Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Electrostatically driven scalable synthesis of MoS2–graphene hybrid films assisted by hydrophobins†
Jasneet Kaur *ab,
Alessandro Vergaracde,
Manuela Rossif,
Alfredo Maria Gravagnuolo
*ab,
Alessandro Vergaracde,
Manuela Rossif,
Alfredo Maria Gravagnuolo cg,
Mohammadhassan Valadan
cg,
Mohammadhassan Valadan a,
Federica Corradoh,
Mariarosaria Contei,
Felice Gesuele
a,
Federica Corradoh,
Mariarosaria Contei,
Felice Gesuele a,
Paola Giardina
a,
Paola Giardina c and
Carlo Altucci*a
c and
Carlo Altucci*a
aDepartment of Physics “Ettore Pancini”, University of Naples Federico II, Naples, Italy. E-mail: jasneet@fisica.unina.it; carlo.altucci@unina.it
bAkal College of Basic Sciences, Eternal University, Baru Sahib, Himachal Pradesh, India. E-mail: jasneet.physics@gmail.com
cDepartment of Chemical Sciences, University of Naples Federico II, Naples, Italy
dCEINGE Biotecnologie Avanzate scarl, Naples, Italy
eInstitute of Biostructures and Bioimaging, CNR, Naples, Italy
fDepartment of Earth, Environment and Resources Sciences, University of Naples Federico II, Naples, Italy
gDivision of Pharmacy and Optometry, Faculty of Biology, Medicine and Health, The University of Manchester, UK
hIstituto Zooprofilattico Sperimentale del Mezzogiorno, Portici, Italy
iIRCCS, SDN, Via E. Gianturco 113, 80143, Naples, Italy
First published on 27th October 2017
Abstract
Liquid processing of 2D crystals offers scalable strategies for the production of 2D materials. Herein, we produce the hybrids of MoS2/graphene, consisting of few-layered nanosheets of luminescent MoS2 and biofunctionalized few-layered graphene assisted by the Vmh2 hydrophobin, a self-assembling adhesive fungal protein, through a green route of production. The functionalization of the graphene flakes assisted by Vmh2 adds surface charge, which enables electrostatic interaction between MoS2 and graphene flakes, leading to the van der Waals coupling. The surface morphology of 2D material based films is analyzed through optical imaging, scanning and transmission electron microscopy. The produced dispersions of MoS2, bGr and the hybrid solutions, are investigated by electrophoretic mobility, UV-Vis, Raman and photoluminescence spectroscopy. Interestingly, the effect of van der Waals interactions between the layers of MoS2 and bGr crystals are evidenced through the significant upshift of 14 cm−1 in the G′ Raman peak of graphene and an upshift of 1.4 cm−1 of the A1g peak of MoS2. Due to the formation of heterostructures, significant quenching of the characteristic photoluminescence emitted from the monolayers of MoS2 was also observed, indicating the charge transfer process occurring between the crystal layers. This approach of scalable synthesis of 2D material based nano-bio hybrids offers economic and eco-friendly solutions to promote novel applications in biosensing and photodetection.
Introduction
The rising interest in two-dimensional (2D) materials, beyond graphene, is to unveil the potential of 2D alternatives and to supplement the gapless feature of graphene, with interesting electronic properties from other analogues such as semiconducting transition metal dichalcogenides (TMDs) and insulating hexagonal boron nitride (hBN).1,2 These novel 2D TMD crystals offer promising applications due to their unique physicochemical features, including a broad absorption spectrum, ultra-thin sheet structures, mechanical flexibility and high optical absorption.3–5 Moreover, since graphene exhibits remarkable electrical and thermal conductivities, optical transparency, elasticity and strength, crucial for flexible electronics, thus research efforts have been dedicated to integrate distinct 2D materials to produce van der Waals heterostructures (vdW), which could offer novel and tailored optoelectronic features with varying functionalities.6–8 Among TMDs, few-layered MoS2 is an interesting candidate with unique properties for a range of electronics and biomedical applications, which can also complement with graphene, since it combines the excellent optical properties of MoS2 with the high mobility and transparency of graphene.9,10 Therefore, the possible heterostructure of MoS2/graphene would form a perfect couple, which can offer novel applications in nanoelectronics and optoelectronics, due to the superlative properties of semiconductor/(semi) metal heterojunction contact.11,12In order to exploit the intriguing properties of vdW heterostructures, the immediate requirement is to develop scalable production route for the exfoliation of high-quality 2D materials through eco-friendly and low-cost techniques. In contrast to conventional epitaxially grown routes for heterostructures, involving micromechanical cleavage followed by critical transfer process, and chemical vapor deposition, the solution processing technique with liquid-phase exfoliation (LPE) offers advantages for scalability and cost-reduction with production of high-quality, few-layered 2D materials based dispersions.13,14 These water-based dispersions consist of 2D nanosheets which can be deposited on the substrates through drop-casting or spin-coating methods.15,16 However, in several literature reports, 2D materials based dispersions are produced by exfoliation of bulk crystals in the presence of toxic organic solvents,17,18 which need to be replaced by eco-friendly and biocompatible solvents for environmental and health prospects.
Biological interfacing of graphene and 2D materials in general has become essential for the sake of biocompatibility, dispersibility and selectivity of innovative hybrid nanomaterials which could be applied to the biotechnological and biomedical fields.19–21 Chemical functionalization of 2D materials can imply noticeable drawbacks such as disrupture of the electronic structure.22 To circumvent these drawbacks milder techniques of functionalization have been implemented. For instance, for graphene23 and MoS2,24 recently interfaced with a biomolecule such as a protein, the functionalization process can be regarded as a biofunctionalization with the formation of a hybrid structure. Because of their wide range of functionalities and high responsiveness to a variety of stimuli, proteins are suitable candidates for bioconjugation of nanomaterials for quite a number of applications.25 In this regard, green synthesis routes of exfoliation should be developed, i.e. exfoliation protocols based in aqueous and possibly eco-friendly solvents, to diminish the impact of nanomaterials in health and environmental issues.
Proteins known as the hydrophobins (HFBs) are amphiphilic and compact/globular proteins of around 3 nm, extracted from fungi, and endowed with peculiar self-assembling mechanisms, high surface activity and propensity to surface adhesion.24,26 Their self-assembled (mono)layers can be used as functional coatings, for the non-covalent immobilization of proteins and nanomaterials, biofunctionalization and stabilization of liquid dispersions of 2D materials.26–29 The class I HFBs, can self-assemble into amyloid-like structures that play relevant biological functions as chemically stable coatings, and therefore they are called functional amyloids.30 Since it has been discovered that they can mask the fungal spores to the immune system they have attracted much interest for their use in the biomedical field.31
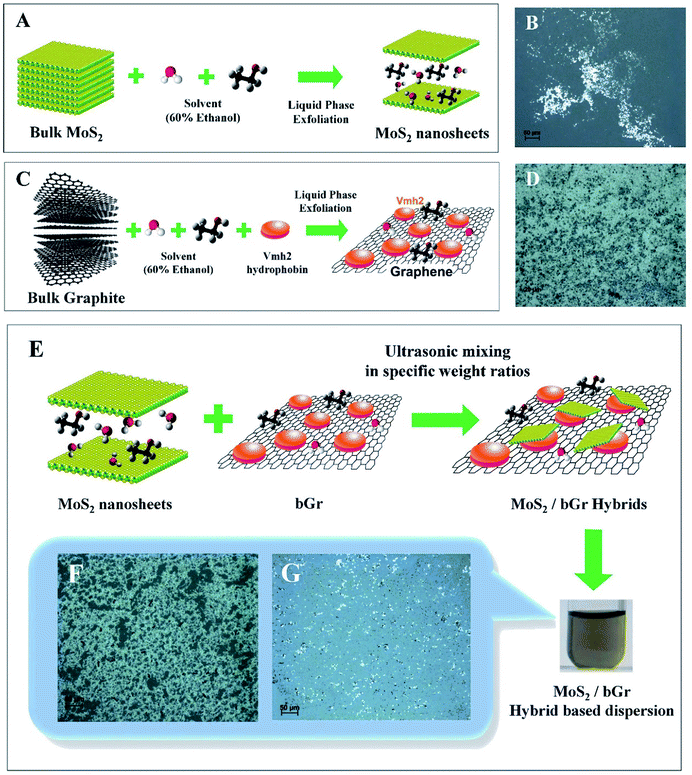
Herein, we produce few-layered, defect-free nanosheets of MoS2, and biofunctionalized graphene (bGr), which are conjugated to a self-assembling protein, dispersed in aqueous solutions, through a green route, shown in Fig. 1(A–D). The protein used is the class I HFB Vmh2 extracted from the edible basidiomycete fungus, Pleurotus ostreatus, commonly known as the oyster mushroom.32,33 This protein plays a crucial role in the stability of both the graphene and MoS2 based dispersion in aqueous alcoholic medium.34,35 In addition, its conjugation to the graphene generates a positive ζ-potential on the surface of nanosheets.36 However, the exfoliated MoS2 nanosheets in the absence of Vmh2 exhibit a negative ζ-potential.24 Here, we take advantage of the oppositely charged surfaces of the nanosheets of MoS2 and bGr as source of electrostatic attraction to lead to attachment between the two types of sheets, followed by vdW interactions between the 2D crystals.37,38
The hybrid structures are further characterized through optimal imaging, scanning electron microscopy, transmission electron microscopy, Raman and photoluminescence (PL) spectroscopy. Interestingly, this novel approach of green-route production of hybrid structures of MoS2/graphene is reliable, reproducible, and offers low-cost technology for mass production of devices based on heterostructures of 2D materials. Moreover, in this combination of MoS2/bGr heterostructure, it forms a semiconductor/metal interface, which is an outstanding platform for photonics and electronic devices, such as in p–n junction diodes and transistors.11,12,32,33 Since in this structure, the Vmh2 protein is also sandwiched between the 2D layers, we name this complex structure as MoS2/bGr hybrid structure, which can have promising applications in bioelectronics, biosensing, bioimaging and photonic devices.9,15,39 In particular, we discuss the applications of MoS2/bGr hybrid structure in biosensing and photodetection.8,34
Experimental
Production of MoS2 and bGr dispersions using green route through LPE
MoS2 powder (Aldrich, 69860, particle size ∼6 μm, density 5.06 g mL−1), was exfoliated in 60% ethanol aqueous solution (35 mg in 5 mL) using a tip sonicator (Bandelin Ultrasound SONOPULS HD3200, maximum power 200 W, working frequency 20 kHz, MS 72 probe, running at 10% amplitude) for 2 hours in cylindrical glass tubes (15 mm diameter, 10 cm height, rounded bottom). The temperature of the dispersion during the sonication was controlled in an ice-water bath.Next, 5 mg of graphite powder (Aldrich, 332461, mesh number of grains +100, >75%, particle size ∼300 μm) in 5 mL of 60% ethanol solution was exfoliated in the presence of Vmh2 (50 μg mL−1), using a tip sonicator (Bandelin, HD3200, MS 72 probe, running at 10% amplitude) for 5 hours in cylindrical glass tubes and cooling in an ice-water bath. The extraction of Vmh2 from P. ostreatus mycelia was carried out as reported elsewhere (see the detailed method in ESI†).23,24 Briefly, P. ostreatus mycelia were treated with 2% sodium dodecyl sulfate (SDS) in a boiling water bath. The residue was freeze-dried and treated with 100% trifluoroacetic acid (TFA) in a water bath sonicator. The supernatant was dried, and then lipids were extracted in a mixture of water–methanol–chloroform 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v. The precipitate was again freeze-dried, treated with TFA, dried in a stream of air, and dissolved in 60% ethanol. To ensure the purity of the protein, the procedure for removal of non-protein contaminants is discussed in ESI† and the purity of the protein was tested by SDS gel electrophoresis, and its conformation by circular dichroism analysis as reported elsewhere.31
1 v/v. The precipitate was again freeze-dried, treated with TFA, dried in a stream of air, and dissolved in 60% ethanol. To ensure the purity of the protein, the procedure for removal of non-protein contaminants is discussed in ESI† and the purity of the protein was tested by SDS gel electrophoresis, and its conformation by circular dichroism analysis as reported elsewhere.31
In Fig. 1, the schematic diagram of MoS2/bGr production is described, in which Fig. 1(A and C) represents the protocol of liquid processing of bulk powders of MoS2 and graphite, along with Vmh2 hydrophobin, by dispersing in the solvent of 60% ethanol, exfoliated through tip sonication, followed by step-wise controlled centrifugation. After sonication, dark dispersions of both MoS2 and bGr samples were produced, consisting of heterogeneous number of layers. Both the dispersions of MoS2 and bGr were centrifuged using step-wise controlled centrifugation (using Eppendorf Centrifuge 5810R, Rotor F-34-6-38) from 40g to 2400g for 45 minutes each step, for size selection and efficient production of monolayer enriched dispersions. The non-exfoliated crystallites were removed with low centrifugal force, 40g for 45 minutes, and the dispersion produced contains few-layered flakes with a wide distribution of size and thickness and a small percentage of monolayers population with varying lateral sizes.
The supernatant was then centrifuged with a higher force of 150g for 45 minutes and the pellets were removed. Further, the supernatant was separated and again centrifuged with a higher centrifugal force of 600g for 45 minutes and finally the associated supernatant was proceeded to the highest centrifugal force of 2400g for 45 minutes. The final dispersions of MoS2 and bGr were drop-casted on the glass substrates for acquiring their morphological analysis through the optical microscope, detailed in ESI† with 50× objective.
Mixing of dispersions to produce MoS2/bGr hybrid based solutions
After the preparation of bGr dispersion, we post-centrifuged the bGr dispersion for 45 minutes at 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000g in a centrifuge (Eppendorf Centrifuge 5415D) and separated the supernatant containing the excess of Vmh2 and 60% ethanol, and the bGr pellet was re-suspended in the MoS2 solution in specific concentrations. Without removing the excess of hydrophobin in solution, the hybrid formation was not efficient, since excess of Vmh2 interacts with MoS2 which acts as a hindrance for the interaction between layers of bGr and MoS2. Thus, this step of post-centrifugation of bGr dispersion is somewhat tricky and crucial for the preparation of the MoS2/bGr hybrid solution.
000g in a centrifuge (Eppendorf Centrifuge 5415D) and separated the supernatant containing the excess of Vmh2 and 60% ethanol, and the bGr pellet was re-suspended in the MoS2 solution in specific concentrations. Without removing the excess of hydrophobin in solution, the hybrid formation was not efficient, since excess of Vmh2 interacts with MoS2 which acts as a hindrance for the interaction between layers of bGr and MoS2. Thus, this step of post-centrifugation of bGr dispersion is somewhat tricky and crucial for the preparation of the MoS2/bGr hybrid solution.
The produced dispersion of MoS2 and bGr were mixed in various weight ratios of MoS2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) bGr = 1
bGr = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, 1
2, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 and 1
4 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 to reach the optimal ratio in order to produce 2D hybrid solutions, and out of these 1
10 to reach the optimal ratio in order to produce 2D hybrid solutions, and out of these 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 turned out to be the best weight ratio of MoS2
2 turned out to be the best weight ratio of MoS2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) bGr, based on the formation of vdW heterostructures, as investigated further by SEM and Raman measurements. The scheme of preparation of the hybrid dispersion is shown in Fig. 1(E). Fig. 1(G) exhibits the optical image of the hybrid sample dried on the glass substrate with weight ratio of MoS2
bGr, based on the formation of vdW heterostructures, as investigated further by SEM and Raman measurements. The scheme of preparation of the hybrid dispersion is shown in Fig. 1(E). Fig. 1(G) exhibits the optical image of the hybrid sample dried on the glass substrate with weight ratio of MoS2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) bGr solutions corresponding to 1
bGr solutions corresponding to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, whereas Fig. 1(F) shows the optical image of the hybrid sample with MoS2
2, whereas Fig. 1(F) shows the optical image of the hybrid sample with MoS2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) bGr weight ratio corresponding to 1
bGr weight ratio corresponding to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10.
10.
Characterizations
Optical extinction spectra were acquired of the dispersions of MoS2 and bGr through UV-Vis spectrophotometer, experimental details are reported in ESI.† The surface morphology of few-layered nanosheets of MoS2, bGr and MoS2/bGr hybrids was characterized by optical microscopy, scanning electron microscopy (SEM) with EDS measurements and transmission electron microscopy (TEM), detailed in ESI.† Raman and PL spectroscopy were performed using a confocal Raman microscope with a laser line at 514 nm.Results and discussion
The green route of production involves the liquid processing of bulk materials, which produces few-layered and high-quality MoS2 and bGr samples. After the production of MoS2 and bGr dispersions, the extinction spectra in the UV-Vis region of the MoS2 and bGr samples were acquired after the final steps of centrifugation and analyzed. The extinction spectra of MoS2 normalized at 350 nm after the final steps of centrifugation of 600g and 2400g are shown in Fig. 2 and bGr absorption spectrum is shown in ESI.† Through this spectral profile, we estimated the mean number of layers per flakes, the mean lateral size and the concentration of the MoS2 nanosheets, based on the formulation,24,35 as shown in Table 1.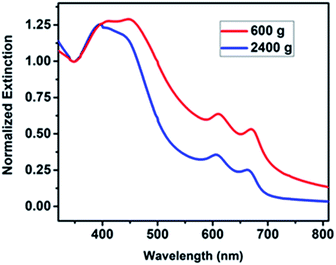 | ||
| Fig. 2 Extinction spectra of MoS2 normalized at 350 nm after centrifugation steps of 600g and 2400g. | ||
![[N with combining macron]](https://www.rsc.org/images/entities/i_char_004e_0304.gif) 〉, mean lateral size per flake 〈
〉, mean lateral size per flake 〈![[L with combining macron]](https://www.rsc.org/images/entities/i_char_004c_0304.gif) 〉 and mean concentration of flakes 〈
〉 and mean concentration of flakes 〈![[C with combining macron]](https://www.rsc.org/images/entities/i_char_0043_0304.gif) 〉 in dispersion estimated by UV-Vis spectroscopy for liquid phase exfoliated MoS2 after centrifugation at 2400g
〉 in dispersion estimated by UV-Vis spectroscopy for liquid phase exfoliated MoS2 after centrifugation at 2400g
| Wavelength of A-exciton | 〈![[N with combining macron]](https://www.rsc.org/images/entities/i_char_004e_0304.gif) 〉 〉 |
〈![[L with combining macron]](https://www.rsc.org/images/entities/i_char_004c_0304.gif) 〉 〉 |
〈![[C with combining macron]](https://www.rsc.org/images/entities/i_char_0043_0304.gif) 〉 〉 |
|---|---|---|---|
| 662 nm | 2.3 layers | 100 nm | 15.4 μg mL−1 |
For the graphene dispersion, the absorbance (Fig. S1†) per unit length (λ = 660 nm) is defined as a function of the produced graphene concentration, which is an important parameter in characterizing the graphene dispersion.36 The absorption coefficient previously established by Lotya and coworkers, (ε = 1390 L g−1 m−1) is related to the absorbance, through the Lambert–Beer law. Thus, the final concentration of the produced graphene dispersion after centrifugation of 2500g is estimated to be 54 μg mL−1. The mean lateral size for bGr dispersion was previously estimated by Raman spectroscopy and reported by Gravagnuolo et al.23 (mean number of layers, 2.9 ± 0.3 layers) after centrifugation at 2500g.
We also estimated the mean lateral size through dynamic light scattering (DLS), using the metrics reported by Lotya et al.36,37 The lateral size was consistent among the UV-Vis and DLS metrics estimation for MoS2 (Tables 1 and 2) and among the DLS metrics estimation and the mean lateral size for bGr previously reported (mean lateral size, 0.49 ± 0.06 μm after centrifugation at 2500g).23 Since the lateral size of the protein is more than one order of magnitude smaller than that of the flakes, its presence on the surface cannot influence the analysis. Furthermore, ζ-potential of the flakes was obtained by electrophoretic mobility measurements of the centrifuged samples of MoS2 and graphene. It was observed that after exfoliation, the flakes of bGr were positively charged; however the flakes of MoS2 were negatively charged, as detailed in Table 2.
![[L with combining macron]](https://www.rsc.org/images/entities/i_char_004c_0304.gif) 〉 estimated by electrophoretic mobility and Dynamic Light Scattering (DLS), for MoS2, bGr and hybrid samples
〉 estimated by electrophoretic mobility and Dynamic Light Scattering (DLS), for MoS2, bGr and hybrid samples
| bGr | MoS2 | Hybrid | |
|---|---|---|---|
| ζ-Potential (mV) | +35.4 ± 0.6 | −22.5 ± 0.5 | +24.5 ± 0.5 |
| Mean lateral size and range (nm) | 550 ± 40 | 125 ± 20 | 586 ± 58 |
Thus, the oppositely charged flakes of MoS2 and bGr become the source of interactions. The ζ-potential of the flakes of the hybrid solution flakes were estimated in the range of +24 mV, which indicates the presence of Vmh2 hydrophobin in the hybrid dispersion, in between the layers of MoS2 and graphene flakes. Moreover, with the addition of excess of Vmh2 (40–50 μL) to the prepared hybrid solution, the ζ-potential of the hybrid flakes has been observed to be enhanced by 10–12 mV, to achieve a higher stability over time for the hybrid dispersions.
The morphology of the films based on 2D materials was studied by using SEM.23 Films were deposited onto SOI (silicon dioxide on silicon) substrates, shown in Fig. 3, with the relevant electron dispersive spectra (EDS) for the elemental analysis.40 The few-layered solutions of 2D materials including MoS2, bGr and the hybrid dispersions were drop-casted onto SOI substrate and dried in air. The samples were investigated initially without metallization for qualitative chemical analyses, after were metalized with gold by using a sputter coater, for morphological analyses and micrographs were acquired to 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000× and 15
000× and 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000× magnification. SEM micrographs confirmed that MoS2 dispersion consists of nanosheets, with mean lateral size in the range of 100–150 nm, whereas bGr flakes were observed comparatively of bigger size, as shown in Fig. 3(A and C). The morphology of MoS2/bGr hybrid sample is shown in Fig. 3(E), which consists of both the types of flakes, with bigger bGr nanosheets interfaced to and surrounded by smaller sized MoS2 nanoflakes. The interfacial contact between the MoS2 and bGr nanosheets is also observed in the hybrid sample through its SEM micrograph.
000× magnification. SEM micrographs confirmed that MoS2 dispersion consists of nanosheets, with mean lateral size in the range of 100–150 nm, whereas bGr flakes were observed comparatively of bigger size, as shown in Fig. 3(A and C). The morphology of MoS2/bGr hybrid sample is shown in Fig. 3(E), which consists of both the types of flakes, with bigger bGr nanosheets interfaced to and surrounded by smaller sized MoS2 nanoflakes. The interfacial contact between the MoS2 and bGr nanosheets is also observed in the hybrid sample through its SEM micrograph.
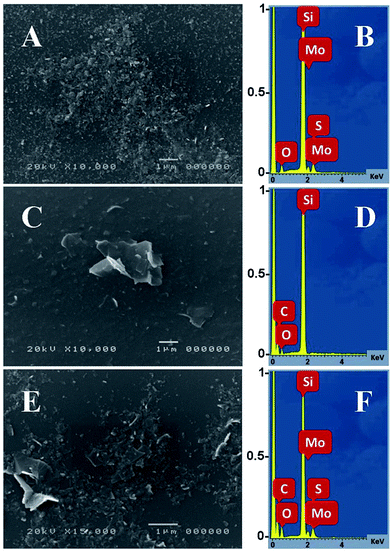 | ||
| Fig. 3 SEM micrographs of MoS2 (A), bGr (C) and hybrids (E) with their respective EDS measurements (B, D and F). | ||
The EDS measurements were made for the elemental analysis, through which the presence of graphene in bGr, was represented by the carbon (C) peak and MoS2 through molybdenum (Mo) and sulphur (S) peaks, shown in Fig. 3(B and D). Presence of the hydrophobin, Vmh2 also contributes to the C and O peaks. Moreover, in case of the hybrid sample, the peaks from all the elements Mo, S, C and O confirm the coexistence of MoS2 and graphene (bGr) in the MoS2/bGr hybrid structure, exhibited in Fig. 3(F). In addition, in all the spectra, there is presence of Si and O peaks from the silica glass substrate (SiO2).
Fig. 4(A–C) show the TEM images of few-layered nanosheets of MoS2 with higher resolution inset of MoS2 nanoflakes, bGr nanosheets and MoS2/bGr hybrids respectively. From TEM images, we confirm the size and morphology of MoS2 nanosheets, the size lying in the 100 nm range, whereas for bGr flakes, the size is bigger, in the 500 nm range. Thus, TEM measurements are consistent with SEM results. Fig. 4(C) exhibits two different types of flakes of MoS2 and bGr in contact with each other. The smaller nanosheets, MoS2, lay on the surface of larger bGr flakes. Interestingly, no nanosheet is observed isolated from flakes, thus evidencing the strong affinity of MoS2 nanosheet for bGr flakes due to their opposite polarity.
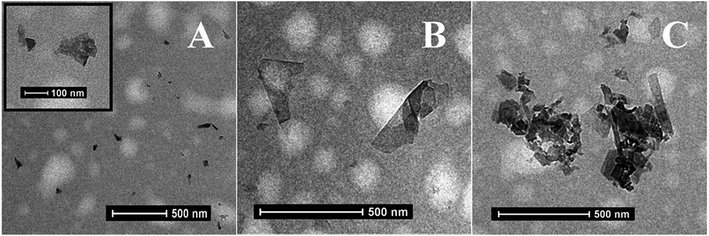 | ||
| Fig. 4 TEM images of MoS2 nanosheets (A) with higher resolution inset, bGr (B) and MoS2/bGr hybrids (C). | ||
The nanostructural and electronic properties of the exfoliated flakes of MoS2, bGr and the produced hybrids, were investigated using Raman and PL spectroscopy at room temperature, which allows the determination of number of layers in MoS2 and graphene. Moreover, Raman spectroscopy is a powerful tool to identify and quantify the quality of the MoS2/bGr interface, to analyze the effect of the heterostructuring on the characteristic features of the parent homostructures of MoS2 and graphene.41–43 Raman spectra are particularly sensitive to materials nanostructuring, since nanoconfinement produces changes in several Raman features, including frequency, intensity and selection rules in most of the materials.44–47 In addition, graphene and MoS2 have been extensively studied, and correlation of Raman features and nanostructuring are reported elsewhere.42–45,48
On excitation with wavelength of 514.5 nm, we collected the Raman spectra shown in Fig. 5. In the low frequency region, (Fig. 5) MoS2 homostructures exhibit two major Raman bands (A1g and E12g) at 406.2 cm−1 and 382.7 cm−1, respectively, which are significantly different from the starting bulk material.48,49 The wavenumber differences between the A1g and E12g (23.5 cm−1) is indicative of a nanostructuring corresponding to 3–4 layers.24 Upon heterostructuring with graphene, the Raman-active phonon modes provide information on the interaction between the two crystals. In particular, the Raman features of MoS2 are modified, with the out-of-plane mode, A1g peak upshifts by 1.4 cm−1, which gives the evidence for the quality of the interfacial contact, whereas E12g is slightly upshifted by 0.5 cm−1, which provides information about the in-plane strain. Furthermore, upon interfacing between the crystals, MoS2 Raman peaks are comparatively broader than those of its hybrid (Fig. 5).
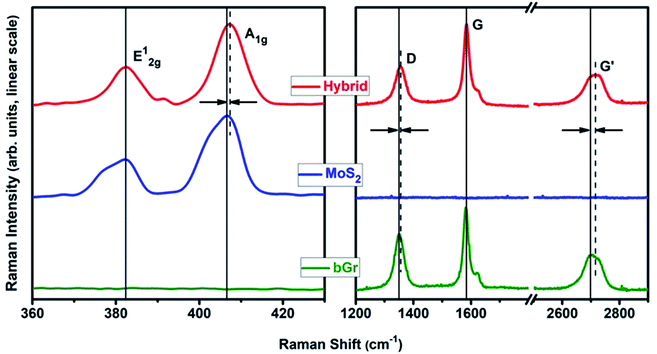 | ||
| Fig. 5 Raman spectra of MoS2, bGr and MoS2/bGr hybrid samples (excitation wavelength of 514 nm, 4 mW at the sample). | ||
The Raman spectra of bGr exhibits a D band at 1350 cm−1 (1354 cm−1, in MoS2/bGr hybrid), a G band at 1582 cm−1 (invariant in the hybrid), and a G′ band at 2700 cm−1 (shifted to 2714 cm−1 in the hybrid). The ratio between the G and G′ band and the shape of the G′ band indicates a bi-layered graphene for the homostructure.23 Thus, in case of MoS2/bGr hybrid structure, the vdW contact is well characterized by the upshift of 14 cm−1 of the G′ band of graphene. Upon interfacing the MoS2 and bGr sheets, the shape and symmetry of band changes, indicating a significant effect of vdW interactions and coupling between the layered materials.
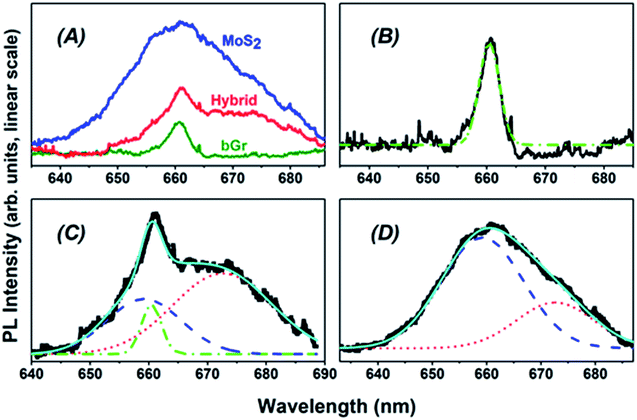
Another significant evidence of electronic coupling between MoS2 and bGr is also apparent from the PL measurements (Fig. 6(A–C)). Note worthily, upon excitation with 514.5 nm, graphene is not photoluminescent, and the band at 661 nm shown in Fig. 6(A) and in the inset of Fig. 6(C) corresponds to a multi-phonon Raman scattering peak of around 4300 cm−1, present both in the graphene homostructure and in MoS2/bGr hybrid when excited with 514.5 nm.50 On the contrary, MoS2 is photoluminescent, confirming the presence of monolayers in MoS2 flakes, which is due to the change from indirect to direct bandgap.51 In particular, in Fig. 6(B) we can identify the exciton (A) and trion (A−) contributions to the emitted band, centered at 659 and 673 nm, respectively.52 These correspond to the energy values of the emitted photon of 1.88 and 1.84 eV, respectively, in good agreement with previous measurement in MoS2 flakes.34
In case of MoS2/bGr hybrid (Fig. 6(C)), PL emission is strongly quenched (an overall <70%) due to the effect of interfacial charge transfer, between the layers of hybrids.41,53 Modifications are also observed in the shape of PL spectra of the hybrid structure, when compared with the parent homostructures. In fact, our analysis based on Gaussian best fits of the A and A− contributions points out that, while for the pure MoS2 flakes the former peak contains a signal nearly three times larger than the latter, in the case of the hybrid this ratio gets ≈0.4, indicating a much stronger quenching of PL for the exciton. Concluding this remark, it can be certainly confirmed a strong electronic interaction and interlayer coupling between the layers of 2D crystals in the MoS2/bGr hybrid structure.
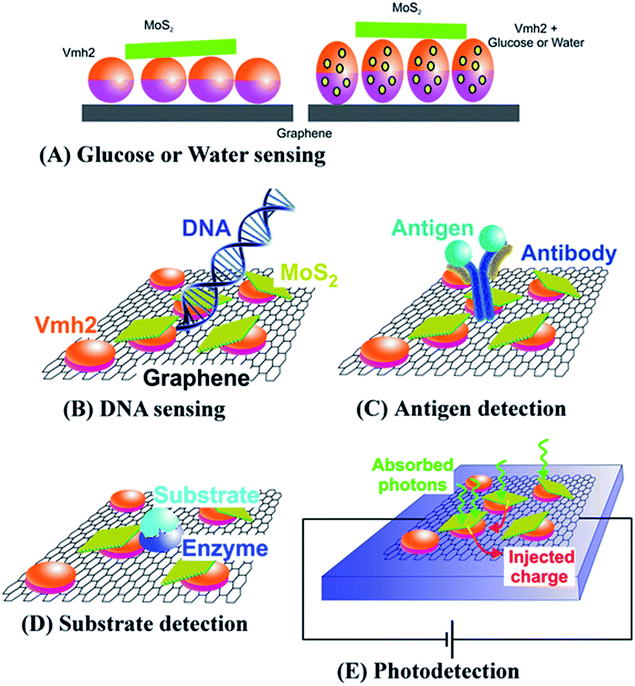
In Fig. 7, we propose some applications of the MoS2/bGr hybrid structures in biosensing and light sensing devices. It is well known that hydrophobin binds some molecules, like water54 or glucose,55 leading to the expansion of the interlayer space between the crystal layers of the hybrid, thus producing variation in the PL yield of MoS2. Therefore MoS2/bGr structure can be employed for water molecular sensing and glucose detection by exciting with an optical source. The principle of this application is shown in Fig. 7(A). Furthermore, as reported by Loan et al.,34 MoS2 and graphene are suitable components for the detection of specific DNA molecules, by exploiting PL quenching due to the presence of monolayered MoS2 flakes in the graphene/MoS2 heterostructure,49 as sketched in Fig. 7(B).
Additionally, in our hybrid system, the presence of Vmh2 hydrophobin makes graphene a bio-functionalized material. This provides the hybrid a biocompatible interface and enables non-covalent conjugation with other proteins, such as antibodies and enzymes, as depicted in Fig. 7(C and D).56,57 When attached to the MoS2/bGr hybrid, these proteins can recognize and bind specific molecules, which changes the PL signal of monolayered MoS2, thus providing a reliable optical method for biosensing applications.9,58 In Fig. 7(E), we illustrate the use of MoS2/bGr hybrid as a micro/nano photodetector. In this application, laser light would be efficiently absorbed by MoS2, thus likely injecting free carriers in the biased conductive graphene layers, which could produce a measurable photocurrent on supplying external bias.59,60
Conclusions
In this work, we have produced dispersions of few-layered, defect-free and luminescent sheets of MoS2, and bilayered biofunctionalized sheets of graphene with a self-assembling protein, i.e. the hydrophobin Vmh2, through the green route of scalable production. Vmh2 is adsorbed on the surface of the graphene sheets, which is used to tune the ζ-potential and the colloidal stability of the produced bGr dispersion. Then the nano-bio hybrid structures of MoS2/bGr are produced by mixing of the dispersions (after removing the unbound Vmh2 by post-centrifugation and removing the supernatant), which are quite stable in liquid phase and can also be drop-casted on various substrates for making 2D heterostructure based films and coatings. Absorption measurements estimate the final concentration for dispersions of bGr and MoS2, mean lateral size and mean thickness of the nanosheets of MoS2. DLS measurements confirm the mean lateral size of the nanosheets. ζ-Potential analysis is performed to estimate the surface potential of the nanosheets, which is negative for MoS2 and positive for bGr due to presence of Vmh2.SEM and TEM imaging exhibits the morphology and texture of the 2D materials based films. Through Raman spectroscopy, the electronic properties of the exfoliated crystals of MoS2 and bGr have been confirmed to that of the pristine materials. Moreover, significant modifications in the Raman features are observed in the MoS2/bGr heterostructure sample, with respect to their homostructures. In particular, shifts are observed in the peak frequencies of MoS2 in A1g peak by +1.4 cm−1 and an upshift of 14 cm−1 is observed in G′ band of hybrids compared with bGr. These are the signatures of electronic interlayer coupling and strain effect produced between layers of 2D structures, which lead to formation of vdW heterostructures. PL emission confirms the presence of monolayers in MoS2 flakes, which is quenched (>70%) due to the effect of interfacial charge transfer, between the layers of MoS2/bGr hybrid materials. Also, changes are observed in the shape of PL spectra other than quenching effect. Multi-phonon Raman scattering peak of graphene around 4300 cm−1 is observed in the spectral range of MoS2 PL emission.
Conclusively, we describe a green and scalable route of production of 2D materials based hybrid structures in which 2D materials layers are coupled by electrostatic vdW interactions. This technique of preparation of nanosheets is versatile, and can be easily extended to other 2D materials interfaced with various biomolecules for a wide range of applications from bioimaging and optoelectronics to optical biosensing. Moreover, this tool can be extended to the fabrication of innovative and application-specific nano-bio-hybrid systems with novel and tailored properties and multi-functionalities.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The research was supported through the project IENA (“Immobilization of Enzymes on hydrophobin-functionalized NAnomaterials”) within the framework of the University research program “Programma per il finanziamento della ricerca di Ateneo” D. R. n. 409 7/02/2017. The authors thank Dr Marinella Pirozzi of the Bioimaging Facility, Institute of Protein Biochemistry (CNR), Naples, for help with TEM experiments. The authors are also obliged to Prof. Lorenzo Marrucci for his financial support and Mr Manjot Singh, PhD student, for his assistance in the experimental protocols, both based at the Department of Physics “Ettore Pancini”, University of Naples “Federico II”, Naples.References
- R. F. Service, Science, 2015, 348, 490–492 CrossRef CAS PubMed.
- G. R. Bhimanapati, Z. Lin, V. Meunier, Y. Jung, J. Cha, S. Das, D. Xiao, Y. Son, M. S. Strano, V. R. Cooper, L. Liang, S. G. Louie, E. Ringe, W. Zhou, S. S. Kim, R. R. Naik, B. G. Sumpter, H. Terrones, F. Xia, Y. Wang, J. Zhu, D. Akinwande, N. Alem, J. A. Schuller, R. E. Schaak, M. Terrones and J. A. Robinson, ACS Nano, 2015, 9, 11509–11539 CrossRef CAS PubMed.
- A. C. Ferrari, F. Bonaccorso, V. Fal'ko, K. S. Novoselov, S. Roche, P. Bøggild, S. Borini, F. H. L. Koppens, V. Palermo, N. Pugno, J. A. Garrido, R. Sordan, A. Bianco, L. Ballerini, M. Prato, E. Lidorikis, J. Kivioja, C. Marinelli, T. Ryhänen, A. Morpurgo, J. N. Coleman, V. Nicolosi, L. Colombo, A. Fert, M. Garcia-Hernandez, A. Bachtold, G. F. Schneider, F. Guinea, C. Dekker, M. Barbone, Z. Sun, C. Galiotis, A. N. Grigorenko, G. Konstantatos, A. Kis, M. Katsnelson, L. Vandersypen, A. Loiseau, V. Morandi, D. Neumaier, E. Treossi, V. Pellegrini, M. Polini, A. Tredicucci, G. M. Williams, B. Hee Hong, J.-H. Ahn, J. Min Kim, H. Zirath, B. J. van Wees, H. van der Zant, L. Occhipinti, A. Di Matteo, I. A. Kinloch, T. Seyller, E. Quesnel, X. Feng, K. Teo, N. Rupesinghe, P. Hakonen, S. R. T. Neil, Q. Tannock, T. Löfwander and J. Kinaret, Nanoscale, 2015, 7, 4598–4810 RSC.
- K. S. Novoselov, V. I. Fal′ko, L. Colombo, P. R. Gellert, M. G. Schwab and K. Kim, Nature, 2012, 490, 192–200 CrossRef CAS PubMed.
- Z. Lin, A. McCreary, N. Briggs, S. Subramanian, K. Zhang, Y. Sun, X. Li, N. J. Borys, H. Yuan, S. K. Fullerton-Shirey, A. Chernikov, H. Zhao, S. McDonnell, A. M. Lindenberg, K. Xiao, B. J. LeRoy, M. Drndić, J. C. M. Hwang, J. Park, M. Chhowalla, R. E. Schaak, A. Javey, M. C. Hersam, J. Robinson and M. Terrones, 2D Mater., 2016, 3, 42001 CrossRef.
- L. Britnell, R. M. Ribeiro, A. Eckmann, R. Jalil, B. D. Belle, A. Mishchenko, Y. Kim, R. V Gorbachev, T. Georgiou, S. V Morozov, A. N. Grigorenko, A. K. Geim, C. Casiraghi, A. H. C. Neto and K. S. Novoselov, Science, 2013, 340, 1311–1314 CrossRef CAS PubMed.
- T. Georgiou, R. Jalil, B. D. Belle, L. Britnell, R. V. Gorbachev, S. V. Morozov, Y.-J. Kim, A. Gholinia, S. J. Haigh, O. Makarovsky, L. Eaves, L. A. Ponomarenko, A. K. Geim, K. S. Novoselov and A. Mishchenko, Nat. Nanotechnol., 2012, 8, 100–103 CrossRef PubMed.
- D. Jariwala, T. J. Marks and M. C. Hersam, Nat. Mater., 2016, 16, 170–181 CrossRef PubMed.
- R. Kurapati, K. Kostarelos, M. Prato and A. Bianco, Adv. Mater., 2016, 28(29), 6052–6074 CrossRef CAS PubMed.
- W. Xia, L. Dai, P. Yu, X. Tong, W. Song, G. Zhang and Z. M. Wang, Nanoscale, 2017, 13, 4324–4365 RSC.
- D. Pierucci, H. Henck, J. Avila, A. Balan, C. Naylor and G. Patriarche, et al., Nano Lett., 2016, 16, 4054–4061 CrossRef CAS PubMed.
- R. Liu, B. Liao, X. Guo, D. Hu, H. Hu, L. Du, H. Yu, G. Zhang, X. Yang and Q. Dai, Nanoscale, 2017, 9, 208–215 RSC.
- J. Shen, Y. He, J. Wu, C. Gao, K. Keyshar, X. Zhang, Y. Yang, M. Ye, R. Vajtai, J. Lou and P. M. Ajayan, Nano Lett., 2015, 15, 5449–5454 CrossRef CAS PubMed.
- J. Kang, V. K. Sangwan, J. D. Wood and M. C. Hersam, Acc. Chem. Res., 2017, 50, 943–951 CrossRef CAS PubMed.
- D. McManus, S. Vranic, F. Withers, V. Sanchez-Romaguera, M. Macucci, H. Yang, R. Sorrentino, K. Parvez, S.-K. Son, G. Iannaccone, K. Kostarelos, G. Fiori and C. Casiraghi, Nat. Nanotechnol., 2017, 12, 343–350 CrossRef CAS PubMed.
- J. Choi, H. Zhang, H. Du and J. H. Choi, ACS Appl. Mater. Interfaces, 2016, 8, 8864–8869 CAS.
- C. Backes, T. M. Higgins, A. Kelly, C. Boland, A. Harvey, D. Hanlon and J. N. Coleman, Chem. Mater., 2017, 29, 243–255 CrossRef CAS.
- A. Jawaid, D. Nepal, K. Park, M. Jespersen, A. Qualley, P. Mirau, L. F. Drummy and R. A. Vaia, Chem. Mater., 2016, 28, 337–348 CrossRef CAS.
- Y. Chen, C. Tan, H. Zhang and L. Wang, Chem. Soc. Rev., 2015, 44, 2681–2741 RSC.
- P. Cicatiello, P. Dardano, M. Pirozzi, A. M. Gravagnuolo, L. De Stefano and P. Giardina, Biotechnol. Bioeng., 2017, 9999, 1–14 Search PubMed.
- K. Yang, Y. Li, X. Tan, R. Peng and Z. Liu, Small, 2013, 9, 1492–1503 CrossRef CAS PubMed.
- T. S. Sreeprasad and V. Berry, Small, 2013, 9, 341–350 CrossRef CAS PubMed.
- A. M. Gravagnuolo, E. Morales-Narváez, S. Longobardi, E. T. Da Silva, P. Giardina and A. Merkoçi, Adv. Funct. Mater., 2015, 25, 2771–2779 CrossRef CAS.
- J. Kaur, A. M. Gravagnuolo, P. Maddalena, C. Altucci, P. Giardina and F. Gesuele, RSC Adv., 2017, 7, 22400–22408 RSC.
- M. Siepi, E. Morales-Narváez, N. Domingo, D. M. Monti, E. Notomista and A. Merkoçi, 2D Mater., 2017, 4, 035007 CrossRef.
- A. M. Gravagnuolo, E. Morales-Narváez, C. R. S. Matos, S. Longobardi, P. Giardina and A. Merkoçi, Adv. Funct. Mat., 2015, 25, 6084–6092 CrossRef CAS.
- P. Laaksonen, M. Kainlauri, T. Laaksonen, A. Shchepetov, H. Jiang, J. Ahopelto and M. B. Linder, Angew. Chem., Int. Ed., 2010, 49, 4946–4949 CrossRef CAS PubMed.
- V. Aimanianda, J. Bayry, S. Bozza, O. Kniemeyer, K. Perruccio, S. R. Elluru, C. Clavaud, S. Paris, A. A. Brakhage, S. V. Kaveri, L. Romani and J. P. Latgé, Nature, 2009, 460, 1117–1121 CrossRef CAS.
- A. M. Gravagnuolo, S. Longobardi, A. Luchini, M. S. Appavou, L. De Stefano, E. Notomista, L. Paduano and P. Giardina, Biomacromolecules, 2016, 17, 954–964 CrossRef CAS PubMed.
- D. L. Cheung, Langmuir, 2012, 28, 8730–8736 CrossRef CAS PubMed.
- S. Longobardi, D. Picone, C. Ercole, R. Spadaccini, L. De Stefano, I. Rea and P. Giardina, Biomacromolecules, 2012, 13, 743–750 CrossRef CAS PubMed.
- J. Y. Kwak, J. Hwang, B. Calderon, H. Alsalman, N. Munoz, B. Schutter and M. G. Spencer, Nano Lett., 2014, 14, 4511–4516 CrossRef CAS PubMed.
- S. Rathi, I. Lee, D. Lim, J. Wang, Y. Ochiai and N. Aoki, Nano Lett., 2015, 15, 5017–5024 CrossRef CAS PubMed.
- P. T. K. Loan, W. Zhang, C. T. Lin, K. H. Wei, L. J. Li and C. H. Chen, Adv. Mater., 2014, 26, 4838–4844 CrossRef CAS PubMed.
- C. Backes, R. J. Smith, N. Mcevoy, N. C. Berner, D. Mccloskey, H. C. Nerl, A. O. Neill, P. J. King, T. Higgins, D. Hanlon, N. Scheuschner, J. Maultzsch, L. Houben, G. S. Duesberg, J. F. Donegan, V. Nicolosi and J. N. Coleman, Nat. Commun., 2014, 5, 4576 CAS.
- M. Lotya, Y. Hernandez, P. J. King, R. J. Smith, V. Nicolosi, L. S. Karlsson, F. M. Blighe, S. De, W. Zhiming, I. T. McGovern, G. S. Duesberg and J. N. Coleman, J. Am. Chem. Soc., 2009, 131, 3611–3620 CrossRef CAS PubMed.
- M. Lotya, A. Rakovich, J. F. Donegan and J. N. Coleman, Nanotechnology, 2013, 24, 265703–265709 CrossRef PubMed.
- K. Zhou, F. Withers, Y. Cao, S. Hu, G. Yu and C. Casiraghi, ACS Nano, 2014, 8, 9914–9924 CrossRef CAS PubMed.
- Q. Liu, B. Cook, M. Gong, Y. Gong, D. Ewing, M. Casper, A. Stramel and J. Wu, ACS Appl. Mater. Interfaces, 2017, 9, 12728–12733 CAS.
- K. Singh, S. Kumar, K. Agarwal, K. Soni, V. R. Gedela and K. Ghosh, Sci. Rep., 2017, 7, 1–12 CrossRef PubMed.
- A. C. Ferrari, J. C. Meyer, V. Scardaci, C. Casiraghi, M. Lazzeri, S. Piscanec, K. S. Novoselov, S. Roth and A. K. Geim, Phys. Rev. Lett., 2006, 97, 187401 CrossRef CAS PubMed.
- H. Li, Q. Zhang, C. C. R. Yap, B. K. Tay, T. H. T. Edwin, A. Olivier and D. Baillargeat, Adv. Funct. Mater., 2012, 22, 1385–1390 CrossRef CAS.
- L. Sirleto, A. Vergara and M. A. Ferrara, Adv. Opt. Photonics, 2017, 9, 169 CrossRef.
- A. Jorio, R. Saito, G. Dresselhaus and M. S. Dresselhaus, Raman Spectroscopy in Graphene Related Systems, 2011 Search PubMed.
- M. Alfè, V. Gargiulo, R. Di Capua, F. Chiarella, J.-N. Rouzaud, A. Vergara and A. Ciajolo, ACS Appl. Mater. Interfaces, 2012, 4, 4491–4498 Search PubMed.
- U. Coscia, G. Ambrosone, F. Gesuele, V. Grossi, V. Parisi, S. Schutzmann and D. K. Basa, Appl. Surf. Sci., 2007, 254(4), 984–988 CrossRef CAS.
- J. Kaur, J. Shah, R. K. Kotnala and K. C. Verma, Ceram. Int., 2012, 38(7), 5563–5570 CrossRef CAS.
- C. Lee, H. Yan, L. Brus, T. Heinz, J. Hone and S. Ryu, ACS Nano, 2010, 4, 2695–2700 CrossRef CAS PubMed.
- X. Zhang, X. Qiao, W. Shi, J. Wu, D. Jiang and P. Tan, Chem. Soc. Rev., 2015, 44, 2757–2785 RSC.
- A. Splendiani, L. Sun, Y. Zhang, T. Li, J. Kim, C. Chim, G. Galli and F. Wang, Nano Lett., 2010, 10, 1271–1275 CrossRef CAS PubMed.
- K. F. Mak, C. Lee, J. Hone, J. Shan and T. Heinz, Phys. Rev. Lett., 2010, 105, 136805 CrossRef PubMed.
- K. F. Mak, K. He, C. Lee, G. H. Lee, J. Hone, T. F. Heinz and J. Shan, Nat. Mater., 2013, 12, 207–211 CrossRef CAS PubMed.
- D. Pierucci, H. Henck, C. H. Naylor, H. Sediri, E. Lhuillier, A. Balan, J. E. Rault, Y. J. Dappe, F. Bertran, P. Le Fèvre, A. T. C. Johnson and A. Ouerghi, Sci. Rep., 2016, 6, 26656 CrossRef CAS PubMed.
- J. Tao, Y. Wang, Y. Xiao, P. Yao, C. Chen, W. Pang, H. Yang, D. Sun, Z. Wang and L. Jing, Carbon N. Y., 2017, 116, 695–702 CrossRef CAS.
- B. Della Ventura, I. Rea, A. Caliò, P. Giardina, A. M. Gravagnuolo, R. Funari, C. Altucci, R. Velotta and L. De Stefano, Appl. Surf. Sci., 2016, 364, 201–207 CrossRef CAS.
- L. De Stefano, I. Rea, E. De Tommasi, I. Rendina, L. Rotiroti, M. Giocondo, S. Longobardi, A. Armenante and P. Giardina, Eur. Phys. J. E, 2009, 30, 181–185 CrossRef CAS PubMed.
- S. Longobardi, A. M. Gravagnuolo, I. Rea, L. De Stefano, G. Marino and P. Giardina, Anal. Biochem., 2014, 449, 9–16 CrossRef CAS PubMed.
- I. Song, C. Park and H. C. Choi, RSC Adv., 2015, 5, 7495–7514 RSC.
- H. Xu, J. Wu, Q. Feng, N. Mao, C. Wang and J. Zhang, Small, 2014, 10, 2300–2306 CrossRef CAS PubMed.
- Z. Huang, W. Han, T. Hongli, R. Long, C. D. Sathish, X. Qi and H. Zhang, 2D Mater., 2015, 2, 35011 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7ra09878b |
| This journal is © The Royal Society of Chemistry 2017 |