Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Mesoporous Ag1(NH4)2PW12O40 heteropolyacids as effective catalysts for the esterification of oleic acid to biodiesel†
Qiu-yun Zhang *abc,
Fang-fang Weiac,
Qian Lia,
Jin-shu Huanga,
Yun-mei Fenga and
Yu-tao Zhanga
*abc,
Fang-fang Weiac,
Qian Lia,
Jin-shu Huanga,
Yun-mei Fenga and
Yu-tao Zhanga
aSchool of Chemistry and Chemical Engineering, Anshun University, Anshun 561000, Guizhou, China. E-mail: sci_qyzhang@126.com
bGuizhou University, Guiyang 550025, Guizhou, China
cEngineering Technology Center of Control and Remediation of Soil Contamination of Provincial Science & Technology Bureau, Anshun, Guizhou 561000, China. E-mail: weiff@iccas.ac.cn
First published on 2nd November 2017
Abstract
Mesoporous Ag1(NH4)2PW12O40 (AgN-PW) was developed by co-doping silver and ammonium ions into phosphotungstic acid as an efficient and stable catalyst for free fatty acid esterification, and its physicochemical properties were derived from X-ray diffraction (XRD), thermogravimetric (TG) analysis, Fourier transform infrared (FT-IR) spectra, N2 adsorption–desorption, scanning electron microscopy (SEM) and transmission electron microscopy (TEM). The final catalyst showed a typical Keggin structure of heteropoly acids. This solid acid exhibited remarkable catalytic activity (nearly 100% conversion) in the esterification of oleic acid with methanol for biodiesel production under optimum synthesis conditions. Furthermore, due to the high activity presented in various esterifications of free fatty acids and non-edible oils with high acid values, the mesoporous AgN-PW material was proven to be an environmentally friendly catalyst for industrial biodiesel production.
1. Introduction
The perpetual demand for fuels worldwide has increased tremendously which partly relies on fossil fuels. However, carbon emissions from fossil fuel burning have also increased which causes global warming. Focusing on this aspect, renewable and clean energy sources are currently in high demand.1–3 Biodiesel has attracted much attention in recent years as an alternative renewable and sustainable energy source which is easy to handle, is nontoxic, carbon-neutral and biodegradable.4 Biodiesel (a fatty acid alkyl ester) is basically produced from free-fatty acids (FFAs), edible oils or animal oils by esterification or transesterification with short-chain alcohols using acid or base catalysts.5 However, one of the big challenges is the high-cost feedstocks of edible vegetable oils and animal oils.6 Therefore, it is necessary to seek inexpensive non-edible oil sources containing high concentrations of FFAs, e.g. Euphorbia lathyris oil,7 Madhuca indica oil,8 Camelina sativa oil9 or waste cooking oils.10 On the other hand, base catalysts are not recommended for the production of biodiesel due to potential saponification.11 Therefore, the acid-catalyzed esterification of FFAs is a safer choice.Although homogeneous acids such as H3PO4, HCl and H2SO4 are relatively cheap, are available and have high catalytic activity, they are usually difficult to separate and purify from products and are corrosive to equipment, leading to an increase in biodiesel production costs and to environmental problems.12 To overcome these drawbacks, different types of solid acid catalyst are used with excellent reusability, convenient product separation, low corrosion, water tolerance and environmental compatibility, offering good potential for the substitution of homogeneous acids. Recently, several solid acid catalysts have been employed in the esterification of FFAs with methanol for biodiesel production, such as picolinic acid-modified 12-tungstophosphoric acid,13 HMCM-36 zeolite,14 Pr-SO3H-functionalized SBA-15,15 sulfated zirconia,16 metal-modified graphene oxide composite catalysts,17 etc.
Heteropoly acids are a kind of environmentally friendly material and possess strong Brønsted acidity, and are used as acid catalysts for dehydration, etherification, esterification and transesterification.13 Moreover, the acid sites of heteropoly acids are more uniform and easier to control than those of other acid catalysts. However, the recovery of heteropoly acids is difficult and complicated, because of their small particle size or their solubility in polar solvents. Recently, the partial substitution of H+ in phosphotungstic acid (HPW) with large monovalent ions such as Cs+, Ag+, K+ and NH4+ has been considered as an effective technique to create major changes in its pore structure to improve the surface area and solubility of the pristine acids.18,19 Narasimharao et al.20 found that CsxH3−xPW12O40 salts were active for the esterification of palmitic acid. Santos et al.21 reported a novel mixed salt of cesium and ammonium derivatives of H3PW with good catalytic activity. However, there are presently no reports available of other cation co-doped heteropoly acids for the esterification of FFAs with methanol for the production of biodiesel.
In this work, we synthesized a phosphotungstic acid co-doped with silver and ammonium ions and tested its catalytic activity in the liquid-phase esterification of oleic acid and methanol for biodiesel production. The effect of various process parameters on the esterification reaction was investigated to optimize the efficiency of this system for biodiesel production. Moreover, the reusability of the catalyst and additional experiments for the catalytic esterification of other free fatty acids and non-edible oils with methanol were also studied.
2. Experimental
2.1. Materials
Phosphotungstic acid (AR), silver nitrate (AR), ammonium carbonate (AR), oleic acid (AR), methanol (AR, >99%), lauric acid (AR, 98%), stearic acid (AR, 98%), myristic acid (AR, 98%), palmitic acid (AR, 98%) and methanol (AR, >99%) were purchased from Sinopharm Chemical Regent Co., Ltd. All other chemicals were of analytical grade and used as received, unless otherwise noted.2.2. Materials preparation and characterization
The ammonium and silver co-doped phosphotungstic acid was prepared according to previous literature21,22 with slight modifications. In a typical procedure, phosphotungstic acid (H3PW12O40, HPW, 2.88 g, 0.2 mol L−1) was dissolved in deionized water with vigorous stirring at room temperature, and then an ammonium carbonate aqueous solution (0.2 mol L−1) was added dropwise, followed by the addition of a silver nitrate (0.2 mol L−1) aqueous solution, and was kept under stirring conditions for over 1 h at room temperature. The obtained mixture was heated at 70 °C for 3 h, and finally dried under vacuum at 110 °C for 12 h to obtain the final co-doped phosphotungstate (Ag1(NH4)2PW12O40) catalyst, which was denoted as AgN-PW. The XRD patterns of the catalysts were obtained using a Rigaku D/max 2000 ultima plus diffractometer (monochromatic nickel filter, Cu Kα radiation). The FT-IR spectra were scanned on a PerkinElmer spectrum100 using the KBr disc technique (4000–400 cm−1). Thermogravimetric analysis (TGA) was performed on a NETZSCH/STA 409 PC Luxx simultaneous thermal analyzer with a heating rate of 5 °C min−1 under an air flow rate of 20 mL min−1. The BET surface area, total pore volume and pore size distribution of the catalyst were determined with a Micromeritics ASAP 2020 V4.02 volumetric adsorption analyzer. Scanning electron microscopy (SEM) images were obtained on a JEOL-6701F scanning electron microscope at 10.0 kV. Transmission electron microscopy (TEM) was carried out on a JEOL 2100F electron microscope operated at 200 kV.2.3. The esterification test
The esterification test was carried out as follows. In a typical procedure, certain amounts of oleic acid, methanol and catalyst were mixed and heated at 70 °C for 4.0 h under vigorous stirring. Upon completion, the catalytic system was cooled down to room temperature immediately, and the catalyst was separated from the reaction mixture by filtration, and purified under reduced pressure to remove excess methanol. The acid value (AV, mg KOH g−1) and the conversion rates could be calculated using the following formulas, by referring to the ISO 660-2009 standard.23,24 The reaction parameters i.e. the amount of catalysts, the molar ratio of oleic acid to methanol, the reaction time and temperature were regulated within a certain range to optimize the conversion of oleic acid.
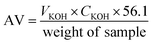 | (1) |
 | (2) |
3. Results and discussion
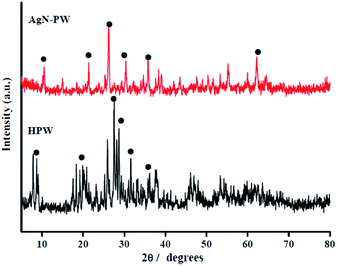
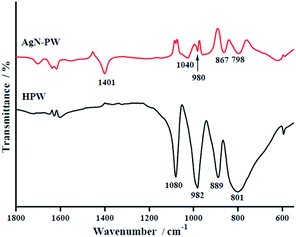
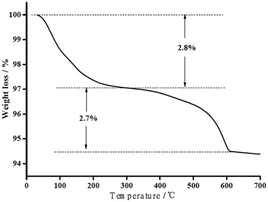
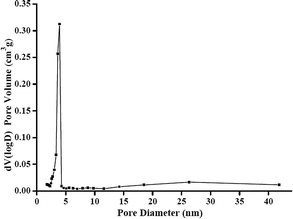
3.1. Characterization
3.2. Catalytic activity of the mesoporous AgN-PW for esterification
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
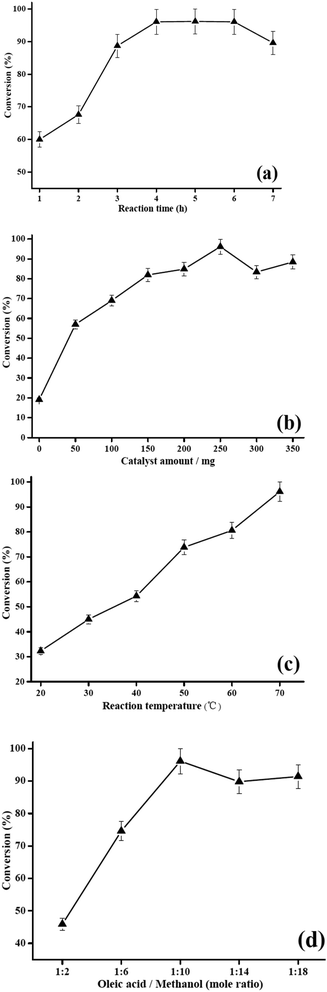
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10, a catalyst loading of 250 mg and a reaction temperature of 70 °C. As shown in Fig. 6a, a sufficient reaction time (1.0 h to 4.0 h) was crucial to obtain a high conversion in the esterification. However, as the reaction time was prolonged to 4.0 h, there was no significant increase in the conversion. This was probably due to the esterification being reversible, which easily took place when the reaction time lasted more than 4.0 h.33 This result indicated that the optimum reaction time for the catalyst was 4.0 h.
10, a catalyst loading of 250 mg and a reaction temperature of 70 °C. As shown in Fig. 6a, a sufficient reaction time (1.0 h to 4.0 h) was crucial to obtain a high conversion in the esterification. However, as the reaction time was prolonged to 4.0 h, there was no significant increase in the conversion. This was probably due to the esterification being reversible, which easily took place when the reaction time lasted more than 4.0 h.33 This result indicated that the optimum reaction time for the catalyst was 4.0 h.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 for 4 h at 70 °C. As shown in Fig. 6b, the conversion rate significantly increased with the amount of catalyst from 50 mg to 250 mg. The biodiesel conversion reached 96.1% with a catalyst amount of 250 mg, this result was due to an increase in the amount of available catalytic active sites as the amount of catalyst increased. However, the conversion rate decreased when the catalyst amount was increased to above 300 mg, and this phenomenon can be explained because the excess catalyst amount made the slurry viscous on account of the emulsification and more products were adsorbed.34,35 Therefore, 250 mg was chosen as the optimum catalyst amount and was used for further investigation in the present research.
10 for 4 h at 70 °C. As shown in Fig. 6b, the conversion rate significantly increased with the amount of catalyst from 50 mg to 250 mg. The biodiesel conversion reached 96.1% with a catalyst amount of 250 mg, this result was due to an increase in the amount of available catalytic active sites as the amount of catalyst increased. However, the conversion rate decreased when the catalyst amount was increased to above 300 mg, and this phenomenon can be explained because the excess catalyst amount made the slurry viscous on account of the emulsification and more products were adsorbed.34,35 Therefore, 250 mg was chosen as the optimum catalyst amount and was used for further investigation in the present research.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10. As shown in Fig. 6c, the oleic acid conversion increased from 32.3% at 20 °C to 96.1% at 70 °C, and higher temperatures may lead to lower yields because of more evaporation of methanol.36 In conclusion, the optimum temperature was approximated to 70 °C.
10. As shown in Fig. 6c, the oleic acid conversion increased from 32.3% at 20 °C to 96.1% at 70 °C, and higher temperatures may lead to lower yields because of more evaporation of methanol.36 In conclusion, the optimum temperature was approximated to 70 °C.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 to 1
2 to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 can result in a remarkable increase in oleic acid conversion. However, lower ratios (e.g. 1
10 can result in a remarkable increase in oleic acid conversion. However, lower ratios (e.g. 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 14 and 1
14 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 18) were found to negatively influence the conversion. Previous studies have suggested that this phenomenon could possibly be attributed to insufficient mixing of the reactants and products with the solid acid catalyst, as well as the decrease in catalyst concentration.37,38 Therefore, the optimum oleic acid to methanol molar ratio for this system was 1
18) were found to negatively influence the conversion. Previous studies have suggested that this phenomenon could possibly be attributed to insufficient mixing of the reactants and products with the solid acid catalyst, as well as the decrease in catalyst concentration.37,38 Therefore, the optimum oleic acid to methanol molar ratio for this system was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10.
10.3.3. Catalyst reusability
Compared with homogeneous inorganic acids, solid acids were a better choice with the advantages of easy separation and good reusability.39 In this case, the mesoporous AgN-PW sample exhibited desirable oleic acid conversion (56.0%) after four successive cycles under the above-mentioned optimal conditions (see Fig. S1†). The observed decline may be due to the blockage of some active sites by residual adsorbed intermediates and products in spite of the sufficient extraction. The indistinctive FT-IR spectra of fresh AgN-PW and the reused catalyst proved that the Keggin structure was not damaged during the esterification of oleic acid and methanol (see Fig. S2†). These results demonstrate the good stability of the AgN-PW composite.3.4. Catalytic performance of AgN-PW for other esterifications
In order to extend the scope of the mesoporous AgN-PW catalyst in biodiesel production, further studies of other FFAs with methanol were investigated (see Table S1†). When lauric acid, myristic acid, palmitic acid, stearic acid and non-edible oils were subjected to the esterification reaction with methanol, high FFA conversions were obtained as well, e.g. 76.0% of stearic acid and 90.7% of non-edible oils, superior to those of other reports.40 These results show that the mesoporous AgN-PW catalyst has great potential to catalyze many types of esterification reactions to meet the demand of synthetic biodiesel.4. Conclusions
In summary, we described a facile synthesis strategy of a mesoporous silver and ammonium co-doped phosphotungstate material with a Keggin structure. This material displayed excellent catalytic performance with an oleic acid conversion of 96.1% in the esterification, under the following optimal conditions: 250 mg of catalyst, a molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 of oleic acid to methanol, a reaction time of 4.0 h and a reaction temperature of 70 °C. This sample displayed good catalytic performance in other FFA esterifications, such as lauric acid, myristic acid, palmitic acid and stearic acid esterification. Moreover, this catalyst could be reused and was stable for a few cycles. Therefore, the mesoporous AgN-PW sample could be a promising catalyst in industrial biodiesel production.
10 of oleic acid to methanol, a reaction time of 4.0 h and a reaction temperature of 70 °C. This sample displayed good catalytic performance in other FFA esterifications, such as lauric acid, myristic acid, palmitic acid and stearic acid esterification. Moreover, this catalyst could be reused and was stable for a few cycles. Therefore, the mesoporous AgN-PW sample could be a promising catalyst in industrial biodiesel production.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by the youth growth S&T personnel foundation of the Guizhou education department (KY [2016]272), the Joint Science and Technology Funds of the Guizhou S&T department, the Anshun city people’s government and Anshun university (LH[2016]7269 and LH[2016]7278), the creative research groups support program of Guizhou education department (No. KY [2017]049), and the 2017 innovative entrepreneurship training program for undergraduates of the Guizhou education department (201710667025).Notes and references
- S. Chu and A. Majumdar, Nature, 2012, 488, 294–303 CrossRef CAS PubMed.
- Q. Y. Zhang, F. F. Wei, D. Luo, P. H. Ma and Y. T. Zhang, China Pet. Process. Petrochem. Technol., 2017, 19, 26–32 Search PubMed.
- F. Zhang, X. F. Tian, M. Shah and W. J. Yang, RSC Adv., 2017, 7, 11403–11413 RSC.
- A. W. Go, S. Sutanto, L. K. Ong, P. L. Tran-Nguyen, S. Ismadji and Y. H. Ju, Renewable Sustainable Energy Rev., 2016, 60, 284–305 CrossRef CAS.
- Q. Y. Zhang, H. Li, X. F. Liu, W. T. Qin, Y. P. Zhang, W. Xue and S. Yang, Energy Technol., 2013, 1, 735–742 CrossRef CAS.
- Rozina, S. Asif, M. Ahmad, M. Zafar and N. Ali, Renewable Sustainable Energy Rev., 2017, 74, 687–702 CrossRef CAS.
- Q. Y. Zhang, F. F. Wei, P. H. Ma, Y. T. Zhang, F. H. Wei and H. L. Chen, Waste Biomass Valorization, 2017 DOI:10.1007/s12649-017-9865-5.
- C. Muthukumaran, R. Praniesh, P. Navamani, R. Swathi, G. Sharmila and N. M. Kumar, Fuel, 2017, 195, 217–225 CrossRef CAS.
- J. Sáez-Bastante, C. Ortega-Román, S. Pinzi, F. R. Lara-Raya, D. E. Leiva-Candia and M. P. Dorado, Bioresour. Technol., 2015, 185, 116–124 CrossRef PubMed.
- N. Shibasaki-Kitakawa, K. Hiromori, T. Ihara, K. Nakashima and T. Yonemoto, Fuel, 2015, 139, 11–17 CrossRef CAS.
- R. Z. Raia, L. S. D. Silva, S. M. P. Marcucci and P. A. Arroyo, Catal. Today, 2017, 289, 105–114 CrossRef CAS.
- A. F. Lee and K. Wilson, Catal. Today, 2015, 242, 3–18 CrossRef CAS.
- S. W. Gong, L. Jing, H. H. Wang, L. J. Liu and Q. Zhang, Appl. Energy, 2014, 134, 283–289 CrossRef CAS.
- R. Purova, K. Narasimharao, N. S. I. Ahmed, S. Al-Thabaiti, A. Al-Shehri, M. Mokhtar and W. Schwieger, J. Mol. Catal. A: Chem., 2015, 406, 159–167 CrossRef CAS.
- S. Jeenpadiphat, E. M. Björk, M. Odén and D. N. Tungasmita, J. Mol. Catal. A: Chem., 2015, 410, 253–259 CrossRef CAS.
- K. Saravanan, B. Tyagi, R. S. Shukla and H. C. Bajaj, Fuel, 2016, 165, 298–305 CrossRef CAS.
- T. M. M. Marso, C. S. Kalpage and M. Y. Udugala-Ganehenege, Fuel, 2017, 199, 47–64 CrossRef CAS.
- F. N. D. C. Gomes, F. M. T. Mendes and M. M. V. M. Souza, Catal. Today, 2017, 279, 296–304 CrossRef CAS.
- M. Safariamin, S. Paul, K. Moonen, D. Ulrichts, F. Dumeignil and B. Katryniok, Catal. Sci. Technol., 2016, 6, 2129–2135 CAS.
- K. Narasimharao, D. R. Brown, A. F. Lee, A. D. Newman, P. F. Siril, S. J. Tavener and K. Wilson, J. Catal., 2007, 248, 226–234 CrossRef CAS.
- J. S. Santos, J. A. Dias, S. C. L. Dias, J. L. de Macedo, F. A. C. Garcia, L. S. Almeida and E. N. C. B. de Carvalho, Appl. Catal., A, 2012, 443–444, 33–39 CrossRef CAS.
- X. Zhou, Z. X. Li, C. Zhang, X. P. Gao, Y. Z. Dai and G. Y. Wang, J. Mol. Catal. A: Chem., 2016, 417, 71–75 CrossRef CAS.
- A. M. Doyle, T. M. Albayati, A. S. Abbas and Z. T. Alismaeel, Renewable Energy, 2016, 97, 19–23 CrossRef CAS.
- G. Raveendra, A. Rajasekhar, M. Srinivas, P. S. Sai Prasad and N. Lingaiah, Appl. Catal., A, 2016, 520, 105–113 CrossRef CAS.
- S. H. Zhu, X. Q. Gao, F. Dong, Y. L. Zhu, H. Y. Zheng and Y. W. Li, J. Catal., 2013, 306, 155–163 CrossRef CAS.
- Y. S. Ren, B. Liu, Z. H. Zhang and J. T. Lin, J. Ind. Eng. Chem., 2015, 21, 1127–1131 CrossRef CAS.
- M. Nyman, F. Bonhomme, T. M. Alam, J. B. Parise and G. M. B. Vaughan, Angew. Chem., Int. Ed., 2004, 43, 2787–2792 CrossRef CAS PubMed.
- D. Y. Zhang, M. H. Duan, X. H. Yao, Y. J. Fu and Y. G. Zu, Fuel, 2016, 172, 293–300 CrossRef CAS.
- A. B. Gawade, M. S. Tiwari and G. D. Yadav, ACS Sustainable Chem. Eng., 2016, 4, 4113–4123 CrossRef CAS.
- S. K. Bhorodwaj and D. K. Dutta, Appl. Catal., A, 2010, 378, 221–226 CrossRef CAS.
- K. Wang, L. Yang, W. Zhao, L. Q. Cao, Z. L. Sun and F. Zhang, Green Chem., 2017, 19, 1949–1957 RSC.
- R. Dutta, S. K. Singh, S. A. Mandavgane and J. D. Ekhe, Appl. Catal., A, 2017, 539, 38–47 CrossRef CAS.
- H. Pan, H. Li, X. F. Liu, H. Zhang, K. L. Yang, S. Huang and S. Yang, Fuel Process. Technol., 2016, 150, 50–57 CrossRef CAS.
- G. Baskar and S. Soumiya, Renewable Energy, 2016, 98, 101–107 CrossRef CAS.
- Q. Y. Zhang, F. F. Wei, Y. T. Zhang, F. H. Wei, P. H. Ma, W. Zheng, Y. T. Zhao and H. L. Chen, J. Oleo Sci., 2017, 66, 491–497 CrossRef CAS PubMed.
- Y. L. Liu, P. B. Zhang, M. M. Fan and P. P. Jiang, Fuel, 2016, 164, 314–321 CrossRef CAS.
- B. Freedman, E. H. Pryde and T. L. Mounts, J. Am. Oil Chem. Soc., 1984, 61, 1638–1643 CrossRef CAS.
- S. Chaveanghong, S. M. Smith, C. Oopathum, C. B. Smith and A. Luengnaruemitchai, Renewable Energy, 2017, 109, 480–486 CrossRef CAS.
- Y. Zhou, S. L. Niu and J. Li, Energy Convers. Manage., 2016, 114, 188–196 CrossRef CAS.
- M. A. R. Melo Júnior, C. E. Albuquerque, J. S. A. Carneiro, C. Dariva, M. Fortuny, A. F. Santo, S. M. S. Egues and A. L. D. Ramos, Ind. Eng. Chem. Res., 2010, 49, 12135–12139 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available: Reusability study of the catalyst, FT-IR spectra of the fresh AgN-PW catalyst and the reused catalyst, and catalytic performance of AgN-PW for other esterification reactions. See DOI: 10.1039/c7ra10554a |
| This journal is © The Royal Society of Chemistry 2017 |