Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
WO3/BiVO4 photoanode coated with mesoporous Al2O3 layer for oxidative production of hydrogen peroxide from water with high selectivity†
Kojiro Fuku *a,
Yuta Miyaseab,
Yugo Miseki
*a,
Yuta Miyaseab,
Yugo Miseki a,
Takahiro Gunjiab and
Kazuhiro Sayama*ab
a,
Takahiro Gunjiab and
Kazuhiro Sayama*ab
aResearch Center for Photovoltaics (RCPV), National Institute of Advanced Industrial Science and Technology (AIST), Central 5, 1-1-1 Higashi, Tsukuba, Ibaraki 305-8565, Japan. E-mail: k.sayama@aist.go.jp
bDepartment of Pure and Applied Chemistry, Tokyo University of Science, 2641 Yamasaki, Noda, Chiba 278-8514, Japan
First published on 11th October 2017
Abstract
A WO3/BiVO4 photoanode coated with various metal oxides demonstrated high selectivity (faradaic efficiency) for hydrogen peroxide (H2O2) generation from water (H2O) under irradiation of simulated solar light in a highly concentrated hydrogen carbonate (KHCO3) aqueous solution. A mesoporous and amorphous aluminium oxide (Al2O3) layer significantly facilitated inhibition of the oxidative degradation of generated H2O2 into oxygen (O2) on the photoanode, resulting in unprecedented H2O2 selectivity (ca. 80%) and the accumulation (>2500 μM at 50C).
Chemical conversions using light energy have been performed in various fields since the discovery of the Honda–Fujishima effect.1–25 Significant efforts have recently been devoted to H2 production by water splitting using inexhaustible light for clean energy conversion processes.1–4,8–29 Photoelectrode systems are widely recognised as a promising technology for H2 production because they operate at an electrolysis voltage lower than the theoretical electrolysis voltage of water (<1.23 V).1,8–29 Visible light-responsive oxide photoanodes with a narrow bandgap energy, such as WO3, BiVO4 and Fe2O3, are desirable for the efficient utilisation of solar light and economical synthetic processes.8–29 Most importantly, numerous efforts have been focused on BiVO4 photoanodes capable of utilising a wide range of light energy (∼520 nm) and achieving efficient O2 generation by water splitting.9–21,28,29,32 A WO3/BiVO4 photoanode that combines BiVO4 with a WO3 underlayer for the efficient transfer of excited electrons on BiVO4 to the F-doped SnO2 conductive glass (FTO) substrate shows exceptional photoelectrochemical performance for water splitting into H2 and O2.10–13,17–20,28,29,32 However, most previous investigations, containing electrochemical reactions, focused solely on the recovery of H2 energy generated on the cathode and little attention was paid to the recovery of the oxidation products simultaneously evolved during water splitting.24–31
H2O2 is an especially versatile and clean oxidation product having the potential to generate instead of O2 from H2O (eqn (1)).
| 2H2O → H2O2 + 2H+ + 2e− (E(H2O2/H2O) = +1.77 V vs. RHE) | (1) |
However, the accumulation of H2O2, generated oxidatively is extremely difficult because degradation of H2O2 into O2 also occurs easily and oxidatively in a conventional photoelectrochemical system i.e. the redox potential of H2O2 degradation is more negative than the redox potential of H2O2 production from H2O (eqn (1) and (2)), resulting in low selectivity for oxidative H2O2 generation.
| H2O2 → O2 + 2H+ + 2e− (E(O2/H2O2) = +0.68 V vs. RHE) | (2) |
Recently, we reported that a photoelectrochemical system combining the WO3/BiVO4 photoanode and aqueous electrolyte of KHCO3 under CO2 bubbling could achieve simultaneous generation and accumulation of H2O2 and H2 from H2O (eqn (3)).28,29 In this system, the aqueous electrolyte of KHCO3 acts as an excellent oxidative catalyst for generating H2O2 from H2O. Moreover, H2O2 could be produced at no external bias on both a WO3/BiVO4 photoanode (from H2O) and an Au cathode (from O2) via a two-photon process (eqn (4)).29
| 2H2O → H2O2 + H2 (two-photon process) | (3) |
| 2H2O + O2 → 2H2O2 (two-photon process) | (4) |
Although the selectivity (faradaic efficiency: η(H2O2)) of reductive H2O2 production from O2 on cathodes such as Au was very high, almost 100%, the maximum selectivity (η(H2O2)) for oxidative H2O2 production on WO3/BiVO4 photoanodes was still low, only ca. 54%. The design of novel photoanodes capable of achieving efficient H2O2 generation and inhibiting oxidative degradation of generated H2O2 is absolutely imperative for building a clean and breakthrough technology, by accumulating H2O2 and H2 with unprecedented H2O2 selectivity using only H2O as the raw material.
Here, we focused on a surface modification of the metal oxide (MeOx) layers on the WO3/BiVO4 photoanode surface to achieve excellent selectivity of generation and accumulation of H2O2 in the KHCO3 aqueous solution under simulated solar light irradiation (Fig. 1). The MeOx layers were prepared by spin-coating of metal organic solutions and calcination. Introducing a porous Al2O3 layer was found to specifically permit oxidative H2O2 generation and accumulation with exceptional selectivity in an aqueous KHCO3 electrolyte because of the blocking effect of oxidative degradation of the generated H2O2 into O2 on the photoanode.
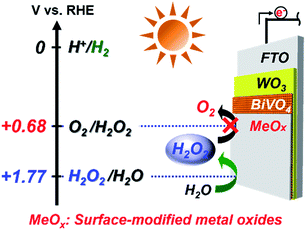 | ||
| Fig. 1 Pattern and energy diagrams for photoelectrochemical H2O2 generation from H2O on WO3/BiVO4/MeOx photoanodes under solar light irradiation. | ||
Details regarding experimental procedures for preparation and photoelectrochemical reactions of photoanodes are provided in the ESI†.
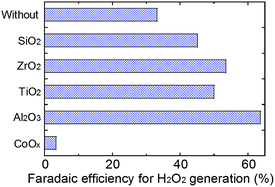
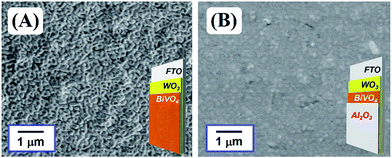
The effects of MeOx layers, modified on the WO3/BiVO4 photoanode, for oxidative H2O2 generation properties were investigated at an applied electric charge of 0.9C (900 s at steady photocurrent of 1 mA) in a 0.5 M KHCO3 aqueous electrolyte. As shown in Fig. 2, all MeOx-coated photoanodes, except CoOx, enhanced the oxidative H2O2 generation compared to a bare WO3/BiVO4 photoanode, and the enhanced effect, ranked by the modified metal oxide, was Al2O3 > ZrO2 > TiO2 > SiO2 ≫ CoOx. Little H2O2 was observed on the CoOx coated photoanode, because CoOx probably decomposed the generated H2O2 quickly, or O2 may be evolved on CoOx directly. It should be noted that the Al2O3 modification on the WO3/BiVO4 photoanode achieved roughly twice the oxidative H2O2 generation compared to the bare WO3/BiVO4 photoanode. The Al2O3 uniformly, smoothly and flatly covered the entire area of the WO3/BiVO4 photoanode as shown in the SEM images (Fig. 3), whereas other MeOx were granularly and uniformly supported on that and possessed any crack holes (Fig. S1; ESI†). It was also confirmed, from XRD measurement (Fig. S2; ESI†), that no diffraction peaks derived from MeOx were observed in all WO3/BiVO4/MeOx photoanodes, suggesting that all tried MeOx modified on WO3/BiVO4 photoanode possess amorphous-like structure. As shown in Fig. S3; ESI,† little change of the light harvesting efficiency (LHE) was also confirmed in tried all photoanodes, suggesting that these MeOx introduced on the WO3/BiVO4 have little effect to light absorption efficiency on WO3/BiVO4 photoanode. The time courses of voltages applied between photoanode and a counter electrode of Pt mesh at steady photocurrent of 1 mA (Fig. 2) in oxidative H2O2 generation reaction were also confirmed (Fig. S4; ESI†). The voltages for applying steady photocurrent of 1 mA slightly increased by introducing MeOx on the WO3/BiVO4 photoanode. In particular, WO3/BiVO4/Al2O3 photoanode, coated uniformly, smoothly and flatly at Al2O3 compared to other MeOx, required highest applied voltage. In order to confirm the effect introducing the Al2O3 on the photoanode in more detail, the photocurrent property of the WO3/BiVO4/Al2O3 photoanode was investigated in a 0.5 M KHCO3 aqueous solution (Fig. S5; ESI†). The bare WO3/BiVO4 photoanode exhibited excellent photocurrent property in all applied voltage ranges as with our past reported example,11,12,28,29 and the photocurrent property slightly decreased by introducing the Al2O3 layer. However, it should be noted that the decreasing degree of the photocurrent property was slight, only ca. 9% and 5% at +1.23 V and +1.77 V vs. RHE, respectively, although the Al2O3, having an insulation property, covered the entire area of the WO3/BiVO4 photoanode. A similar phenomenon has also been observed in O2 and H2 generation through water splitting on a photoanode coated with amorphous-like Ta2O5.32 In addition, it was confirmed, from the N2 absorption and desorption measurement of MeOx particles (Fig. S6; ESI†), that almost all MeOx possess mesoporous structure at a pore size of ca. 4–20 nm. In particular, a pore size of the Al2O3 was ca. 4.7 nm. The thicknesses of Al2O3 calculated from the coating amount on the WO3/BiVO4 photoanode by XRF measurement were ca. 100 nm (0.055 mg cm−2). In order to also investigate the effects of dense Al2O3 on WO3/BiVO4 on the oxidative H2O2 generation, increasing Al2O3 amount on WO3/BiVO4 photoanode was performed by decreasing the spin coating number (500 rpm) of precursor solution of EMOD solved in butyl acetate containing ethylcellulose when introducing Al2O3 layers. The thickness of Al2O3 introduced at 500 rpm calculated from the XRF measurement was ca. 127 nm (0.070 mg cm−2), suggested that the thickness increases with decreasing the spin coating number. As shown in Fig. S7; ESI,† little change of the H2O2 generation amounts was observed on these WO3/BiVO4/Al2O3 photoanodes prepared at 500 and 1000 rpm, indicating that increasing Al2O3 on WO3/BiVO4 photoanode has little effect on the oxidative H2O2 generation. In subsequent experiments, WO3/BiVO4/Al2O3 photoanode prepared at 1000 rpm was utilized as the photoanode. These results indicate that the specific effect enhancing oxidative H2O2 generation property was achieved on the WO3/BiVO4 though the mesoporous and amorphous Al2O3 layer covered uniformly, smoothly and flatly the entire area.
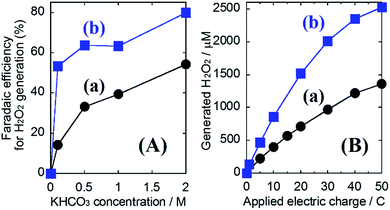
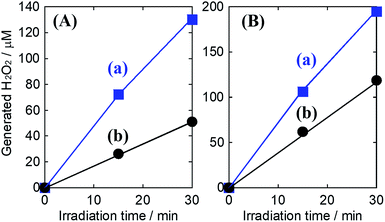
To track the specific performance enhancing effect of generating H2O2 by introducing the Al2O3 layer, the concentration dependency of KHCO3 aqueous electrolytes on the oxidative H2O2 generation property was investigated at an applied electric charge of 0.9C (Fig. 4(A)). We have already reported that the oxidative H2O2 generation property on the WO3/BiVO4 photoanode was improved with increasing concentration of KHCO3, which acts as an effective catalyst for H2O2 generation via the two-electron oxidation of H2O.28 Even in the case of using the WO3/BiVO4/Al2O3 photoanode, the selectivity (η(H2O2)) for H2O2 generation was significantly enhanced with increasing concentration of KHCO3, and the η(H2O2) in the 2.0 M KHCO3 aqueous solution reached ca. 80% at 0.9C, whereas that using the bare WO3/BiVO4 photoanode was ca. 54%. It should be noted that the selectivity (η(H2O2) = ca. 53%) on the WO3/BiVO4/Al2O3 photoanode in lowly concentrated KHCO3 (0.1 M) was comparable to that (ca. 54%) on the bare WO3/BiVO4 photoanode in highly concentrated KHCO3 (2.0 M). This suggests that the Al2O3 could effectively be contributing to oxidative H2O2 generation from H2O even in the lowly concentrated KHCO3. Moreover, as shown in Fig. 4(B), the excellent H2O2 generation property on the WO3/BiVO4/Al2O3 photoanode compared to the WO3/BiVO4 photoanode was significantly maintained even at high electric charge up to 50C. As a result, the accumulation amount, using the WO3/BiVO4/Al2O3 photoanode, reached >2500 μM at 50C, while that using the bare WO3/BiVO4 photoanode was >1300 μM at 50C. The dependency of the applied voltage on the oxidative H2O2 generation was investigated to confirm the effect of the Al2O3 coating in detail (Fig. S8; ESI†). A small change in H2O2 generation performance was observed in all ranges of applied voltages (0.8–1.8 V), suggesting that the enhanced effect of introducing an Al2O3 layer is independent of the voltages applied between a photoanode as the working electrode and a Pt mesh as counter electrode using the aqueous electrolyte of the KHCO3.
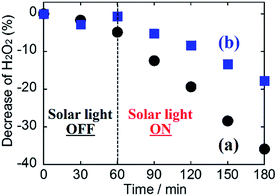
Although little development with regards to highly selective H2O2 generation via two-photon oxidation of H2O and accumulation using photoanodes has been reported, our method of Al2O3 coating on the WO3/BiVO4 photoanode produced tremendous improvement in selective H2O2 generation and accumulation from H2O in a KHCO3 aqueous electrolyte. It is speculated that the specific enhancement of selectivity for H2O2 generation on the WO3/BiVO4/Al2O3 photoanode may be caused by a blocking effect, on the mesoporous Al2O3 layer, that inhibits oxidative H2O2 degradation into O2 on the BiVO4. To investigate the blocking effect on the Al2O3 layer, a degradation property test of H2O2 was performed in a 2.0 M KHCO3 aqueous solution containing H2O2 (550 μM) in the presence of the bare WO3/BiVO4 or WO3/BiVO4/Al2O3 photoanodes in presence or absence of simulated solar light irradiation in an ice bath (below 5 °C). In both cases, as shown in Fig. 5, almost all the initial amount of H2O2 was maintained in the dark condition, however, the H2O2 amount drastically decreased with irradiation by simulated solar light, suggesting that the H2O2 was decomposed by photocarriers (excited electrons and holes) produced on the BiVO4. It should be noted that the H2O2 degradation in the presence of the WO3/BiVO4/Al2O3 photoanode was dramatically inhibited compared to the degradation in the presence of a bare WO3/BiVO4 photoanode. The oxidative H2O2 generation test was also confirmed in a 2.0 M KHCO3 aqueous electrolyte, initially containing H2O2 (210 μM) on the bare WO3/BiVO4 and WO3/BiVO4/Al2O3 photoanodes, to track the generated H2O2 degradation behaviour in more detail (Fig. 6). The generated rates of H2O2 were reduced by the initial addition of H2O2 in both cases of presence or absence of Al2O3. However, the decreasing rate of H2O2 generation was significantly inhibited, from ca. 61% to ca. 39%, by introducing the Al2O3 layer on the WO3/BiVO4 photoanode. These results suggest that introducing the Al2O3 layer significantly contributed to the highly selective H2O2 generation and accumulation from H2O, with a high photocurrent property, by a blocking effect that inhibited the oxidative degradation of generated H2O2. The mechanism of blocking effect is proposed that the H2O2 generated on the BiVO4 in the WO3/BiVO4/Al2O3 photoanode diffuses in electrolyte of KHCO3 aqueous solution through mesoporous of the Al2O3, and contact of the H2O2 diffused in electrolyte with the BiVO4 covered uniformly and smoothly Al2O3 may be significantly inhibited compared with that with bare BiVO4, resulting in the formation of effective inhibition of oxidative H2O2 degradation. Furthermore, there may be other possible mechanisms such as a blocking effect of a direct O2 evolution site via a 4-photon process covering by Al2O3, or an enrichment effect resulting from the increasing KHCO3 concentration around the photoanode based on the acid–base adsorption between HCO3− (a weak base) and the weakly acidic sites on the Al2O3 surface, related to the good η(H2O2) in lower KHCO3 concentration, as shown in Fig. 4(A). The tracking and contribution of these other mechanisms, on the Al2O3 layer, is currently under investigation.
Conclusions
In summary, various metal oxides were coated onto a WO3/BiVO4 photoanode to enhance the selectivity (faradaic efficiency) of oxidative H2O2 generation, in an aqueous electrolyte of KHCO3, from water under solar light irradiation. Among the various metal oxides, the Al2O3 coating, which produced a mesoporous and amorphous structure on the WO3/BiVO4 photoanode, achieved excellent oxidative H2O2 generation at a selectivity of ca. 80% and an accumulation of >2500 μM (50C). Interestingly, the Al2O3-coated WO3/BiVO4 photoanode dramatically inhibited oxidative degradation of H2O2 generated on the WO3/BiVO4 photoanode after introducing the Al2O3 layer. This study contributes to developing a promising design for a clean H2O2 production system that uses only water as the raw material under solar light irradiation. More effective dreamy H2O2 generation, at an excellent selectivity close to 100%, can be expected by modifying the surface-treatment technology, and it is currently under investigation.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The present work was partially supported by JSPS KAKENHI Grant Number 26810105 and the International Joint Research Program for Innovative Energy Technology. We thank Dr Etsuko Fujita (Brookhaven National Laboratory) for helpful discussions.Notes and references
- A. Fujishima and K. Honda, Nature, 1972, 238, 37 CrossRef CAS PubMed
.
- Z. G. Zou, J. H. Ye, K. Sayama and H. Arakawa, Nature, 2001, 414, 625 CrossRef CAS PubMed
.
- A. Kudo and Y. Miseki, Chem. Soc. Rev., 2009, 38, 253 RSC
.
- K. Maeda, K. Teramura, D. Lu, T. Takata, N. Saito, Y. Inoue and K. Domen, Nature, 2006, 440, 295 CrossRef CAS PubMed
.
- K. Fuku, K. Hashimoto and H. Kominami, Chem. Commun., 2010, 46, 5118 RSC
.
- K. Fuku, T. Kamegawa, K. Mori and H. Yamashita, Chem.–Asian J., 2012, 7, 1366 CrossRef CAS PubMed
.
- K. Fuku, R. Hayashi, S. Takakura, T. Kamegawa, K. Mori and H. Yamashita, Angew. Chem., Int. Ed., 2013, 52, 7446 CrossRef CAS PubMed
.
- B. D. Alexander, P. J. Kulesza, I. Rutkowska, R. Solarska and J. Augustynski, J. Mater. Chem., 2008, 18, 2298 RSC
.
- K. Sayama, A. Nomura, Z. Zou, R. Abe, Y. Abe and H. Arakawa, Chem. Commun., 2003, 2908 RSC
.
- P. Chatchai, Y. Murakami, S. Kishioka, A. Y. Nosaka and Y. Nosaka, Electrochim. Acta, 2009, 54, 1147 CrossRef CAS
.
- R. Saito, Y. Miseki and K. Sayama, Chem. Commun., 2012, 48, 3833 RSC
.
- I. Fujimoto, N. Wang, R. Saito, Y. Miseki, T. Gunji and K. Sayama, Int. J. Hydrogen Energy, 2014, 39, 2454 CrossRef CAS
.
- X. Shi, I. Y. Choi, K. Zhang, J. Kwon, D. Y. Kim, J. K. Lee, S. H. Oh, J. K. Kim and J. H. Park, Nat. Commun., 2014, 5, 4775 CrossRef CAS PubMed
.
- T. W. Kim and K. S. Choi, Science, 2014, 343, 990 CrossRef CAS PubMed
.
- Y. Liu, Y. Guo, L. T. Schelhas, M. Li and J. W. Ager III, J. Phys. Chem. C, 2016, 120, 23449 CAS
.
- L. H. Hess, J. K. Cooper, A. Loiudice, C. M. Jiang, R. Buonsanti and I. D. Sharp, Nano Energy, 2017, 34, 375 CrossRef CAS
.
- J. Su, L. Guo, N. Bao and C. A. Grimes, Nano Lett., 2011, 11, 1928 CrossRef CAS PubMed
.
- P. M. Rao, L. Cai, C. Liu, I. S. Cho, C. H. Lee, J. M. Weisse, P. Yang and X. Zheng, Nano Lett., 2014, 14, 1099 CrossRef CAS PubMed
.
- I. Grigioni, K. G. Stamplecoskie, D. H. Jara, M. V. Dozzi, A. Oriana, G. Cerullo, P. V. Kamat and E. Selli, ACS Energy Lett., 2017, 2, 1362 CrossRef CAS
.
- K. Mase, M. Yoneda, Y. Yamada and S. Fukuzumi, ACS Energy Lett., 2016, 1, 913 CrossRef CAS
.
- F. M. Toma, J. K. Cooper, V. Kunzelmann, M. T. McDowell, J. Yu, D. M. Larson, N. J. Borys, C. Abelyan, J. W. Beeman, K. M. Yu, J. Yang, L. Chen, M. R. Shaner, J. Spurgeon, F. A. Houle, K. A. Persson and I. D. Sharp, Nat. Commun., 2016, 7, 12012 CrossRef PubMed
.
- J. Y. Kim, G. Magesh, D. H. Youn, J. W. Jang, J. Kubota, K. Domen and J. S. Lee, Sci. Rep., 2013, 3, 2681 CrossRef PubMed
.
- Q. Mi, A. Zhanaidarova, B. S. Brunschwig, H. B. Gray and N. S. Lewis, Energy Environ. Sci., 2012, 5, 5694 CAS
.
- J. C. Hill and K. S. Choi, J. Phys. Chem. C, 2012, 116, 7612 CAS
.
- K. Ueno and H. Misawa, NPG Asia Mater., 2013, 5, e61 CrossRef CAS
.
- K. Fuku, N. Wang, Y. Miseki, T. Funaki and K. Sayama, ChemSusChem, 2015, 8, 1593 CrossRef CAS PubMed
.
- H. G. Cha and K. S. Choi, Nat. Chem., 2015, 7, 328 CrossRef CAS PubMed
.
- K. Fuku and K. Sayama, Chem. Commun., 2016, 52, 5406 RSC
.
- K. Fuku, Y. Miyase, Y. Miseki, T. Funaki, T. Gunji and K. Sayama, Chem.–Asian J., 2017, 12, 1111 CrossRef CAS PubMed
.
- K. Fuku, Y. Miyase, Y. Miseki, T. Gunji and K. Sayama, ChemistrySelect, 2016, 1, 5721 CrossRef CAS
.
- T. Shiragami, H. Nakamura, J. Matsumoto, M. Yasuda, Y. Suzuri, H. Tachibana and H. Inoue, J. Photochem. Photobiol., A, 2015, 313, 131 CrossRef CAS
.
- R. Saito, Y. Miseki, W. Nini and K. Sayama, ACS Comb. Sci., 2015, 17, 592 CrossRef CAS PubMed
.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental section, SEM images, XRD spectra, LHE spectra, applied voltage properties, I–V characteristic of photoanodes, pore size distribution of the MeOx particles, effect of Al2O3 amount on WO3/BiVO4 and dependence of applied voltage. See DOI: 10.1039/c7ra09693c |
| This journal is © The Royal Society of Chemistry 2017 |