Selective functionalization of complex heterocycles via an automated strong base screening platform†
Sobhana Babu
Boga
*a,
Melodie
Christensen
*b,
Nicholas
Perrotto
b,
Shane W.
Krska
a,
Spencer
Dreher
a,
Matthew T.
Tudge
b,
Eric R.
Ashley
b,
Marc
Poirier
b,
Mikhail
Reibarkh
b,
Yong
Liu
b,
Eric
Streckfuss
a,
Louis-Charles
Campeau
b,
Rebecca T.
Ruck
b,
Ian W.
Davies
b and
Petr
Vachal
a
aDepartment of Discovery Chemistry, MRL, Merck & Co., Inc., Rahway, NJ, USA. E-mail: sobhana.babu.boga@merck.com
bDepartment of Process Research & Development, MRL, Merck & Co., Inc., Rahway, NJ, USA. E-mail: melodie.christensen@merck.com
First published on 16th May 2017
Abstract
Knochel–Hauser bases, derived from 2,2,6,6-tetramethylpiperidinyl (TMP) metal amides, offer exceptional selectivity and functional group tolerance in the regioselective metalation of arenes and heteroarenes. The selectivity, stability and yield of these reactions are highly dependent on the nature of the base, additive and deprotonation temperature. We have developed and validated an automated micro-scale high throughput experimentation (HTE) approach to rapidly optimize base and temperature matrices. We describe the application of this approach to the regioselective functionalization of a variety of complex heterocycles and extension to the preparation of organometallic reagents for transition metal catalyzed cross-coupling screens.
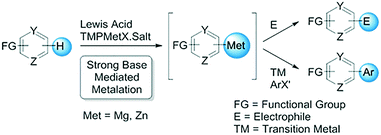
Strong base mediated metalation and subsequent functionalization of arenes and heteroarenes represent a powerful tool in the synthesis of compounds of pharmaceutical interest.1 Highly stable and tunable 2,2,6,6-tetramethylpiperidine (TMP)-based organometallic amides, also known as Knochel–Hauser bases or metallic TMP bases, combine exceptional selectivity with high functional group tolerance in the regioselective metalation of arenes and heteroarenes.2 In addition, the formation of frustrated Lewis pairs between metallic TMP bases and Lewis acids has been shown to accelerate metalation rates.3 The resulting organometallic species can be functionalized with a variety of electrophiles or utilized directly in transition metal catalyzed cross-coupling reactions (Fig. 1).4
It is well-established that the regioselectivities and yields of metalation reactions can be influenced by the nature of the base, utilization of additives and reaction temperature.5 A wide range of temperatures, from cryogenic to ambient conditions, are typical for such transformations. Given the diverse array of reported conditions, the correct choice of base, additive and temperature for regioselective metalation of a given complex substrate may be difficult to predict. Thus, we envisioned that systematic and comprehensive screening of these variables through automated high throughput experimentation (HTE)6 would enable rapid definition of the optimal set of conditions and, in some cases, reveal conditions that afford unexpected selectivity.
An effective automated strong base and temperature screening workflow would require multiple transfer steps of volatile organic mixtures and pyrophoric reagents while maintaining meticulous low temperature control and robust agitation under rigorously inert conditions. In addition, the screen would need to consume only milligram quantities of high-value intermediates. Finally, the protocol would need to be robust and the results scalable. Herein, we describe the development, validation and application of the first automated micro-scale strong base and temperature screening platform for selective functionalization of complex heterocycles.
We identified the Chemspeed SWING liquid handling robotic system, outfitted with a commercially available tumble stirrer and high capacity chiller, as satisfying all of our requirements. Typical screens evaluated six bases across four substrates at four temperatures, but the protocol could easily be modified to explore variables such as stoichiometry and solvent (Fig. 2). The only manual steps in the workflow involved substrate mixture preparation and analysis plate handling. The automated reaction set-up and quench steps were carried out sequentially, row by row, for each temperature. In order to track the formation of organometallic species, we used iodine as a diagnostic electrophile. Screens were carried out in 96-well block assemblies using glass vial inserts.‡ Only 20 μmol of substrate was consumed per vial and overall run times spanned 20 hours.
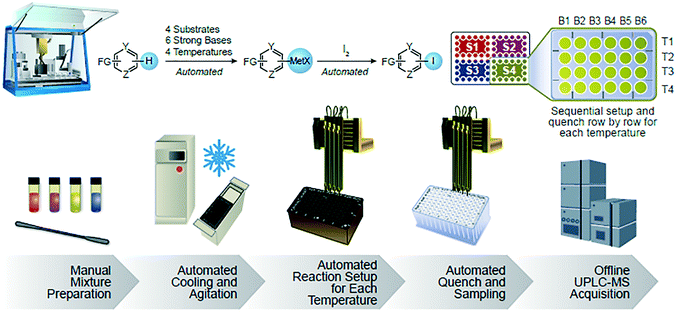 | ||
| Fig. 2 Automated base and temperature screening workflow. Six bases (B1–B6) screened across four substrates (S1–S4) at four temperatures (T1–T4). | ||
Once the workflow was established, we set out to validate the protocol using a selection of substrates well-known in the literature for regioselective deprotonation with hindered main group amide bases (Table 1 entries 1a through 1c). In our first experiment, we screened the regioselective metalation of chromone 1a. Consistent with literature precedent, TMP2Zn·2MgCl2·2LiCl afforded C(2)-zincation at −45 °C, while TMPZnCl·LiCl afforded C(3)-zincation at 0 °C (Fig. 3).7 Notably, we uncovered that TMP2Zn afforded C(3)-zincation at 25 °C, plausibly through complex induced proximity effect. In our second experiment, we screened the metalation of coumarin 1b. We were able to reproduce literature findings that TMP2Zn·2MgCl2·2LiCl offered C(3)-zincation at 25 °C.8 In our third experiment, we screened the regioselective metalation of N,N-dimethyluracil 1c. A recent literature report indicated that C(5)-magnesiation could be achieved with TMPMgCl·LiCl at −40 °C, while C(6)-zincation could be achieved with TMP2Zn·2MgCl2·2LiCl at −30 °C.9 The screen confirmed these findings, but also revealed a temperature-dependent regioselectivity using TMP2Zn·2MgCl2·2LiCl such that that the C(5)-zincate was favored over the C(6)-zincate at 25 °C. This is noteworthy because the improved thermal stability of the C(5)-zincate would render it a more desirable intermediate over the magnesiate in a variety of applications. All the results were in good agreement with literature reports and, in two instances, uncovered novel conditions for regioselective metalation of the substrates screened.
| Substrate | Product yield% isolateda (assay)b | Optimal base & reaction conditiona |
|---|---|---|
| a Optimal metalation conditions identified in HTE screens were reproduced on 0.34 mmol scale and treated with iodine to determine product structures and isolated yields. b Assay yields at 3 h on 0.02 mmol HTE scale are provided for comparison. c See Fig. 3 for screen results. d See ESI for screen results. | ||
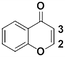 1a
1a
|
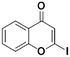 2a, 63% (81%)
2a, 63% (81%) |
TMP2Zn·2MgCl2·2LiCl −45 °C, 3 hc |
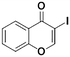 3a, 55% (75%)
3a, 55% (75%) |
TMPZnCl·LiCl 0 °C, 3 hc | |
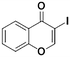 3a, 80% (85%)
3a, 80% (85%) |
TMP2Zn 25 °C, 3 hc | |
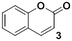 1b
1b
|
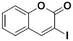 2b, 56% (72%)
2b, 56% (72%) |
TMP2Zn·2MgCl2·2LiCl 25 °C, 3 hd |
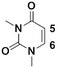 1c
1c
|
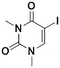 2c, 48% (68%)
2c, 48% (68%) |
TMP2Zn·2MgCl2·2LiCl 25 °C, 3 hd |
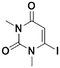 3c, 35% (42%)
3c, 35% (42%) |
TMP2Zn·2MgCl2·2LiCl −45 °C, 3 hd | |
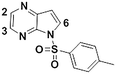 1d
1d
|
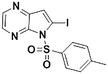 2d, 47% (64%)
2d, 47% (64%) |
TMPMgCl·LiCl −45 °C, 3 hd |
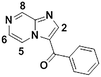 1e
1e
|
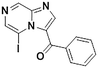 2e, 23% (7%)
2e, 23% (7%) |
TMPMgCl·LiCl −45 °C, 16 hd |
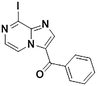 3e, 36% (28%)
3e, 36% (28%) |
TMPZnCl·LiCl −45 °C, 16 hd | |
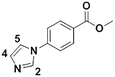 1f
1f
|
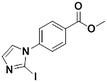 2f, 44% (89%)
2f, 44% (89%) |
TMPZnCl·LiCl −20 °C, 16 hd |
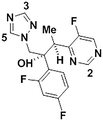 1g
1g
|
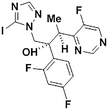 2g, 30% (60%)
2g, 30% (60%) |
MeLi, 30 min TMP2Zn 0 °C, 16 hd |
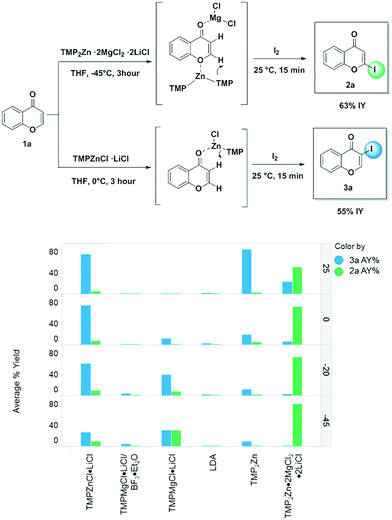 | ||
| Fig. 3 Results of validation screen with chromone 1a. Note: Assay yields determined by UPLC-MS at 210 nm using 4,4′-di-tert-butylbiphenyl as an internal standard. Reported yields average 4 replicates (see ESI† for quadruplicate results). | ||
We next applied our automated screening approach to a variety of complex heterocycles containing functional groups commonly observed in drug-like molecules. Each contained more than one site in which directed metalation could occur, rendering it difficult to formulate accurate predictions around regioselectivity (Table 1 entries 1d through 1g). We first screened pyrrolopyrazine derivative 1d, for which TMPMgCl·LiCl afforded C(6)-magnesiation at −45 °C, likely through the ortho-directing effect of the sulfonyl protecting group.10 Screening imidazopyrazine 1e afforded regioselective metalation at two different positions at −45 °C, with TMPMgCl·LiCl offering C(5)-magnesiation and TMPZnCl·LiCl offering C(8)-zincation. C(5)-magnesiation was likely driven by complex induced proximity effect, while C(8)-zincation occurred at the most acidic proton. Compound 1f underwent zincation at the most acidic C(2) position with TMPZnCl·LiCl, and the resulting C(2)-zincate was demonstrated to be stable across a wide temperature range from −45 to 0 °C. Finally, we screened the metalation of voriconazole 1g. This screen was particularly challenging due to the presence of an acidic proton. It has been shown that compounds containing acidic protons can be pre-treated with methyllithium prior to metalation to deprotonate all acidic positions and enable efficient C–H metalation.11 We incorporated this deprotonation step into our protocol and successfully identified conditions for C(5)-zincation at the triazole ring. The identification of such unique and diverse conditions for this series of metalations highlights both the utility and importance of this novel automated screening tool.
Organometallics generated through directed metalation have found wide use in transition metal catalyzed cross-coupling reactions.4 We sought to extend our technology to prepare viable intermediates for Negishi reactions. To this end, we screened the metalation of dichloropyridine compounds 1h and 1i to enable arylation at the C(4) positions.12 Even though the results were in good agreement with literature with respect to base, the screen allowed us to fine-tune the metalation temperatures.13 Upon implementing the optimal conditions identified in the screen on gram scale, 1h and 1i underwent facile deprotonation to cleanly afford C(4)-metalates. Transmetallation with ZnCl2 afforded stable zincates that were arylated via Pd catalyzed Negishi cross-coupling in excellent yields (Fig. 4). This application demonstrates the direct translation of our automated screening tool to building structures of increasing complexity.
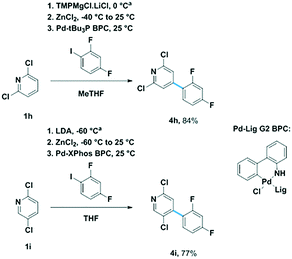 | ||
| Fig. 4 C(4)-arylation of compounds 1h and 1i. aSee ESI† for screen results. | ||
In conclusion, we developed a fully automated micro-scale HTE screening platform for evaluating base and temperature matrices in the regioselective metalation of complex heterocycles. The technology was validated on examples from the literature as well as applied to a variety of previously unreported complex heterocycles. We consistently found that, through comprehensive base and temperature screening, we were able to identify conditions that provided optimal selectivity, stability and yield across a diverse array of substrates. Furthermore, the optimization was achieved in a very short period of time, consuming only milligram quantities of substrate per reaction. Finally, we extended the utility of this technology to prepare organometallic reagents for transition metal catalyzed cross-coupling reactions. Future work will integrate strong base and temperature screens with transition metal catalyzed cross-coupling screens in a single automated workflow.
Acknowledgements
We thank Daniel A. DiRocco (Merck) for helpful discussions, Donald V. Conway (Merck) for engineering support and Natalya Pissarnitski (Merck) for purification of samples.Notes and references
- (a) V. Snieckus, Chem. Rev., 1990, 90, 879 CrossRef CAS; (b) M. Schlosser, Angew. Chem., Int. Ed., 2005, 44, 376 CrossRef CAS PubMed; (c) N. Boudet, J. R. Lachs and P. Knochel, Org. Lett., 2007, 26, 5525 CrossRef PubMed; (d) H. Naka, J. V. Morey, J. Haywood, D. J. Eisler, M. McPartlin, F. García, H. Kudo, Y. Kondo, M. Uchiyama and A. E. H. Wheatley, J. Am. Chem. Soc., 2008, 130, 16193 CrossRef CAS PubMed; (e) C. Despotopoulou, L. Klier and P. Knochel, Org. Lett., 2009, 11, 3326 CrossRef CAS PubMed; (f) F. M. Piller and P. Knochel, Org. Lett., 2009, 11, 445 CrossRef CAS PubMed; (g) C. J. Rohbogner, S. Wirth and P. Knochel, Org. Lett., 2010, 12, 1984 CrossRef CAS PubMed.
- (a) Y. Kondo, M. Shilai, M. Uchiyama and T. Sakamoto, J. Am. Chem. Soc., 1999, 121, 3539 CrossRef CAS; (b) A. Krasovskiy, V. Krasovskaya and P. Knochel, Angew. Chem., Int. Ed., 2006, 45, 2958 CrossRef CAS PubMed; (c) R. E. Mulvey, F. Mongin, M. Uchiyama and Y. Kondo, Angew. Chem., Int. Ed., 2007, 46, 3802 CrossRef CAS PubMed; (d) M. L. Hlavinka and J. R. Hagadorn, Organometallics, 2007, 26, 4105 CrossRef CAS; (e) S. H. Wunderlich and P. Knochel, Angew. Chem., Int. Ed., 2007, 46, 7685 CrossRef CAS PubMed; (f) H. Naka, M. Uchiyama, Y. Matsumoto, A. E. H. Wheatley, M. McPartlin, J. V. Morey and Y. Kondo, J. Am. Chem. Soc., 2007, 129, 1921 CrossRef CAS PubMed; (g) S. H. Wunderlich and P. Knochel, Org. Lett., 2008, 10, 4705 CrossRef CAS PubMed; (h) M. Mosrin and P. Knochel, Org. Lett., 2009, 11, 1837 CrossRef CAS PubMed; (i) M. Mosrin, T. Bresser and P. Knochel, Org. Lett., 2009, 11, 3406 CrossRef CAS PubMed; (j) M. Mosrin, G. Monzon, T. Bresser and P. Knochel, Chem. Commun., 2009, 5615 RSC; (k) M. Hedidi, G. Bentabed-Ababsa, A. Derdour, Y. S. Halauko, O. A. Ivashkevich, V. E. Matulis, F. Chevallier, T. Roisnel, V. Dorcet and F. Mongin, Tetrahedron, 2016, 72(17), 2196 CrossRef CAS; (l) D. R. Armstrong, E. Crosbie, E. Hevia, R. E. Mulvey, D. L. Ramsay and S. D. Robertson, Chem. Sci., 2014, 5(8), 3031 RSC.
- (a) M. Jaric, B. A. Haag, A. Unsinn, K. Karaghiosoff and P. Knochel, Angew. Chem., Int. Ed., 2010, 49, 5451 CrossRef CAS PubMed; (b) K. Groll, S. M. Manolikakes, X. Mollat du Jourdin, M. Jaric, A. Bredihhin, K. Karaghiosoff, T. Carell and P. Knochel, Angew. Chem., Int. Ed., 2013, 52, 6776 CrossRef CAS PubMed.
- (a) J. Board, J. Cosman, S. Singh, T. Rantanen and V. Snieckus, Platinum Met. Rev., 2013, 57, 234 CrossRef; (b) T. Klatt, J. T. Markiewicz, C. Sämann and P. Knochel, J. Org. Chem., 2014, 79, 4235 CrossRef PubMed; (c) T. J. Greshock, K. P. Moore, R. T. McClain, A. Bellomo, C. K. Chung, S. D. Dreher, P. S. Kutchukian, Z. Peng, I. W. Davies, P. Vachal, M. Ellwart, S. M. Manolikakes, P. Knochel and P. G. Nantermet, Angew. Chem., Int. Ed., 2016, 55, 13714 CrossRef CAS PubMed.
- B. Haag, M. Mosrin, H. Ila, V. Malakhov and P. Knochel, Angew. Chem., Int. Ed., 2011, 50, 9794 CrossRef CAS PubMed.
- (a) J. R. Schmink, A. Bellomo and S. Berritt, Aldrichimica Acta, 2013, 46, 71 Search PubMed; (b) A. B. Santanilla, E. R. Regalado, T. Pereira, M. Shevlin, K. Bateman, L. C. Campeau, J. Schneeweis, S. Berritt, Z.-C. Shi, P. Nantermet, Y. Liu, R. Helmy, C. J. Welch, P. Vachal, I. W. Davies, T. Cernak and S. D. Dreher, Science, 2015, 347, 49 CrossRef PubMed; (c) T. Cernak, K. D. Dykstra, S. Tyagarajan, P. Vachal and S. W. Krska, Chem. Soc. Rev., 2016, 45, 546 RSC; (d) M. Christensen, A. Nolting, M. Shevlin, M. Weisel, P. E. Maligres, J. Lee, R. K. Orr, C. W. Plummer, M. T. Tudge, L. C. Campeau and R. T. Ruck, J. Org. Chem., 2016, 81, 824 CrossRef CAS PubMed; (e) M. Shevlin, M. R. Friedfeld, H. Sheng, N. A. Pierson, J. M. Hoyt, L. C. Campeau and P. J. Chirik, J. Am. Chem. Soc., 2016, 138, 3562 CrossRef CAS PubMed; (f) J. A. Selekman, D. Roberts, V. Rosso, J. Qiu, J. Nolfo, Q. Gao and J. Janey, Org. Process Res. Dev., 2016, 20, 70 CrossRef CAS; (g) J. A. Selekman, K. Tran, Z. Xu, M. Dummeldinger, S. Kiau, J. Nolfo and J. Janey, Org. Process Res. Dev., 2016, 20, 1728 CrossRef CAS.
- L. Klier, T. Bresser, T. A. Nigst, K. Karaghiosoff and P. Knochel, J. Am. Chem. Soc., 2012, 134, 13584 CrossRef CAS PubMed.
- S. Wunderlich, PhD Thesis, Ludwig-Maximilians-Universität (DE), 2010 Search PubMed.
- L. Klier, E. Aranzamendi, D. Ziegler, J. Nickel, K. Karaghiosoff, T. Carell and P. Knochel, Org. Lett., 2016, 18, 1068 CrossRef CAS PubMed.
- T. C. Leboho, J. P. Michael, W. A. L. van Otterlo, S. F. van Vuuren and C. B. de Koning, Bioorg. Med. Chem. Lett., 2009, 19, 4948 CrossRef CAS PubMed.
- M. Jaric, B. A. Haag, S. M. Manolikakes and P. Knochel, Org. Lett., 2011, 13, 2306 CrossRef CAS PubMed.
- (a) C. J. Rohbogner, S. H. Wunderlich, G. C. Clososki and P. Knochel, Eur. J. Org. Chem., 2009, 11, 1781 CrossRef; (b) K. Snégaroff, T. T. Nguyen, N. Marquise, Y. S. Halauko, P. J. Harford, T. Roisnel, V. E. Matulis, O. A. Ivashkevich, F. Chevallier, A. E. H. Wheatley, P. C. Gros and F. Mongin, Chem. – Eur. J., 2011, 17, 13284 CrossRef PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7re00057j |
‡ General procedure for automated HTE screens. A PFA sealed 96-well metal block with glass vial inserts was cooled to −45 °C under N2 atmosphere, with 300 rpm agitation, on a Chemspeed SWING robot. To the first row of each quadrant was dispensed a different heterocycle in THF (20 μmol, 0.20 M, 100 μl). Upon 5 min hold, a solution of BF3·Et2O in toluene was dispensed to applicable wells (30 μmol, 1.5 M, 20 μl). Upon 20 min hold, six strong base solutions were dispensed across the first row of each quadrant. The plate was aged for 3 h and a solution of I2 in THF was dispensed across the first row of each quadrant (30 μmol, 0.50 M, 60 μl). The plate was aged for 10 minutes and the first row of each quadrant was quenched with a solution of AcOH in a 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of ACN:DMSO (528 μmol, 0.88 M, 600 μl). These dispense steps were then repeated sequentially at −20, 0 and 25 °C in the second, third and fourth rows of each quadrant. Finally, 28 μl from the reaction plate was transferred to a collection plate containing 700 μl of ACN. 1 mixture of ACN:DMSO (528 μmol, 0.88 M, 600 μl). These dispense steps were then repeated sequentially at −20, 0 and 25 °C in the second, third and fourth rows of each quadrant. Finally, 28 μl from the reaction plate was transferred to a collection plate containing 700 μl of ACN. |
| This journal is © The Royal Society of Chemistry 2017 |