Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Selectivity of a QCM gas sensor modified by ZnSn(OH)6 via analysis of adsorption thermodynamics and kinetics
J. Xie ab,
H. Wang
ab,
H. Wang *ab,
X. X. Liubd,
M. Duanac,
J. L. Tangac and
Y. Y. Wangac
*ab,
X. X. Liubd,
M. Duanac,
J. L. Tangac and
Y. Y. Wangac
aState Key Laboratory of Oil and Gas Reservoir Geology and Exploitation, Southwest Petroleum University (SWPU), Chengdu 610500, China. E-mail: senty78@126.com
bThe Center of New Energy Materials and Technology, School of Materials Science and Engineering, Southwest Petroleum University (SWPU), Chengdu 610500, China
cCollege of Chemistry and Chemical Engineering, Southwest Petroleum University (SWPU), Chengdu 610500, China
dXiaogan Huagong Gaoli Electron Co., Ltd., Xiaogan 432000, China
First published on 5th October 2017
Abstract
In this paper, a novel analysis approach was employed to achieve the selectivity of a quartz crystal microbalance (QCM) sensor. A QCM sensor chip modified by ZnSn(OH)6 (ZSH) was used to identify gases (CO, CH4 and O2) with different volume fractions. The combination of adsorption thermodynamics and adsorption kinetics was firstly used to identify gas species. The relationship between the adsorption thermodynamic parameter (adsorbed mass amount in molar) and the adsorption dynamic parameter (adsorption rate constant, k) was used to characterize the selectivity and concentration of gases. It showed that the method is useful but it still has some limitations. Another approach was also proposed. The kinetic parameter t90 and thermodynamic parameter Δm were employed as characteristic parameters to determine the species and concentration of a gas. The results showed that three kinds of gas with different volume fractions were located in different regions of the t90-Δm two-dimensional graph. We can identify single gases with different volume fractions by enriching the t90-Δm graph with additional data. It is believed that this new analysis method would be promising in gas species identification with QCM sensors.
Introduction
Gas sensors have attracted considerable research interest due to their latent applications in many fields, including environmental monitoring, medical diagnosis, public security, agriculture and food production. Although many techniques and methods have been used in gas sensors, it is still urgent to develop new gas sensors with the functions of reliability, small size, low-cost, and system portability.1–3In recent years, quartz crystal microbalances (QCMs) have been widely used as gas sensors due to their ultra-high sensitivity in mass variation in the nano-gram level. N. V. Quy4 reported enhanced NH3 gas sensing properties of a QCM sensor by increasing the length of vertically orientated ZnO nanorods. I. E. Tothill5 developed an immune-sensor for the detection of the food pathogen campylobacter jejuni by a QCM. S. Öztürka6 showed that Pd-doped ZnO nanorods have been synthesized as QCM sensors of volatile organic compounds (VOCs) at room temperature. J. He7 studied graphene oxide as QCM sensing layers for the detection of formaldehyde and so on.
Although QCMs are powerful in recording frequency and mass variations, it is still hard to distinguish in detail the species of adsorbed gas, which always hampers the development and application of this technique. Generally, QCM sensors with a modified surface exhibit high sensitivity to one or several kinds of gas. However, selectivity is the disadvantage of QCM sensors. Most of the previous research studies discussed typical gas sensing based on the adsorption quantity (commonly frequency variation, ΔF, or adsorbed mass, Δm). The maximum variations of frequency or adsorbed mass were commonly used as a characteristic parameter to explain the adsorption behaviour. In addition, recording the adsorption of gas on a QCM sensor can lead to a dynamic adsorption process and be interpreted as a thermodynamic parameter, which is far from our expectation for the identification of gas species and concentration determination. In fact, the adsorption behaviour of various gases based on QCMs typically involves both adsorption thermodynamics and adsorption kinetics. Different kinds of gas molecule should exhibit different adsorption kinetics, even in the same molar concentration. The combination of adsorption thermodynamics and kinetics would thus be an important way of identifying unknown gases.
To the best of our knowledge, there are no published papers about the combination of adsorption thermodynamics and kinetics of QCM sensors to identify species and concentrations of gases. Herein, a QCM chip modified by zinc hydroxystannate (ZnSn(OH)6, ZSH)8–10 was used to measure three kinds of gas (CO, CH4 and O2). Based on our preliminary research, the QCM sensor coated with ZSH was found to exhibit good sensitivity properties to single gases, such as CO and CH4.11,12 However, when the QCM chip was exposed to a mixture of gases, it was hard to identify the type and concentration of a gas. Therefore, in this paper, the reason why ZSH was chosen to modify QCM chips as gas sensors was that a new analysis approach would be utilized to distinguish the species of gas compared with conventional methods based on the same sensing materials. Adsorption thermodynamics and kinetics were analysed simultaneously using new methods in order to achieve QCM sensor selectivity.
Results and discussion
The crystal structures and morphologies of the as-obtained samples were investigated by XRD and SEM, respectively. Fig. 1(a) shows that all peaks perfectly coincided with those of standard ZSH with a perovskite structure (JCPDS no. 20-1455), affirming that the as-synthesized sample has a typical face-centered cubic crystal structure. The sharp and strong intensity of the diffraction peaks of ZSH imply that the phase of ZSH is well crystallized. The morphology of the sample in Fig. 1(b) characterized by SEM shows that a large number of cubic crystals with side lengths of approximately 202 nm were uniformly distributed on the surface of the substrate. Meanwhile, some nanopores were found on the surface of the cubic ZSH, which are beneficial for gas adsorption.11,12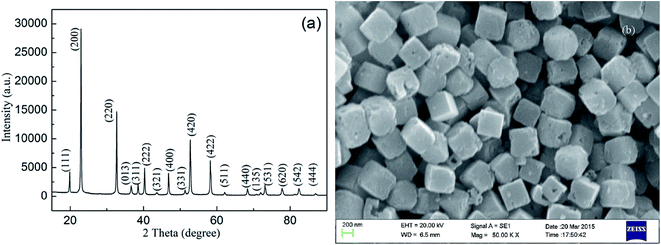 | ||
| Fig. 1 (a) XRD pattern of the sample, matching that of standard ZSH (JCPDS 20-1455). (b) Typical SEM image of the synthesized ZSH. | ||
Fig. 2 shows the response of the ZSH film modified QCM sensor in different concentrations of CO, CH4 and O2. The data are presented as the mass variation versus time. The QCM sensor is very sensitive to the three tested gases. It is obvious that the adsorbed mass rises with an increase of all the gas volume fractions. The processes of adsorption and desorption exhibit good reversibility, which indicates that ZSH is a promising material for the detection of gases. Meanwhile, this also demonstrates that the adsorbed mass presents significant differences for typical gases (CO, CH4 and O2) at the same concentration. Specifically, CO displays a superior adsorption ability to other gases while the smallest adsorbed mass was observed when exposed to O2.
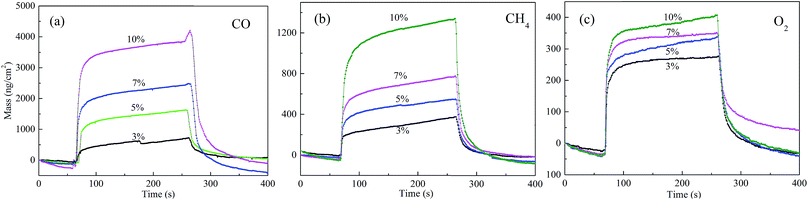 | ||
| Fig. 2 Response and recovery curves of the QCM sensor to different gases with different volume fractions. | ||
In most of the previous research studies, data similar to that shown above had been presented to characterize the adsorption of the gas sensor, from which only limited information can be acquired. In particular it is very hard to distinguish the gas species, or to characterize the selectivity. It is well known that the adsorption rate and the equilibrium adsorption amount are determined by both typical gas molecules and sensor materials. Therefore, we can identify the gas species by establishing adsorption kinetics and thermodynamic characteristic parameters.
More detailed information can be obtained from further data analysis of adsorption processes. The adsorption process of the three gases on the sensor follows a second-order model.13–15 As for the adsorption process, if it obeys a second-order adsorption model, it commonly conforms to the following equation:
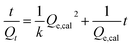 | (1) |
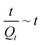 relationship of 3% O2, in which the plot shows good linearity. It demonstrates that the adsorption process of 3% O2 on the sensor surface is a second-order adsorption. Hence, the intercept and slope in the plot shown in Fig. 3(a) can be obtained from the following equations:
relationship of 3% O2, in which the plot shows good linearity. It demonstrates that the adsorption process of 3% O2 on the sensor surface is a second-order adsorption. Hence, the intercept and slope in the plot shown in Fig. 3(a) can be obtained from the following equations:
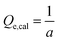 | (2) |
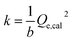 | (3) |
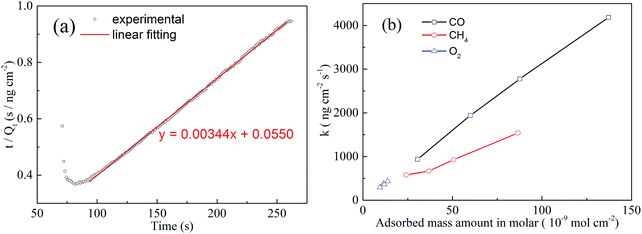 | ||
| Fig. 3 (a) Fitting of the adsorption process by a second-order equation of 3% O2. (b) Plane coordinates to interpret the gas type and concentration. | ||
Herein, the relationship between the adsorption thermodynamic parameter (adsorbed mass amount in molar) and the adsorption dynamic parameter (adsorption rate constant, k) has been used to try to characterize the selectivity. Fig. 3(b) presents the behaviour of k and the adsorbed mass amount in molar of the three tested gases with different concentrations. All the dots in the figure corresponding to a specific gas are linear. The plot also reveals that a gas with specific characteristics and concentration can be located at a certain position of the plane coordinates, such that different gases are located at different zones of the figure. CO varies from the bottom left corner to the top right corner of the figure, CH4 just stands right behind CO, and O2 is located at the bottom left corner of the figure. As for an unknown gas which should, of course, be one of the three gases, it is possible to characterize its concentration and type by inputting the analysed data into the plane coordinates. However, the method still has its limitations. As we can see from Fig. 3(b), CO and O2 are almost along the same line. If we widened the range of the concentration, the data might then overlap. Moreover, the data of CH4 and CO were very close at low concentrations.
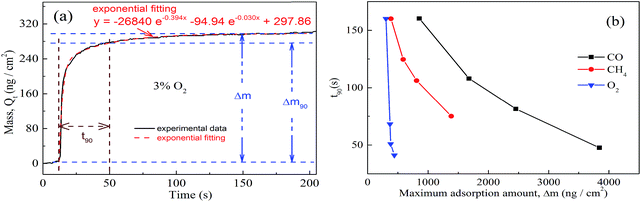
Hence, another new analysis method has also been proposed, which is based on characteristic parameters of adsorption thermodynamics and kinetics as well. t90-Δm of various gases, in which t90 refers to the time taken to reach 90% of the maximum adsorption amount and Δm is the maximum adsorption amount, has been utilized innovatively for the identification of unknown gases as shown in Fig. 4.
The kinetics of the gas adsorption process are based on the fitting of mass–time curves. Fig. 4(a) shows the adsorbed mass (Qt)–time curves and the exponential fitting results. The exponential fitting used in the figure corresponds to the following equation:
| Qt = Qe,exp + A1e−k1t + A2e−k2t | (4) |
t90-Δm is a competitive method to combine kinetic and thermodynamic processes, as is shown in Fig. 4(b). It can be seen that CO/CH4/O2 with different volume fractions are located in different regions in the t90-Δm two-dimensional graph. Hence, we can identify single gases with different volume fractions by enriching the t90-Δm map via measurements of more kinds of gas to get a t90-Δm database. This new analysis method can achieve the selectivity of QCM sensors.
Experimental
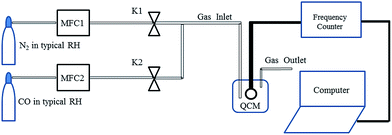
In a typical synthesis procedure, 1 mmol of analytical grade zinc acetate (Zn(CH3COO)2·2H2O) was dissolved into 40 mL of deionized water until Zn(CH3COO)2·2H2O was dissolved completely by stirring at room temperature (25 °C). Then 1 mmol SnCl4·5H2O was added into the Zn(CH3COO)2·2H2O solution. Lastly, sodium hydroxide (NaOH) was added to the above mixture solution under magnetic stirring for 30 min. The homogeneous solution was quickly transferred into a Teflon-lined stainless steel autoclave with a capacity of 50 mL, it was heated at 80 °C for 20 h, and then cooled down naturally. The products were washed with absolute ethanol and double distilled water three times and kept in suspension conditions as final products for further characterization and gas sensing testing. X-ray powder diffraction (XRD, X’Pert PRO) using CuKα radiation (λ = 0.1548 nm) was used for determining the phase purity and crystal structure of the product. The morphology was characterized by scanning electron microscopy (SEM, EVO MA15). The gas-sensing properties of ZSH were studied with a QCM sensor system at room temperature in dry conditions.In detail, a ZSH-based CO sensor was prepared by the following steps: a gold-coated QCM was cleaned with ethanol for 20 min. Then the QCM was rinsed in deionized water and dried at room temperature in a dry nitrogen atmosphere. The suspension was dropped onto the QCM and the coating was dried in a furnace at 80 °C for 1 h. Fig. 5 shows a two channel flow system, which was used to detect QCM signals when exposed to volume fractions of typical gases (CO, CH4 and O2) at room temperature (25 °C) in dry conditions to study the adsorption and desorption processes. Two mass flow controller (MFC) valves were used to dilute the CO concentrations with N2. The MFC system was used to change the ratio in the testing chamber to obtain the desired gas volume fraction.
Conclusions
In summary, a ZSH-modified QCM sensor was used for detecting different concentrations of CO, CH4 and O2. The following conclusions can be drawn:(1) The ZSH-modified QCM sensor was very sensitive to different concentrations of CO, CH4 and O2. The adsorbed mass presented significant differences for gases at the same concentration. CO displayed a superior adsorption ability to other gases, while the sensor was least sensitive to O2.
(2) The relationship between the adsorption thermodynamic parameter (adsorbed mass amount in molar) and the adsorption dynamic parameter (adsorption rate constant, k) can be used to characterize the selectivity and concentration of a gas. But it still has some limitations.
(3) The relationship of characteristic parameters, t90-Δm, was useful to determine the species and concentrations of gases. This new analysis method would be promising in gas species identification with QCM sensors.
Conflicts of interest
We declare that there exists no conflict in the submission of this manuscript, and all the authors have seen the manuscript and approved submission to the journal. I would like to declare on behalf of my co-authors that the work described is original research that has not been published previously, and is not under consideration for publication elsewhere, in whole or in part.Acknowledgements
This work was financially supported by the National Science Foundation of China (51641108).Notes and references
- A. Kaushik, R. Kumar, S. K. Arya, M. Nair, B. D. Malhotra and S. Bhansali, Chem. Rev., 2015, 115(11), 4571 CrossRef CAS PubMed.
- D. Compagnone, M. Faieta, D. Pizzoni, C. D. Natale, R. Paolesse, T. V. Caelenberg, B. Beheydt and P. Pittia, Sens. Actuators, B, 2015, 207, 1114–1120 CrossRef CAS.
- M. Procek, A. Stolarczyk, T. Pustelny and E. Maciak, Sensors, 2015, 15, 9563 CrossRef CAS PubMed.
- V. A. Minh, L. A. Tuan, T. Q. Huy, V. N. Hung and N. V. Quy, Appl. Surf. Sci., 2013, 265, 458 CrossRef CAS.
- N. A. Masdor, Z. Altintas and I. E. Tothill, Biosens. Bioelectron., 2016, 78, 328 CrossRef CAS PubMed.
- S. Öztürka, A. Kösemen, Z. A. Kösemen, N. Kılınc, Z. Z. Öztürk and M. Penza, Sens. Actuators, B, 2016, 222, 280–289 CrossRef.
- M. Yang and J. He, Sens. Actuators, B, 2016, 228, 486–490 CrossRef CAS.
- C. Chen, X. Zheng, J. Yang and M. Wei, Phys. Chem. Chem. Phys., 2014, 16, 20073–20078 RSC.
- W. H. Feng, Z. X. Pei, Z. B. Fang, M. L. Huang, M. L. Lu, S. X. Weng, Z. Y. Zheng, J. Hu and P. Liu, J. Mater. Chem. A, 2014, 2, 7802–7811 CAS.
- H. Zhang, P. Song, D. Han and H. Yan, Sens. Actuators, B, 2015, 209, 384 CrossRef CAS.
- H. Wang, X. X. Liu, J. Xie, M. Duan and J. L. Tang, J. Alloys Compd., 2016, 657, 691–696 CrossRef CAS.
- H. Wang, X. X. Liu, J. Xie, M. Duan and J. L. Tang, Chin. Chem. Lett., 2016, 27, 464–466 CrossRef CAS.
- R. Tseng, P. Wu, F. Wu and R. Juang, Chem. Eng. J., 2014, 237, 153–161 CrossRef CAS.
- Y. Liu, Colloids Surf., A, 2008, 320, 275–278 CrossRef CAS.
- T. Shahwan, J. Environ. Chem. Eng., 2014, 2, 1001–1006 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2017 |