Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Regioselective alkylation of carbohydrates and diols: a cheaper iron catalyst, new applications and mechanism†
Bo Ren *,
Ningning Yan and
Lu Gan
*,
Ningning Yan and
Lu Gan
College of Chemistry & Chemical Engineering, Xinyang Normal University, Nanhu Road 237, Xinyang, Henan 464000, P. R. China. E-mail: renbo@xynu.edu.cn
First published on 28th September 2017
Abstract
As an extension of our previous research on the regioselective protection of carbohydrates and diols, we developed an iron catalyst, Fe(dibm)3 (dibm = diisobutyrylmethane), which has an unusually broad catalytic scope in the selective monoalkylation of diols and carbohydrates. However, even though the iron reagent is green, the cost to prepare the catalyst is high, which is not conducive to large-scale application, and the mechanism of the catalytic reaction is still not completely clear. In this study, we have developed a much cheaper iron catalyst, Fe(dipm)3 (dipm = dipivaloylmethane), which has a similar catalytic efficiency in the regioselective alkylation of carbohydrates and diols. The mechanism of the iron-catalyzed selective alkylation was also studied and a new detailed reaction mechanism for the iron catalysis was proposed.
Introduction
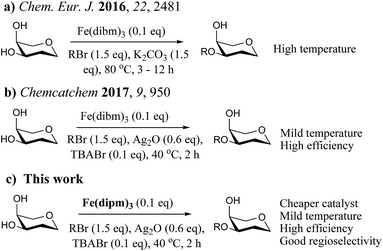
Regioselective protection is very important in organic chemistry especially in carbohydrate chemistry.1–5 Regioselective alkylation is always the most important reaction in the synthesis of oligosaccharide building blocks due to the stability of alkyl groups.6–11 Many metal compounds and organic small molecules have been employed in regioselective alkylation methods, including tin(IV)-,12–16 boron(IV)-,17,18 silver(I)-,19,20 and nickel(II)-based complexes21 as well as the iron catalyst that we have reported.22,23 Organotin compounds were the most widely used reagents for selective alkylation reactions for many years, but their use must be limited due to their potential toxicity.24,25 Taylor's group developed the organoboron catalyzed regioselective alkylation of carbohydrates, which is a good way to avoid the use of a toxic catalyst. However, the scope this reaction limited its applicability.17,18 Methods that use metal salts can only achieve good selectivity for a small subset of substrates.19–21In our previous study, we reported a Fe(dibm)3 (dibm, diisobutyrylmethane) catalyst that has an unusually broad substrate scope in the selective alkylation of carbohydrates, diols and polyols (Scheme 1a).22 This reaction could be used to completely replace the toxic organotin reagents in the regioselective protection of carbohydrates and polyols since iron is less expensive and non-toxic.26,27 Because this method is conducted at high temperature, we initially developed iron-catalyzed regioselective alkylation reactions with Ag2O and tetrabutylammonium bromide (TBAB) as co-catalysts, which can efficiently proceed to completion in 2–3 hours at 40 °C (Scheme 1b).23 Although iron is cheap and abundant, the organic ligand dibm is very expensive, which is an obstacle in the synthesis of this high-efficiency catalyst. We have been aware of this problem for a long time, and the key to solving this problem is to clarify the reaction mechanism to design a cheaper and more efficient iron catalyst.
After many control experiments, we successfully found a much cheaper iron catalyst, Fe(dipm)3 (dipm = dipivaloylmethane), which costs 80% less than the Fe(dibm)3 catalyst (Scheme 1c) but has almost the same catalytic efficiency. To the best of our knowledge, this is one of the cheapest iron catalyst that can replace the excellent Fe(dibm)3 catalyst in the regioselective alkylation of carbohydrates and polyols. It can also take the place of organotin catalysts and could be used on a large scale.
Results and discussion
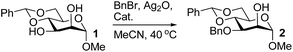
Organotin reagents have been studied for a long time, and their mechanism in regioselective reactions has been hypothesized to be controlled by complex stannylene dimer or polymer structures.16,28 In 2012, Dong's group predicted that any reagent that could form cyclic dioxolane-type intermediates with hydroxyl groups could achieve similar selectivities to the organotin reagents.15 One of the best examples is the organoboron catalyst developed by Taylor's group.17,18 Another example may be the Fe(dibm)3 catalyst we developed, which shows excellent regioselectivities in selective alkylations. We have only proposed that the iron catalyst could form cyclic dioxolane/dioxane-type intermediates with the hydroxyl groups like the organotin reagents, but we have not explained how it forms the intermediates or why other iron reagents could not achieve similar regioselectivities.First we chose methyl 4,6-O-benzylidene-α-D-mannopyranoside, 1, as the substrate to carefully verify some of our hypotheses and found a better and cheaper iron catalyst (Table 1). Thus, 1 was treated with 1.1 equiv. of BnBr and 0.6 equiv. of Ag2O in the presence of a catalyst and 0.1 equiv. of TBAB as a co-catalyst in acetonitrile at 40 °C for 2–3 h. The isolated yields were low when FeCl3 and FeBr3 were used as the catalysts (Table 1, entries 2–3), which is consistent with previous reports.22 To our delight, when Fe(acac)3 was used as the catalyst, 3-O-benzylated mannoside, 2, was isolated in 90% yield (Table 1, entry 6). This is in contrast with our previous reports making us wonder whether the ligands affected the catalysis efficiency. To test this hypothesis, Zn(acac)2 and Cu(acac)2 were used as catalysts. To our surprise, product 2 was isolated in yields near 90% (Table 1, entries 4–5). Then we changed the ligands of iron catalysts. We then decided to test other ligands on iron. We made or bought three iron catalysts, Fe(dbzm)3 (Fe(dbzm)3 = tris(dibenzoylmethanato)iron), Fe(dibm)3, and Fe(dipm)3 (dipm = dipivaloylmethane), all of which were found to catalyze the reaction with good regioselectivity (Table 1, entries 7–10). To test whether the ligands can catalyze the reaction, four 1,3-diketone ligands (benzoylacetone, acetylacetone, dibm and dipm) were also tested as catalysts. To our surprise, all the ligands reacted with the alkylation reagents, so the isolated yields were near 50%, which is the same as it is without any catalyst (Table 1, entries 11–15).
| Entry | Various conditions | Isolated yield (%) |
|---|---|---|
| a Reactant (50 mg), BnBr (1.1 eq.), cat. (0.05–0.1 eq.), Ag2O (0.6 eq.), TBAB (0.1 eq.), MeCN (1 mL), 2–3 h.b Poor or no selectivity.c Low conversion (5–10% of 2). | ||
| 1 | Optimized conditiona | 93 |
| 2 | 0.1 eq. FeCl3 | 70b |
| 3 | 0.1 eq. FeBr3 | 65b |
| 4 | 0.1 eq. Zn(acac)2 | 90 |
| 5 | 0.1 eq. Cu(acac)2 | 91 |
| 6 | 0.1 eq. Fe(acac)3 | 90 |
| 7 | 0.1 eq. Fe(dbzm)3 | 90 |
| 8 | 0.1 eq. Fe(dibm)3 | 92 |
| 9 | 0.1 eq. Fe(dipm)3 | 93 |
| 10 | 0.05 eq. Fe(dipm)3 | 88 |
| 11 | 0.1 eq. benzoylacetone | 40b |
| 12 | 0.1 eq. acetylacetone | 42b |
| 13 | 0.1 eq. dibm | 50b |
| 14 | 0.1 eq. dipm | 50b |
| 15 | Without cat. | 50b |
| 16 | Without Ag2O | —c |
| 17 | Reaction at rt | 70b |
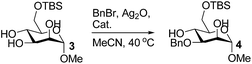
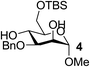
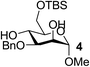
On the chance that substrate 1 was not representative of all carbohydrates, we changed to methyl 6-O-(tert-butyldimethylsilyloxy)-α-D-mannopyranoside, 3, as the substrate to verify our hypothesis (Table 2). To our amazement, when 3 was subjected to the same reaction conditions, the three synthesized or purchased iron catalysts, Fe(dbzm)3, Fe(dibm)3, and Fe(dipm)3, all afforded isolated yields of the desired product near 90% (Table 2, entries 7–10). However, when Fe(dbzm)3 was used as the catalyst, some benzoylated carbohydrate by-product was observed. All other catalysts afforded poor regioselectivities. Therefore, only the Fe(dipm)3 catalyst has the same catalytic efficiency as what has been reported for Fe(dibm)3. In addition, a big advantage is that the dipm ligand cost 20% as much as the dibm ligand, which makes the catalyst easier to acquire.
| Entry | Various conditions | Isolated yield (%) |
|---|---|---|
| a Reactant (50 mg), BnBr (1.1 eq.), cat. (0.05–1.0 eq.), Ag2O (0.6 eq.), TBAB (0.1 eq.), MeCN (1 mL), 2–3 h.b Poor or no selectivity.c Low conversion (5–10% of 4). | ||
| 1 | Optimized conditiona | 91 |
| 2 | 0.1 eq. FeCl3 | 60b |
| 3 | 0.1 eq. FeBr3 | 55b |
| 4 | 0.1 eq. Zn(acac)2 | 53b |
| 5 | 0.1 eq. Cu(acac)2 | 54b |
| 6 | 0.1 eq. Fe(acac)3 | 52b |
| 7 | 0.1 eq. Fe(dbzm)3 | 85 |
| 8 | 0.1 eq. Fe(dibm)3 | 91 |
| 9 | 0.1 eq. Fe(dipm)3 | 91 |
| 10 | 0.05 eq. Fe(dipm)3 | 88 |
| 11 | 0.1 eq. benzoylacetone | 40b |
| 12 | 0.1 eq. acetylacetone | 42b |
| 13 | 0.1 eq. dibm | 51b |
| 14 | 0.1 eq. dipm | 53b |
| 15 | Without cat. | 50b |
| 16 | Without Ag2O | —c |
| 17 | Reaction at rt | 61b |
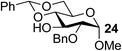
To test the catalytic efficiency and substrate scope of the Fe(dibm)3 catalyst, this new method was further tested with cis-carbohydrates and with other alkylating agents (Table 3). For all the tested substrates, the equatorial hydroxyl group was selectively alkylated in isolated yields of 80–98% in 2–3 hours. Substrates 1 and 3 were alkylated with different alkylating reagents in good isolated yields (Table 3, entries 1–10). To verify the catalytic efficiency of Fe(dibm)3, we treated substrates 1 and 3 with K2CO3 as the base in 80 °C, as we have previously reported;22 both desired products were isolated yields of 89% (Table 3, entries 2 and 8). The unprotected glycosides were alkylated in a mixed solvent system (MeCN–DMF, 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) due to their poor solubility in MeCN alone (Table 3, entries 14–16). All the tests showed that the Fe(dipm)3 catalyst had almost the same catalytic efficiency as has been reported for Fe(dibm)3.
1) due to their poor solubility in MeCN alone (Table 3, entries 14–16). All the tests showed that the Fe(dipm)3 catalyst had almost the same catalytic efficiency as has been reported for Fe(dibm)3.
| Entry | Conditions | Reactant | Product | Isolated yield (%) |
|---|---|---|---|---|
| a Reaction conditions: (A) 50 mg substrate, 1 mL MeCN, 0.1 eq. Fe(dipm)3, 0.6 eq. Ag2O, 0.1 eq. TBAB, 1.1 eq. BnBr/PMBCl/4-Br-BnBr/allyl bromide, 40 °C, 2 h. (B) 50 mg substrate, 1 mL MeCN, 0.1 eq. Fe(dipm)3, 1.5 eq. K2CO3, 1.1 eq. BnBr, 80 °C, 12 h. (C) 50 mg substrate, 1 mL MeCN + 0.1 mL DMF, 0.1 eq. Fe(dipm)3, 0.6 eq. Ag2O, 0.1 eq. TBAB, 1.1 eq. BnBr, 40 °C, 3 h. | ||||
| 1 | A | 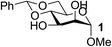 |
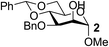 |
93 |
| 2 | B | 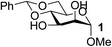 |
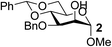 |
89 |
| 3 | A | 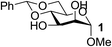 |
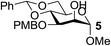 |
98 |
| 4 | A | 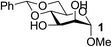 |
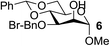 |
94 |
| 5 | A | 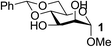 |
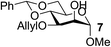 |
94 |
| 6 | A | 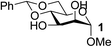 |
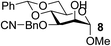 |
85 |
| 7 | A | 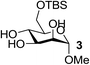 |
 |
91 |
| 8 | B | 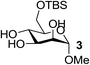 |
 |
89 |
| 9 | A | 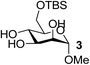 |
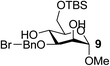 |
82 |
| 10 | A | 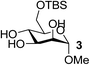 |
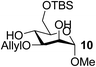 |
92 |
| 11 | A | 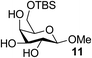 |
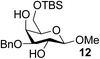 |
94 |
| 12 | A | 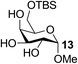 |
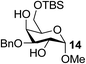 |
91 |
| 13 | A | 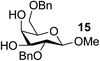 |
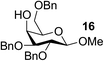 |
91 |
| 14 | C | 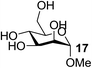 |
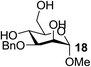 |
80 |
| 15 | C | 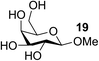 |
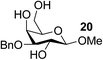 |
90 |
| 16 | C | 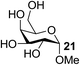 |
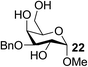 |
85 |
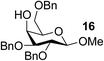
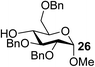
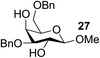
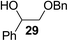
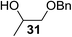
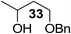
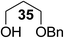
The Fe(dipm)3 catalyst was further used in the alkylation of 1,2- or 1,3-diols and carbohydrates with other configurations (Table 4). For the glycosides containing 1,3-diols (23 and 25) and non-carbohydrate 1,2- and 1,3-diols (28, 30, 32, and 34), the terminal hydroxyl group was alkylated in 75–93% isolated yields (Table 4, entries 1, 2 and 4–7). Thus, we can selectively alkylate the primary hydroxyl groups in 1,2- or 1,3-diols with the optimized conditions. As reported, trans-carbohydrates were not isolated in good yields with Ag2O (Table 4, entry 8).17 Then, we tested replacing the organotin reagent with Fe(dipm)3 in the production of dibenzylated carbohydrate 27, which was obtained in 81% isolated yield (Table 4, entry 3).14 All tests showed that this method could achieve isolated yields that were just as high as those obtained with Fe(dibm)3 and may be able to replace the toxic organotin reagents in the future.
| Entry | Conditions | Reactant | Product | Isolated yield (%) |
|---|---|---|---|---|
| a Reaction conditions: (A) 50 mg substrate, 1 mL MeCN, 0.1 eq. Fe(dipm)3, 0.6 eq. Ag2O, 0.1 eq. TBAB, 1.1 eq. BnBr/PMBCl/4-Br-BnBr/allyl bromide, 40 °C, 3 h. (D) (1) 50 mg substrate, 1 mL MeCN, 0.1 eq. Fe(dipm)3, 0.6 eq. Ag2O, 0.1 eq. TBAB, 1.1 eq. BnBr, 40 °C, 3 h. (2) Add 1.5 eq. NaOH and 1.5 eq. BnBr, 40 °C, 3 h. | ||||
| 1 | A | 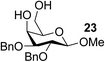 |
 |
86 |
| 2 | A | 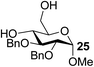 |
 |
78 |
| 3 | D | 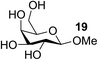 |
 |
81 |
| 4 | A | 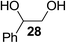 |
 |
75 |
| 5 | A | 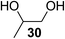 |
 |
93 |
| 6 | A | 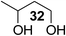 |
 |
86 |
| 7 | A | 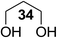 |
 |
90 |
| 8 | A | 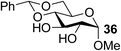 |
 |
64 |
In our previous report, we only proposed that a cyclic dioxolane-type intermediate with two hydroxyl groups plays a key role in the catalytic cycle. However, this was over simplified, and there was little evidence to support such a mechanism. Additionally, we did not address the formation of the intermediates and why similar regioselectivities could not be achieved with other iron reagents.
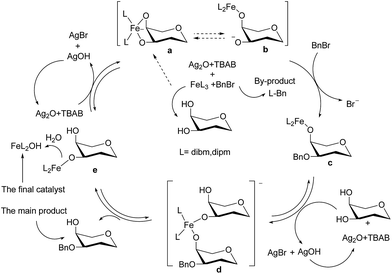
To elucidate the mechanism and its key controlling factors, we conducted many control experiments. Initially, we speculated that the 1,3-diketone ligands may be the key factor controlling the efficiency of this catalyst. However, the control experiments showed that the ligands did not catalyzed the reaction; they the simply reacted with the BnBr under basic conditions (Fig. S2†), and we realized that this was the reason other iron catalysts were not delivering the same regioselectivities. We always thought the Fe(dibm)3 catalyst could be recycled like organoboron catalysts, meaning the Fe(dibm)3 catalyst was not changed during the reaction. However, our recycling experiments were puzzling; the catalyst could only be recycled 2–3 times, and the isolated yield dropped with every cycle. The only reasonable explanation is that the catalyst had changed or degraded in some way. Therefore, we believe that BnBr reacted with the ligand on the Fe(dibm)3 or Fe(dibm)3 catalysts, which caused the iron catalyst to form a cyclic dioxolane intermediate with adjacent hydroxyl groups. Each time the iron catalyst went through the catalytic cycle, it was sequentially converted from Fe(L)3 to Fe(L)2OH, FeL(OH)2 and Fe(OH)3, which caused its catalytic efficiency to decrease after 2–3 cycles because it could no longer form the cyclic dioxolane intermediates with the substrates. This also explains why we could not isolate the stable cyclic dioxolane intermediates, as was possible with the organotin reagents. BnBr immediately reacted with the intermediate after activation of the iron catalyst. This theory also explains why other iron catalysts did not perform as well in the catalytic regioselective alkylation of carbohydrates. Some ligands, such as benzoylacetone and acetylacetone, are not stable under these reaction conditions; they are easily decomposed by the higher temperatures, or the ligand is consumed by reaction with the alkylation reagents. In contrast, some ligands are too stable to activate the catalyst. Only the appropriate ligands, such as dibm and dipm, can properly activate the reaction; they are neither too stable nor too reactive, which ensures the iron catalyst is activated and can be recycled.
Therefore, we proposed the detailed mechanism for the Fe(dibm)3- or Fe(dibm)3-catalyzed reactions shown in (Scheme 2). First, BnBr reacted with the ligand on the iron catalyst, and at the same time, the cyclic dioxolane intermediate a formed between the substrate and Fe+(L)2. Intermediate a then reacted with BnBr to form c, and then, c transferred the iron catalyst to another substrate as it proceeded to form d via the deprotonation of the hydroxyl group by Ag2O and TBAB. The main alkylated product was released during the conversion of d to e, and finally, e was converted to a by deprotonation with Ag2O and TBAB. After the completion of the reaction, the iron catalyst was turned into Fe(L)2OH by residual water in silica gel during flash column chromatography.
Conclusions
We successfully found a much cheaper iron catalyst, Fe(dipm)3 (dipm = dipivaloylmethane), which costs only 20% as much as Fe(dibm)3 but has almost the same catalytic efficiency. To the best of our knowledge, this is one of the cheapest iron catalyst that can replace the excellent Fe(dibm)3 catalyst in regioselective alkylations of carbohydrates and polyols. It can also take the place of organotin catalysts, and it could be used on a large scale. After carefully studying these reactions, we elucidated the mechanism, which can explain all the unusual results in the study.Experimental section
General
All commercially available starting materials and solvents were of reagent grade and used without further purification. Chemical reactions were monitored with thin-layer chromatography using precoated silica gel 60 (0.25 mm thickness) plates. 1H NMR and 13C NMR spectra were recorded using a Bruker Avance 400 instrument or a Bruker DMX 500 instrument at 298 K in CDCl3, using the residual signals from CHCl3 (1H: δ = 7.25 ppm; 13C: δ = 77.2 ppm) as the internal standard. 1H NMR peak assignments were made by first-order analysis of the spectra, supported by standard 1H–1H NMR correlation spectroscopy (COSY).General method for regioselective alkylation of diols and polyols
(1) The substrates (50 mg) were allowed to react with RX (alkylation reagents) (1.1 eq.) in dry acetonitrile (1 mL) or a mixed solvent (MeCN/DMF: 10/1) at 40 °C for 2–3 h in the presence of Ag2O (0.6 eq.), TBAB (0.1 eq.) and Fe(dipm)3 (0.1 eq.). The reaction mixtures were filtered and directly purified by flash column chromatography (hexanes/EtOAc = 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 1
1 to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to afford the pure products.
1) to afford the pure products.
(2) Diol and polyol reactants (50 mg) were allowed to react with RX (alkylation reagents) (1.1 eq.) in 1 mL of dry acetonitrile at 80 °C for 8 h, in the presence of Fe(dipm)3 (0.1 eq.) and K2CO3 (1.5 eq.). After cooling and evaporating the solvent, the reaction mixture was directly purified by flash column chromatography (hexanes–EtOAc 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 1
1 to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), affording the pure products.
1), affording the pure products.
The procedure for producing the Fe(dibm)3 catalyst was reported in ref. 29. In addition, the Fe(dipm)3 catalyst was prepared by the same procedure as was used for Fe(dibm)3.
Spectroscopic data of the known products were in accordance with those reported in the literature.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This study was supported by the Henan Key Scientific Research Project (18A150015) and the Nanhu Scholars Program for Young Scholars of XYNU. This study was also supported by the Doctoral Research Foundation of Xinyang Normal University (Grant Number: 16070). The authors also thank Dr Shitao Fu of HUST for support with the NMR instruments.References
- C. Wang, J. Lee, S. Luo, S. S. Kulkarni, Y. Huang, C. Lee, K. Chang and S. Hung, Nature, 2007, 446, 896–899 CrossRef CAS PubMed.
- G. Xiao, G. A. Cintron-Rosado, D. A. Glazier, B. Xi, C. Liu, P. Liu and W. Tang, J. Am. Chem. Soc., 2017, 139, 4346–4349 CrossRef CAS PubMed.
- P. Peng, M. Linseis, R. F. Winter and R. R. Schmidt, J. Am. Chem. Soc., 2016, 138, 6002–6009 CrossRef CAS PubMed.
- B. Ren, M. Rahm, X. L. Zhang, Y. X. Zhou and H. Dong, J. Org. Chem., 2014, 79, 8134–8142 CrossRef CAS PubMed.
- K. L. M. Hoang and X. Liu, Nat. Commun., 2014, 5, 5051–5060 CrossRef CAS PubMed.
- Y. Park, K. C. Harper, N. Kuhl, E. E. Kwan, R. Y. Liu and E. N. Jacobsen, Science, 2017, 355, 162–166 CrossRef CAS PubMed.
- Y. Wu, D. Xiong, S. Chen, Y. Wang and X. Ye, Nat. Commun., 2017, 8, 14851 CrossRef CAS PubMed.
- Q. Zhang, E. R. van Rijssel, M. T. C. Walvoort, H. S. Overkleeft, G. A. van der Marel and J. D. C. Codée, Angew. Chem., Int. Ed., 2015, 54, 7670–7673 CrossRef CAS PubMed.
- D. Schmidt, F. Schuhmacher, A. Geissner, P. H. Seeberger and F. Pfrengle, Chem.–Eur. J., 2015, 21, 5709–5713 CrossRef CAS PubMed.
- D. Zhu, K. N. Baryal, S. Adhikari and J. Zhu, J. Am. Chem. Soc., 2014, 136, 3172–3175 CrossRef CAS PubMed.
- P. Peng and R. R. Schmidt, Acc. Chem. Res., 2017, 50, 1171–1183 CrossRef CAS PubMed.
- M. Giordano and A. Iadonisi, J. Org. Chem., 2014, 79, 213–222 CrossRef CAS PubMed.
- H. Xu, Y. Lu, Y. Zhou, B. Ren, Y. Pei, H. Dong and Z. Pei, Adv. Synth. Catal., 2014, 356, 1735–1740 CrossRef CAS.
- Y. Zhou, J. Li, Y. Zhan, Z. Pei and H. Dong, Tetrahedron, 2013, 69, 2693–2700 CrossRef CAS.
- H. Dong, Y. Zhou, X. Pan, F. Cui, W. Liu, J. Liu and O. Ramström, J. Org. Chem., 2012, 77, 1457–1467 CrossRef CAS PubMed.
- T. B. Grindley, Adv. Carbohydr. Chem. Biochem., 1998, 53, 17–142 CrossRef CAS PubMed.
- D. Lee, C. L. Williamson, L. Chan and M. S. Taylor, J. Am. Chem. Soc., 2012, 134, 8260–8267 CrossRef CAS PubMed.
- L. Chan and M. S. Taylor, Org. Lett., 2011, 13, 3090–3093 CrossRef CAS PubMed.
- B. Ren, M. Wang, J. Liu, J. Ge and H. Dong, ChemCatChem, 2015, 7, 761–765 CrossRef CAS.
- S. Malik, V. A. Dixit, P. V. Bharatam and K. P. R. Kartha, Carbohydr. Res., 2010, 345, 559–564 CrossRef CAS PubMed.
- U. B. Gangadharmath and A. V. Demchenko, Synlett, 2004, 2191–2193 CAS.
- B. Ren, O. Ramstrom, Q. Zhang, J. Ge and H. Dong, Chem.–Eur. J., 2016, 22, 2481–2486 CrossRef CAS PubMed.
- B. Ren, J. Lv, Y. Zhang, J. Tian and H. Dong, ChemCatChem, 2017, 9, 950–953 CrossRef CAS.
- S. M. Jenkins, K. Ehman and S. Barone, Dev. Brain Res., 2004, 151, 1–12 CrossRef CAS PubMed.
- M. M. Whalen, B. G. Loganathan and K. Kannan, Environ. Res., 1999, 81, 108–116 CrossRef CAS PubMed.
- I. Bauer and H. J. Knolker, Chem. Rev., 2015, 115, 3170–3387 CrossRef CAS PubMed.
- B. D. Sherry and A. Fuerstner, Acc. Chem. Res., 2008, 41, 1500–1511 CrossRef CAS PubMed.
- S. David and A. Thiéffry, Tetrahedron Lett., 1981, 22, 2647–2650 CrossRef CAS.
- J. C. Lo, J. Gui, Y. Yabe, C. Pan and P. S. Baran, Nature, 2014, 516, 343–348 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7ra10220h |
| This journal is © The Royal Society of Chemistry 2017 |