coating on the Li-ion diffusion during the charge/discharge process, the impedance spectra under different discharge states for NiCo2O4 and NiCo2O4@TiO2 electrodes are continuously measured, shown in Fig. 12(a and b). According to eqn (5) and (6), the corresponding Li+-ion diffusion coefficients of NiCo2O4 and NiCo2O4@TiO2 electrodes are calculated. Fig. 12(c) presents lithium-ion diffusion behaviors during the lithium-ion insertion process. Both electrodes demonstrate similar lithium-ion diffusion behavior. On the whole, NiCo2O4@TiO2 electrode exhibits larger Li+ diffusion coefficients than those of the bare one, indicating that TiO2-coating does readily facilitate the Li+ diffusion in composites. It is worth noting that NiCo2O4@TiO2 electrode has much larger diffusion coefficients of lithium-ions in the voltage range from 1.0 to 0.01 V, which might be attributed to the lithium ion insertion in TiO2. It is expected that lithium-inserted LixTiO2 anatase would turn from an insulator into an electronic conductor during the Li+ insertion possess, resulting in enhanced electron-transfer in composites.33 As a result, TiO2 coating on NiCo2O4 is potentially beneficial for the improved rate capability as well as the structural integrity of the composite.
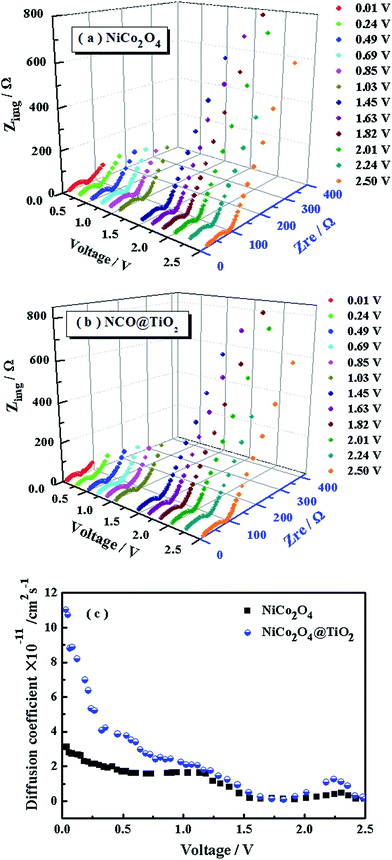 | ||
| Fig. 12 (a, b) The impedance spectra and (c) corresponding Li-ion of diffusion coefficients of NiCo2O4 and NiCo2O4@TiO2 electrodes under different discharge states. | ||
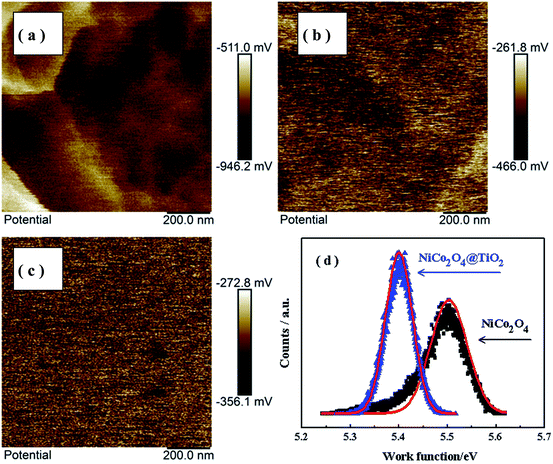
Kelvin probe atomic force microscopy is used to study the influence of TiO2-coating on the Li-ion kinetic behavior in composites. Fig. 13(a and b) shows the surface potential maps over a scan area of 200 nm × 200 nm of NiCo2O4 and NiCo2O4@TiO2 powders before cycling. Fig. 13(c) presents the surface potential image of Au foil acting as reference sample. According to our prior work,45 the work functions of NiCo2O4 and NiCo2O4@TiO2 powders are calculated based on the surface potential profiles and the corresponding results are shown in Fig. 13(d). Here, the work functions of the SFM-tip (ϕtip) is calibrated by Au foil, whose work function (ϕAu) is 5.31 eV. It is found that NiCo2O4@TiO2 has a smaller work function (∼5.41 eV) than that (∼5.51 eV) of the NiCo2O4. The measured work function of NiCo2O4 is close to the reported value (5.53 eV).46,47 The larger work function suggests the more energy required for electrons to escape from the composites. As a result, the electrochemical performances of the composites are enhanced with surface-modified with TiO2-coating. These obtained results are consist with the analysis of EIS measurements.
The reduced work function of NiCo2O4@TiO2 could be explained phenomenologically based on the energy-band model. As shown in Fig. 14(a), electrons transfer occurs from TiO2 to NiCo2O4 until the Fermi levels are aligned due to the smaller work function (∼4.5 eV) of anatase TiO2.48 As a result, the TiO2 is positively charged and the NiCo2O4 is negatively charged near its surface due to electrostatic induction because of electrostatic induction. Meanwhile, a corresponding electric field (E) is built up between them, shown in Fig. 14(b). Such electric field could facilitate Li-ion diffusion from positively-charged TiO2 to negatively-charged NiCo2O4, and electron transfer from NiCo2O4 to TiO2 across heterojunction interfaces. With the help of the electric field, more electrons in NiCo2O4 matrix would transfer through TiO2 rather than NiCo2O4/NiCo2O4 interface during the lithium insertion process. Moreover, TiO2 coated on NiCo2O4 could effectively suppress the pulverization of NiCo2O4 matrix due to the volume change in the charge/discharge process. As a result, the electrochemical performances are enhanced.
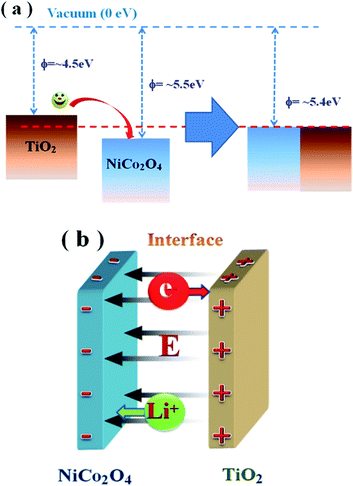 | ||
| Fig. 14 (a) The energy-level model for explaining the improved electron transfer in NiCo2O4@TiO2 electrodes; (b) a built electric field (E) between NiCo2O4 and TiO2. | ||
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work is supported by a grant from Key Project of Department of Science & Technology of Fujian Province (No. 2014H0020), Program for New Century Excellent Talents in University of Fujian Province (No. JA14069) and Project A of Education Bureau of Fujian Province (No. JA12067).References
- M. Armand and J.-M. Tarascon, Building better batteries, Nature, 2008, 451, 652–657 CrossRef CAS PubMed.
- J. B. Goodenough, Electrochemical energy storage in a sustainable modern society, Energy Environ. Sci., 2014, 7, 14–18 CAS.
- R. A. Huggins, Review—a new class of high rate, long cycle life, aqueous electrolyte battery electrodes, J. Electrochem. Soc., 2017, 164, A5031–A5036 CrossRef CAS.
- J. Lu, Z. H. Chen, Z. F. Ma, F. Pan, A. L. Curtiss and K. Amine, The role of nanotechnology in the development of battery materials for electric vehicles, Nat. Nanotechnol., 2016, 11, 1031–1038 CrossRef CAS PubMed.
- G. E. Blomgren, The development and future of lithium ion batteries, J. Electrochem. Soc., 2017, 164, A5019–A5025 CrossRef CAS.
- Z. Y. Wang, L. Zhou and X. W. Lou, Metal oxide hollow nanostructures for lithium-ion batteries, Adv. Mater., 2012, 24, 1903–1911 CrossRef CAS PubMed.
- M. V. Reddy, G. V. S. Rao and B. V. R. Chowdari, Metal oxides and oxysalts as anode materials for Li ion batteries, Chem. Rev., 2013, 113, 5364–5457 CrossRef CAS PubMed.
- M. Freire, N. V. Kosova, C. Jordy, D. Chateigner, O. I. Lebedev, A. Maignan and V. Pralong, A new active Li-Mn-O compound for high energy density Li-ion batteries, Nat. Mater., 2016, 15, 173–177 CrossRef CAS PubMed.
- H. Buqa, D. Goers, M. Holzapfel, M. E. Spahr and P. Novák, High rate capability of graphite negative electrodes for lithium-ion batteries, J. Electrochem. Soc., 2005, 152, A474–A481 CrossRef CAS.
- J. F. Li, S. L. Xiong, Y. R. Liu, Z. C. Ju and Y. T. Qian, High electrochemical performance of monodisperse NiCo2O4 mesoporous microspheres as an anode material for Li-ion batteries, ACS Appl. Mater. Interfaces, 2013, 5, 981–988 CAS.
- B. B. Jiang, C. P. Han, B. Li, Y. J. He and Z. Q. Lin, In-situ crafting of ZnFe2O4 nanoparticles impregnated within continuous carbon network as advanced anode materials, ACS Nano, 2016, 10, 2728–2735 CrossRef CAS PubMed.
- R. C. Jin, H. Jiang, Y. X. Sun, Y. Q. Ma, H. H. Li and G. Chen, Fabrication of NiFe2O4/C hollow spheres constructed by mesoporous nanospheres for high-performance lithium-ion batteries, Chem. Eng. J., 2016, 303, 501–510 CrossRef CAS.
- X. Q. Chen, Y. M. Zhang, H. B. Lin, P. Xia, X. Cai, X. G. Li, X. P. Li and W. S. Li, Porous ZnMn2O4 nanospheres: facile synthesis through microemulsion method and excellent performance as anode of lithium ion battery, J. Power Sources, 2016, 312, 137–145 CrossRef CAS.
- G. Zhou, C. Wu, Y. H. Wei, C. C. Li, Q. W. Lian, C. Cui, W. F. Wei and L. B. Chen, Tufted NiCo2O4 nanoneedles grown on carbon nanofibers with advanced electrochemical property for lithium ion batteries, Electrochim. Acta, 2016, 222, 1878–1886 CrossRef CAS.
- C. Zhang and J. S. Yu, Morphology-tuned synthesis of NiCo2O4-Coated 3D graphene architectures used as binder-free electrodes for lithium-ion batteries, Chem.–Eur. J., 2016, 22, 4422–4430 CrossRef CAS PubMed.
- Y. Lei, J. Li, Y. Wang, L. Gu, Y. Chang, H. Yuan and D. Xiao, Rapid microwave-assisted green synthesis of 3D hierarchical flower-shaped NiCo2O4 microsphere for high-performance supercapacitor, ACS Appl. Mater. Interfaces, 2014, 6, 1773–1780 CAS.
- A. K. Mondal, D. W. Su, S. Q. Chen, K. Kretschmer, X. Q. Xie, H. J. Ahn and G. X. Wang, A microwave synthesis of mesoporous NiCo2O4 nanosheets as electrode materials for lithium-ion batteries and supercapacitors, ChemPhysChem, 2015, 16, 169–175 CrossRef CAS PubMed.
- L. L. Li, S. J. Peng, Y. L. Cheah, P. F. Teh, J. Wang, G. Wee, Y. Ko, C. L. Wong and M. Srinivasan, Electrospun porous NiCo2O4 nanotubes as advanced electrodes for electrochemical capacitors, Chem.–Eur. J., 2013, 19, 5892–5898 CrossRef CAS PubMed.
- H. R. Kou, X. F. Li, H. Shan, L. L. Fan, B. Yan and D. J. Li, An optimized Al2O3 layer for enhancing the anode performance of NiCo2O4 nanosheets for sodium-ion batteries, J. Mater. Chem. A, 2017, 5, 17881–17888 CAS.
- Z. H. Chen, I. Belharouak, Y. K. Sun and K. Amine, Titanium-based anode materials for safe lithium-ion batteries, Adv. Funct. Mater., 2013, 23, 959–969 CrossRef CAS.
- J. H. Lee, M. H. Hon, Y. W. Chung and I. C. Leu, The effect of TiO2 coating on the electrochemical performance of ZnO nanorod as the anode material for lithium-ion battery, Appl. Phys. A, 2011, 102, 545–550 CrossRef CAS.
- T. Li, X. Li, Z. Wang, H. Guo and Y. Li, A novel NiCo2O4 anode morphology for lithium-ion batteries, J. Mater. Chem. A, 2015, 3, 11970–11975 CAS.
- J. Xu, D. W. Su, W. Z. Bao, Y. F. Zhao, X. Q. Xie and G. X. Wang, Rose flower-like NiCo2O4 with hierarchically porous structures for highly reversible lithium storage, J. Alloys Compd., 2016, 684, 691–698 CrossRef CAS.
- F. C. Zheng, D. Q. Zhu and Q. W. Chen, Facile fabrication of porous NixCo3−xO4nanosheets with enhanced electrochemical performance as anode materials for li-ion batteries, ACS Appl. Mater. Interfaces, 2014, 6, 9256–9264 CAS.
- Y. D. Mo, Q. Ru, X. Songa, L. Y. Guo, J. F. Chen, X. H. Hou and S. J. Hu, The sucrose-assisted NiCo2O4@C composites with enhanced lithium-storage properties, Carbon, 2016, 109, 616–623 CrossRef CAS.
- A. K. Mondal, D. W. Su, S. Q. Chen, X. Q. Xie and G. X. Wang, Highly porous NiCo2O4 nanoflakes and nanobelts as anode materials for lithium-ion batteries with excellent rate capability, ACS Appl. Mater. Interfaces, 2014, 6, 14827–14835 CAS.
- H. Zheng, S. Xu, L. Li, C. Feng and S. Q. Wang, Synthesis of NiCo2O4 microellipsoids as anode material for lithium-ion batteries, J. Electron. Mater., 2016, 45, 4966–4972 CrossRef CAS.
- L. B. Ma, X. P. Shen, Z. Y. Ji, X. Q. Cai, G. X. Zhu and K. M. Chen, Porous NiCo2O4 nanosheets/reduced graphene oxide composite: facile synthesis and excellent capacitive performance for supercapacitors, J. Colloid Interface Sci., 2015, 440, 211–218 CrossRef CAS PubMed.
- Y. Zhang, W. Wu, K. Zhang, C. H. Liu, A. F. Yu, M. Z. Peng and J. Y. Zhai, Raman study of 2D anatase TiO2 nanosheets, Phys. Chem. Chem. Phys., 2016, 18, 32178–32184 RSC.
- R. X. Ge, M. Ma, X. Ren, F. L. Qu, Z. A. Liu, G. Du, A. M. Asiri, L. Chen, B. Zheng and X. Sun, A NiCo2O4@Ni–Co–Ci core–shell nanowire array as an efficient electrocatalyst for water oxidation at near-neutral pH, Chem. Commun., 2017, 53, 7812–7815 RSC.
- M. C. Biesinger, L. W. Lau, A. R. Gerson and R. S. C. Smart, Resolving surface chemical states in XPS analysis of first row transition metals oxides and hydroxides: Sc, Ti, V, Cu and Zn, Appl. Surf. Sci., 2010, 257, 887–898 CrossRef CAS.
- Y. D. Mo, Q. Ru, X. Song, S. J. Hu, L. Y. Guo and X. Q. Chen, 3-dimensional porous NiCo2O4 nanocomposite as a high-rate capacity anode for lithium-ion batteries, Electrochim. Acta, 2015, 176, 575–585 CrossRef CAS.
- E. M. Lotfabad, P. Kalisvaart, K. Cui, A. Kohandehghan, M. Kupsta, B. Olsena and D. Mitlin, ALD TiO2 coated silicon nanowires for lithium ion battery anodes with enhanced cycling stability and coulombic efficiency, Phys. Chem. Chem. Phys., 2013, 15, 13646–13657 RSC.
- W. J. H. Borghols, D. Lutzenkirchen-Hecht, U. Haake, E. R. H. van Eck, F. M. Mulder and M. Wagemaker, The electronic structure and ionic diffusion of nanoscale LiTiO2 anatase, Phys. Chem. Chem. Phys., 2009, 11, 5742–5748 RSC.
- S. N. Liu, J. Wu, J. Zhou, G. Z. Fang and S. Q. Liang, Mesoporous NiCo2O4 nanoneedles grown on three dimensional graphene networks as binder-free electrode for high-performance lithium-ion batteries and supercapacitors, Electrochim. Acta, 2015, 176, 1–9 CrossRef CAS.
- G. H. Chen, J. Yang, J. J Tang and X. Y. Zhou, Hierarchical NiCo2O4 nanowire arrays on Ni foam as an anode for lithium-ion batteries, RSC Adv., 2015, 5, 23067–23072 RSC.
- G. X. Gao, H. B. Wu, S. J. Ding and X. W. Lou, Preparation of carbon-coated NiCo2O4@SnO2 hetero-nanostructures and their reversible lithium storage properties, Small, 2015, 11, 432–436 CrossRef CAS PubMed.
- Y. J. Chen, J. Zhu, B. H. Qu, B. A. Lun and Z. Xu, Graphene improving lithium-ion battery performance by construction of NiCo2O4/graphene hybrid nanosheet arrays, Nano Energy, 2014, 3, 88–94 CrossRef CAS.
- Y. J. Chen, M. Zhuo, J. W. Deng, Z. Xu, Q. H. Li and T. H. Wang, Graphene improving lithium-ion battery performance by construction of NiCo2O4/graphene hybrid nanosheet arrays, J. Mater. Chem. A, 2014, 2, 4449–4456 CAS.
- J. B. Cheng, Y. Lu, K. W. Qiu, H. L. Yan, J. Y. Xu, L. Han, X. M. Liu, J. S. Luo, J. K. Kim and Y. S. Luo, Hierarchical core/shell NiCo2O4@NiCo2O4 nanocactus arrays with dual-functionalities for high performance supercapacitors and Li-ion batteries, Sci. Rep., 2015, 5, 12099 CrossRef PubMed.
- Y. Chen, J. Zhu, B. Qu, B. Lu and Z. Xu, Graphene improving lithium-ion battery performance by construction of NiCo2O4/graphene hybrid nanosheet arrays, Nano Energy, 2014, 3, 88–94 CrossRef CAS.
- C. F. Zhang and J. S. Yu, Morphology-tuned synthesis of NiCo2O4-coated 3D graphene architectures used as binder-free electrodes for lithium-ion batteries, Chem.–Eur. J., 2016, 22, 4422–4430 CrossRef CAS PubMed.
- L. L. Li, Y. Cheah, Y. W. Ko, P. Teh, G. Wee, C. L. Wong, S. J. Peng and M. Srinivasan, The facile synthesis of hierarchical porous flower-like NiCo2O4 with superior lithium storage properties, J. Mater. Chem. A, 2013, 1, 10935–10941 CAS.
- W. Shi, H. T. Zhao and B. G. Lu, Core–shell ZnCo2O4@TiO2 nanowall arrays as anodes for lithium ion batteries, Nanotechnology, 2017, 28, 165403 CrossRef PubMed.
- L. C. Chen, Y. M. Yang, Z. S. Wang, J. Y. Zhang, Q. L. Su, Y. Chen, Y. B. Lin and Z. G. Huang, Enhanced electrochemical performances and thermal stability of LiNi1/3Co1/3Mn1/3O2 by surface modification with YF3, J. Alloys Compd., 2017, 711, 462–472 CrossRef CAS.
- K. K. Naik, R. T Khare, R. V Gelamo, M. A More, R. Thapa, D. J. Late and C. S. Rout, Enhanced electron field emission from NiCo2O4nanosheet arrays, Mater. Res. Express, 2015, 2, 095011 CrossRef.
- C. Zhang, X. P. Geng, S. L. Tang, M. S. Deng and Y. W. Du, NiCo2O4@rGO hybrid nanostructures on Ni foam as high-performance supercapacitor electrodes, J. Mater. Chem. A, 2017, 5, 5912–5919 CAS.
- L. X. Zheng, S. C. Han, H. Liu, P. P. Yu and X. S. Fang, Hierarchical MoS2 nanosheet@TiO2 nanotube array composites with enhanced photocatalytic and photocurrent performances, Small, 2016, 12, 1527–1536 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2017 |