Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Tuning the physical properties of malleable and recyclable polyimine thermosets: the effect of solvent and monomer concentration†
Chengpu Zhu,
Cally Xi,
William Doro,
Tianyi Wang,
Xin Zhang,
Yinghua Jin and
Wei Zhang *
*
Department of Chemistry and Biochemistry, University of Colorado, Boulder, CO 80309, USA. E-mail: wei.zhang@colorado.edu
First published on 16th October 2017
Abstract
The morphological, mechanical, and thermal properties of polyimine films were investigated under different reaction conditions. Polyimines with Young's modulus of 0.03–2.04 GPa could be obtained from the same monomer combinations. Our study shows that polyimine properties are determined by the combined effect of solvent choice and polymerization rate.
Dynamic polymers linked by reversible bonds have attracted great attention due to their self-healing properties and responsiveness to external stimuli.1–3 Recently, Covalent Adaptable Network (CAN) polymers that are crosslinked by dynamic covalent bonds4–6 have emerged as a novel class of dynamic polymers.7,8 They behave like classic thermosets at room temperature, but can undergo microscopic stress relaxation and materials flow under external stimuli (e.g. heat, light). Therefore, they exhibit combined properties of classic thermosets and thermoplastics: they have excellent mechanical properties of thermosets due to the crosslinked nature of polymer networks, yet they are reprocessible, repairable and recyclable like thermoplastics due to the reversible chemical bonds connecting monomers. A variety of CANs have been developed utilizing reversible Diels–Alder (DA) reactions,9–11 transesterification,12–19 olefin metathesis,20,21 disulfide chemistry22–27 and Schiff base reactions.28–32 Among them, polyimines prepared from simple imine condensation33 are of particular interest since they can achieve stress relaxation and material flow in the absence of a transition metal catalyst, and can be reshaped, reprocessed, and repaired upon application of only heat or water, the greenest possible approaches available so far. In addition, the commercial availability of various amines and aldehydes promises ready tunability of the polyimine properties through judicious selection of monomers. Previously, we have reported inexpensive, catalyst-free polyimine network materials that exhibit Arrhenius-like malleability in response to heat and can be recycled and reshaped at an ambient temperature using only water.29 Through variation of diamine monomers, we were able to obtain polyimines with a wide range of thermal, mechanical, and moisture-induced properties, such as semicrystalline polymers with high strength and thermal stability, and elastomeric materials with low temperature malleability.32 Such polyimines have been used as binder materials to form fully recyclable carbon fiber reinforced composites (CFRCs) that exhibit excellent moldability, weldability and easy repair of delamination damage through a simple heat-pressing process.30 Recovery of full-length fiber, as well as complete recovery and reuse of the polyimine binder materials has been achieved by taking advantage of the reversibility of imine bonds under external trigger, in this case the addition of excess monomer. The malleability of polyimines has also enabled dry processing of a thin electrolyte membrane for solid state lithium batteries through hot iso-static pressing of polyimine powder and the glass-ceramic solid electrolyte.31
During the process of preparing polyimine samples for various applications, we found that the solvent has a profound effect on the morphology and mechanical properties of the materials. Strong defect-free polymer film or powder can be formed from the same monomer combinations but in different reaction solvents. For example, polymerization of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.9
0.9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.4 molar ratio of terephthaldehyde, diethylenetriamine, and triethylenetetramine in mixed solvents of dichloromethane, ethyl acetate and ethanol provided a homogeneous polymer film after slow evaporation of the solvents, whereas polymer powder was obtained when ethyl acetate was used as the sole solvent. This result shows that reaction conditions are critical to obtain a polymer with suitable properties. Herein, we present how the reaction conditions (solvent choice and monomer concentration) affect the morphology, mechanical properties, and moisture sensitivity of polyimine films.
1.4 molar ratio of terephthaldehyde, diethylenetriamine, and triethylenetetramine in mixed solvents of dichloromethane, ethyl acetate and ethanol provided a homogeneous polymer film after slow evaporation of the solvents, whereas polymer powder was obtained when ethyl acetate was used as the sole solvent. This result shows that reaction conditions are critical to obtain a polymer with suitable properties. Herein, we present how the reaction conditions (solvent choice and monomer concentration) affect the morphology, mechanical properties, and moisture sensitivity of polyimine films.
Aprotic and protic solvents with different polarities were used as the reaction media, and the properties of resulting polyimines were systematically studied. Solvents directly interact with monomers and polymer chains to influence both reaction kinetics and polymer film formation and further the physical properties of materials.
In a typical procedure, the monomers, terephthaldehyde (1), ethylenediamine (2), triethylenetetramine (3), in a 1.00![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.30
0.30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
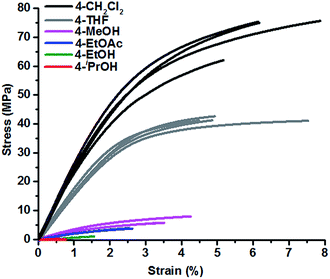
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.47 molar ratio, were dissolved in a solvent and the solution was added to a box-shaped tray (9 cm × 9 cm × 2 cm) made by folding silicone-coated paper. The solvent was then allowed to evaporate in a fume hood under ambient conditions. A total of six different solvents, methylene chloride (CH2Cl2), tetrahydrofuran (THF), ethyl acetate (EtOAc), methanol (MeOH), ethanol (EtOH), and isopropanol (iPrOH) were tested (Scheme 1). In CH2Cl2 and THF, homogeneous polymer films with smooth surfaces were obtained, whereas in other solvents, films with rough surfaces formed. In all cases, the polyimine films (4) were heat-pressed at 78 °C for 3 h, at 95 °C for 1 h, and finally at 105 °C for 1 h before being subjected to various property tests. The mechanical properties of the polyimine samples were investigated by measuring stress–strain curves (Fig. 1). As shown in Table 1, a wide range of tensile strength (69 MPa to 0.2 MPa) was observed for the polyimines prepared in different solvents. The polymer films, 4-CH2Cl2 and 4-THF, obtained in CH2Cl2 and THF exhibit significantly higher tensile strength, 69 MPa and 42 MPa, respectively, compared to the samples prepared in other solvents. The polyimine samples obtained in this study generally don't deform much before break, showing elongation at break from 0.7% to 6.4%. The polyimine 4-iPrOH prepared in isopropyl alcohol exhibits the worst performance, weak and brittle, with tensile strength of 0.23 MPa (300 times lower than 4-CH2Cl2) and elongation at break of 0.7%. Table 1 summarizes the solvent properties and mechanical properties of polyimines. It appears that vapor pressure of the solvents and surface tension have some effect on the mechanical properties of the resulting polyimine samples. The order of tensile strength of polyimines (4-CH2Cl2 > 4-THF > 4-MeOH > 4-EtOAc > 4-EtOH > 4-iPrOH) follows the order of vapor pressure and surface tension of the solvents (CH2Cl2 > THF > MeOH, EtOAc > EtOH > iPrOH).
0.47 molar ratio, were dissolved in a solvent and the solution was added to a box-shaped tray (9 cm × 9 cm × 2 cm) made by folding silicone-coated paper. The solvent was then allowed to evaporate in a fume hood under ambient conditions. A total of six different solvents, methylene chloride (CH2Cl2), tetrahydrofuran (THF), ethyl acetate (EtOAc), methanol (MeOH), ethanol (EtOH), and isopropanol (iPrOH) were tested (Scheme 1). In CH2Cl2 and THF, homogeneous polymer films with smooth surfaces were obtained, whereas in other solvents, films with rough surfaces formed. In all cases, the polyimine films (4) were heat-pressed at 78 °C for 3 h, at 95 °C for 1 h, and finally at 105 °C for 1 h before being subjected to various property tests. The mechanical properties of the polyimine samples were investigated by measuring stress–strain curves (Fig. 1). As shown in Table 1, a wide range of tensile strength (69 MPa to 0.2 MPa) was observed for the polyimines prepared in different solvents. The polymer films, 4-CH2Cl2 and 4-THF, obtained in CH2Cl2 and THF exhibit significantly higher tensile strength, 69 MPa and 42 MPa, respectively, compared to the samples prepared in other solvents. The polyimine samples obtained in this study generally don't deform much before break, showing elongation at break from 0.7% to 6.4%. The polyimine 4-iPrOH prepared in isopropyl alcohol exhibits the worst performance, weak and brittle, with tensile strength of 0.23 MPa (300 times lower than 4-CH2Cl2) and elongation at break of 0.7%. Table 1 summarizes the solvent properties and mechanical properties of polyimines. It appears that vapor pressure of the solvents and surface tension have some effect on the mechanical properties of the resulting polyimine samples. The order of tensile strength of polyimines (4-CH2Cl2 > 4-THF > 4-MeOH > 4-EtOAc > 4-EtOH > 4-iPrOH) follows the order of vapor pressure and surface tension of the solvents (CH2Cl2 > THF > MeOH, EtOAc > EtOH > iPrOH).
| Solvents | Solvent propertiesb | Polyimine properties | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| vp (kPa) | μ (Debye) | η (mPa s) | γ (mN m−1) | Poly-imines | Tensile strength (MPa) | Elongation at break (%) | Young's modulus (GPa) | Tg (°C) | Weightd gain (%) | |
| a The vapor pressure (25 °C) is denoted with vp, the dipole moment (25 °C) with μ, the viscosity (25 °C) with η, and the surface tension (25 °C) with γ unless otherwise indicated. Total five samples were examined for each polyimine prepared in a different solvent.b All the data are from CRC Handbook of Chemistry and Physics, ed. W. M. Haynes, 97th edn, CRC Press, Boca Raton, FL, 2017, except as noted.c From Dean, J. A. Lange's Handbook of Chemistry, McGraw-Hill, New York, 15th edn., 1999.d Weight gain after soaking in DI water for 24 h at room temperature. | ||||||||||
| CH2Cl2 | 58.2 | 1.60 | 0.41 | 27.2 | 4-CH2Cl2 | 69 ± 6 | 6.4 ± 1.3 | 1.10 ± 0.01 | 86 | 6.5 |
| THF | 21.6 | 1.75 | 0.46 | 26.5c | 4-THF | 42 ± 1 | 4.6 ± 0.2 | 1.55 ± 0.01 | 80 | 14 |
| EtOAc | 12.6 | 1.78 | 0.42 | 23.4 | 4-EtOAc | 3.8 ± 0.7 | 2.3 ± 0.3 | 0.163 ± 0.001 | 115 | 139 |
| MeOH | 16.9 | 1.70 | 0.54 | 22.1 | 4-MeOH | 6.6 ± 0.8 | 3.8 ± 0.5 | 0.281 ± 0.001 | 108 | 59 |
| EtOH | 7.87 | 1.69 | 1.07 | 22.0 | 4-EtOH | 1.2 ± 0.1 | 1.4 ± 0.1 | 0.088 ± 0.002 | 117 | 106 |
| iPrOH | 6.02 | n/a | 2.04 | 20.9 | 4-iPrOH | 0.23 ± 0.01 | 0.7 ± 0.1 | 0.029 ± 0.001 | 81 | 262 |
It has been known that solvent retention in polymer films can cause plasticization and significantly affect the physical properties.34 To investigate the possible inclusion of the solvents in polyimine samples, we conducted thermal gravimetric analysis (TGA). TGA shows <2% weight loss at 200 °C and <3% weight loss before their decomposition (232–243 °C), indicating the presence of a minimal amount of residual solvents in the polyimine films (Fig. S3†). Therefore, the effects of retained solvents on the mechanical properties of polyimines would be negligible.
Since mechanical properties are closely related to molecular weight and crosslinking density of polymers, we next studied the possible solvent effect on the monomer conversion. It should be noted that the same monomers and crosslinker in the same stoichiometric ratio were used in all the entries, therefore the crosslink density is mainly controlled by the monomer conversion in different solvents. We estimated the aldehyde conversion by analyzing 13C magic-angle spinning solid-state NMR spectra of the best-performing 4-CH2Cl2 and the worst-performing 4-iPrOH (Fig. S4 and S5†). Our analysis shows that 98% aldehyde conversion was achieved in CH2Cl2, whereas only 94% aldehyde conversion was reached in isopropyl alcohol. The polyimines are formed through condensation of equimolar quantities of amine and aldehyde groups (step-growth polymerization), thus the 4% lower conversion of aldehyde monomers would decrease the polymerization degree and average molecular weight by ∼3 fold, which has significant negative effect on the mechanical properties. This result is consistent with our mechanical test, which shows much poorer mechanical properties of 4-iPrOH compared to those of 4-CH2Cl2.
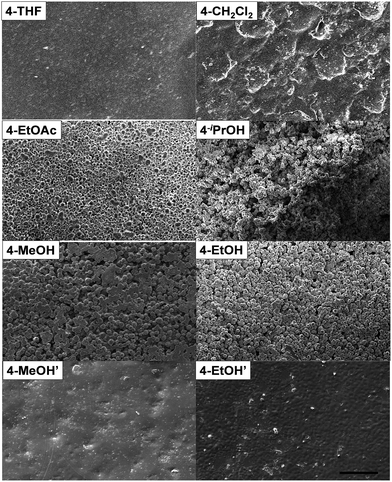
We next examined the morphological features of the polyimine samples, which are also known to greatly affect mechanical properties of polymers. The polyimine morphologies were investigated by analyzing scanning electron microscopy (SEM) images of both the surface and cross section. As shown in Fig. 2, the SEM images of the surfaces of polyimine films show drastically different morphologies. The polyimine films 4-THF and 4-CH2Cl2 prepared in THF and CH2Cl2 show coherent smooth surfaces, whereas the surfaces of polyimines 4-EtOAc, 4-iPrOH, 4-MeOH, 4-EtOH prepared in EtOAc, iPrOH, MeOH, and EtOH are relatively rough, consisting of agglomerated micron-sized spherical particles likely caused by phase separation. The polyimine morphologies shown in cross section SEM images (Fig. S1†) agree well with their corresponding surface morphologies, indicating these samples have homogeneous phases. Flattened particles were observed on the surface of some samples (e.g. 4-MeOH, Fig. 2), but not at cross section, suggesting it is likely caused by the contact point pressure during heat-press. It is plausible that agglomerates in polyimines 4-EtOAc, 4-iPrOH, 4-MeOH, 4-EtOH loosely bound to the neighboring particles mostly through van der Waals forces, causing their poor mechanical performances. Such morphological differences directly affect moisture sensitivity of polyimines. 4-THF and 4-CH2Cl2 with relatively seamless smooth surfaces show only 14% and 6% weight gain when they were soaked in deionized (DI) water for 24 h. By contrast, polyimines 4-MeOH, 4-EtOH, 4-EtOAc, 4-iPrOH, which show obvious surface porosities, absorb DI water of 59–262 wt% when treated under the same conditions.
Our study shows that solvents have significant effects on the surface roughness, film morphology and moisture sensitivity. In all the cases, the polymerization started as homogeneous solution. In CH2Cl2 and THF, no phase separation was observed and the mixtures remained clear throughout the polymerization process until the evaporation of the solvents to form transparent and relatively tough films. In protic solvents MeOH, EtOH and iPrOH, milky gels were formed in the course of polymerization and brittle opaque films were obtained. Obvious polymerization-induced phase separation (yellow precipitation) was observed in EtOAc, resulting in a polyimine film with powdery composition. Although the imine linkages are reversible and theoretically allow materials flow at certain conditions, our attempts to obtain homogeneous films similar to 4-THF and 4-CH2Cl2 by post-treatment of the polyimine films (heat pressing under high pressure and high temperature above their Tg) were unsuccessful. Heat-pressing of 4-iPrOH film at a pressure of 8.9 MPa (170 °C, 10 min) led to a brittle film with dark brown colour. We did not try longer pressing time or higher temperature, since the change of the film colour from bright yellow to dark brown is likely due to the occurrence of possible side reactions. At this stage, we have not yet developed suitable conditions to erase the solvent effect by simple post-treatment method (e.g. heating), and changing the reaction solvent appears a more viable approach to engineer the properties of polyimine films. Likely, imine exchange reactions are limited to contact points and the system is unable to reach global materials flow under the applied conditions.
Since the dynamics of phase separation and gel-formation are controlled by reaction rate and monomer concentrations in the early stage, and solvent diffusion and interfacial tension in the late stage, we studied the effect of monomer concentration on the polymer morphology in different solvents. We increased the monomer concentration by two folds and carried out the polymerization under the same conditions as previously described. In CH2Cl2, higher monomer concentration induced more rapid imine condensation, which is exothermic, leading to boiling of the solvent and formation of polyimine film with many entrapped bubbles. In EtOAc, at increased monomer concentration, the reaction was rapid and immediate, and massive precipitation was observed upon mixing the monomers. Interestingly, in contrast to the results from low monomer concentration experiments in MeOH and EtOH, at higher monomer concentration, transparent and homogeneous films (4-MeOH′ and 4-EtOH′) were obtained (see SEM images in Fig. 2), which show excellent mechanical properties. The tensile strength of 4-MeOH′ and 4-EtOH′ are 39 MPa and 33 MPa, which are increased 6-fold and 28-fold compared to those of 4-MeOH and 4-EtOH, respectively (Fig. 3). They also exhibit greatly enhanced Young's moduli, 1.80 GPa and 2.04 GPa (up to 22 fold increase) for 4-MeOH′ and 4-EtOH′, respectively. Correspondingly, 4-MeOH′ and 4-EtOH′ show much reduced moisture sensitivity compared to 4-MeOH (12 wt% vs. 59 wt%) and 4-EtOH (22 wt% vs. 106 wt%). Our study clearly indicates that the solvent properties and reaction kinetics are critical to the dynamics of phase separation and morphology evolution of polyimine films and thus their mechanical properties. Although, vapor pressure of the solvents and surface tension could have some effect on the mechanical properties of the resulting polyimine samples as mentioned previously, it is more likely a combined effect of various parameters including reaction rate, diffusion of solvent, solubility of polymers, and interactions between polymers and solvents.
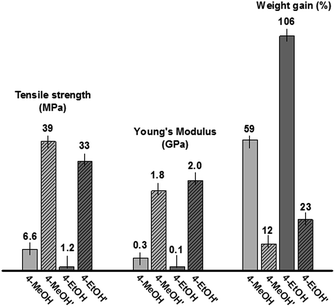 | ||
| Fig. 3 Comparison of tensile strength, young's modulus and weight gain (after soaking in DI water for 24 h) of 4-MeOH, 4-EtOH, 4-MeOH′, and 4-EtOH′. | ||
Since solvents have significant effects on reaction kinetics,35 we next monitored the reaction progress in different deuterated solvents, CD2Cl2, THF-D8, and CD3OD using model compounds, 4-bromobenzaldehyde and ethylenediamine by 1H NMR spectroscopy under the same monomer concentrations as the polyimine formation. The monomer conversions were then calculated based on 1H NMR signal integration and plotted against time as shown in Fig. 4. Our study shows that the reaction rate is the highest in methanol, in which the polymerization provides weak and brittle film with micrometer-range heterogeneity. Much lower reaction rate was observed in THF, in which smoother polyimine film with higher mechanical properties can be obtained. These results indicate that phase separation and chemical reaction are in competition, and the morphology is determined by the combined effect of solvent choice (e.g. diffusion) and polymerization rate. At high reaction rate, the growth of polymer network is fast, leading to rapid sol–gel transition prior to phase-separation. At very low reaction rate, the growth of polymer network is slow and the reaction mixture remain homogeneous for prolonged time due to the mutual solubility of constituents, leading to delayed phase-separation. However, for intermediate rates, the phase separation can be initiated prior to sol–gel transition, leading to much coarser phase-separated domains as shown in 4-EtOAc, 4-iPrOH, 4-MeOH, 4-EtOH.
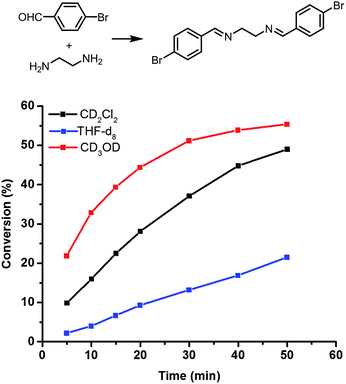 | ||
| Fig. 4 Imine condensation reaction progress monitored by 1H NMR spectroscopy using condensation between 4-bromobenzaldehyde and ethylenediamine as a model reaction. | ||
These polyimines can be completely degraded by adding diamine monomer, and recycled to form new batch of polyimine films. They can also be welded and reshaped in any desirable shape for multiple times by simple application of heat. As a proof of concept demonstration, we reshaped a strip of polyimine 4-EtOH′ by simple heating. As shown in Fig. 5, the polyimine strip can be repeatedly reshaped to a bent circle or a helical fusilli-like object multiple times upon heating and cooling. The sample was twisted into a fusilli shape at high temperature, and was subsequently cooled down to a hard thermoset object. It can be twisted back to the original straight shape by heating and cooling.
In conclusion, we have demonstrated how solvent choice and monomer concentration affect polyimine morphology and mechanical properties. Monomer concentration and solvent properties directly control reaction rates and influence solvent diffusion into polymer chains and interfacial interactions during the polymerization progress, thus determining the morphology evolution and properties of polymers. A range of mechanical properties can be obtained in different solvents from the same monomer composition. Tough polyimine materials with excellent Young's modulus of ∼2 GPa was obtained in ethanol, highlighting the great potential of these malleable and recyclable thermosets in materials development. We also briefly demonstrated the malleability of a polyimine sample by repeatedly reshaping it between a circular shape and a fusilli-shape. The knowledge gained in this study would be of great importance to future development of polyimine thermoset materials targeting various potential applications.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors thank Prof. Yifu Ding and Prof. Christopher Bowman for providing instrumental support to the project. This work is supported by Colorado AIA grant program and K. C. Wong Education Foundation.References
- R. J. Wojtecki, M. A. Meador and S. J. Rowan, Nat. Mater., 2011, 10, 14–27 CrossRef CAS PubMed
.
- D. Roy, W. L. A. Brooks and B. S. Sumerlin, Chem. Soc. Rev., 2013, 42, 7214–7243 RSC
.
- N. Roy, B. Bruchmann and J. M. Lehn, Chem. Soc. Rev., 2015, 44, 3786–3807 RSC
.
- S. J. Rowan, S. J. Cantrill, G. R. L. Cousins, J. K. M. Sanders and J. F. Stoddart, Angew. Chem., Int. Ed., 2002, 41, 898–952 CrossRef PubMed
.
- P. T. Corbett, J. Leclaire, L. Vial, K. R. West, J.-L. Wietor, J. K. M. Sanders and S. Otto, Chem. Rev., 2006, 106, 3652–3711 CrossRef CAS PubMed
.
- Y. Jin, C. Yu, R. J. Denman and W. Zhang, Chem. Soc. Rev., 2013, 42, 6634–6654 RSC
.
- C. J. Kloxin, T. F. Scott, B. J. Adzima and C. N. Bowman, Macromolecules, 2010, 43, 2643–2653 CrossRef CAS PubMed
.
- C. J. Kloxin and C. N. Bowman, Chem. Soc. Rev., 2013, 42, 7161–7173 RSC
.
- X. X. Chen, M. A. Dam, K. Ono, A. Mal, H. B. Shen, S. R. Nutt, K. Sheran and F. Wudl, Science, 2002, 295, 1698–1702 CrossRef CAS PubMed
.
- J. S. Park, T. Darlington, A. F. Starr, K. Takahashi, J. Riendeau and H. T. Hahn, Compos. Sci. Technol., 2010, 70, 2154–2159 CrossRef CAS
.
- M. Q. Zhang and M. Z. Rong, Polym. Chem., 2013, 4, 4878–4884 RSC
.
- D. Montarnal, M. Capelot, F. Tournilhac and L. Leibler, Science, 2011, 334, 965–968 CrossRef CAS PubMed
.
- M. Capelot, M. Unterlass, F. Tournihac and L. Leibler, ACS Macro Lett., 2012, 1, 789–792 CrossRef CAS
.
- K. Yu, P. Taynton, W. Zhang, M. L. Dunn and H. J. Qi, RSC Adv., 2014, 4, 48682–48690 RSC
.
- Z. Pei, Y. Yang, Q. Chen, E. M. Terentjev, Y. Wei and Y. Ji, Nat. Mater., 2014, 13, 36–41 CrossRef CAS PubMed
.
- K. Yu, P. Taynton, W. Zhang, M. L. Dunn and H. J. Qi, RSC Adv., 2014, 4, 10108–10117 RSC
.
- M. Capelot, D. Montarnal, F. Tournilhac and L. Leibler, J. Am. Chem. Soc., 2012, 134, 7664–7667 CrossRef CAS PubMed
.
- Z. Q. Pei, Y. Yang, Q. M. Chen, Y. Wei and Y. Ji, Adv. Mater., 2016, 28, 156–160 CrossRef CAS PubMed
.
- C. Ding, P. S. Shuttleworth, S. Makin, J. H. Clark and A. S. Matharu, Green Chem., 2015, 17, 4000–4008 RSC
.
- Y. X. Lu, F. Tournilhac, L. Leibler and Z. B. Guan, J. Am. Chem. Soc., 2012, 134, 8424–8427 CrossRef CAS PubMed
.
- J. A. Neal, D. Mozhdehi and Z. Guan, J. Am. Chem. Soc., 2015, 137, 4846–4850 CrossRef CAS PubMed
.
- J. J. Griebel, N. A. Nguyen, A. V. Astashkin, R. S. Glass, M. E. Mackay, K. Char and J. Pyun, ACS Macro Lett., 2014, 3, 1258–1261 CrossRef CAS
.
- L. Imbernon, E. K. Oikonomou, S. Norvez and L. Leibler, Polym. Chem., 2015, 6, 4271–4278 RSC
.
- A. Rekondo, R. Martin, A. Ruiz de Luzuriaga, G. Cabanero, H. J. Grande and I. Odriozola, Mater. Horiz., 2014, 1, 237–240 RSC
.
- Z. Q. Lei, H. P. Xiang, Y. J. Yuan, M. Z. ROng and M. Q. Zhang, Chem. Mater., 2014, 26, 2038–2046 CrossRef CAS
.
- R. Martin, A. Rekondo, A. Ruiz De Luzuriaga, G. Cabanero, H. Grande and I. Odriozola, J. Mater. Chem. A, 2014, 2, 5710–5715 CAS
.
- M. Pepels, I. Filot, B. Klumperman and H. Goossens, Polym. Chem., 2013, 4, 4955–4965 RSC
.
- A. Chao, J. Negulescu and D. H. Zhang, Macromolecules, 2016, 49, 6277–6284 CrossRef CAS
.
- P. Taynton, K. Yu, R. K. Shoemaker, Y. H. Jin, H. J. Qi and W. Zhang, Adv. Mater., 2014, 26, 3938–3942 CrossRef CAS PubMed
.
- P. Taynton, H. G. Ni, C. P. Zhu, K. Yu, S. Loob, Y. H. Jin, H. J. Qi and W. Zhang, Adv. Mater., 2016, 28, 2904–2909 CrossRef CAS PubMed
.
- J. M. Whiteley, P. Taynton, W. Zhang and S. H. Lee, Adv. Mater., 2015, 27, 6922–6927 CrossRef CAS PubMed
.
- P. Taynton, C. P. Zhu, S. Loob, R. Shoemaker, J. Pritchard, Y. H. Jin and W. Zhang, Polym. Chem., 2016, 7, 7052–7056 RSC
.
- D. N. Crisan, O. Creese, R. Ball, J. L. Brioso, B. Martyn, J. Montenegro and F. Fernandez-Trillo, Polym. Chem., 2017, 8, 4576–4584 RSC
.
- M. L. Sanyang, S. M. Sapuan, M. Jawaid, M. R. Ishak and J. Sahari, Polymers, 2015, 7, 1106–1124 CrossRef CAS
.
- A. F. Abdel-Magid, K. G. Carson, B. D. Harris, C. A. Maryanoff and R. D. Shah, J. Org. Chem., 1996, 61, 3849–3862 CrossRef CAS PubMed
.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures, mechanical and thermal property tests, SEM image and NMR spectra. See DOI: 10.1039/c7ra10956c |
| This journal is © The Royal Society of Chemistry 2017 |