Open Access Article
Open Access ArticleC(sp3)–C(sp2) cross-coupling of alkylsilicates with borylated aryl bromides – an iterative platform to alkylated aryl- and heteroaryl boronates†
Brandon A.
Vara‡
,
Matthieu
Jouffroy‡
and
Gary A.
Molander
*
Roy and Diana Vagelos Laboratories, Department of Chemistry, University of Pennsylvania, 231 South 34th Street, Philadelphia, Pennsylvania 19104-6323, USA. E-mail: gmolandr@sas.upenn.edu
First published on 9th September 2016
Abstract
The attractive field of iterative cross-coupling has seen numerous advances, although almost exclusively in the union of sp2-hybridized partners. Conspicuously absent from this useful synthetic manifold is the inclusion of sp3-hybridized pronucleophiles that can undergo transmetalation under mild conditions. Described here is the use of primary and secondary ammonium alkylsilicates, which undergo facile C(sp3)–C(sp2) cross-coupling with borylated aryl bromide partners under photoredox/nickel dual catalysis conditions. This operationally simple procedure allows the production of alkylated small molecules possessing boronate ester (BPin, Bneopentyl, BMIDA) functional handles. Because of the extremely mild reaction conditions and the innocuous byproduct generated upon fragmentative oxidation of silicates, the corresponding borylated compounds were isolated in good to excellent yields. Aryl bromides bearing unprotected boronic acids are also generally tolerated for the first time and prove useful in multistep syntheses. Unlike many previously reported photoredox/Ni dual cross-couplings, the C(sp3)–C(sp2) bonds were forged using a transition metal-free photocatalyst, allowing a substantial increase in sustainability as well as a cost reduction. Because the developed Ni-catalyzed cross-coupling does not require discrete boron speciation control, as in many popular orthogonal Pd-based methods, this protocol represents a significant advance in atom- and step-economy.
Introduction
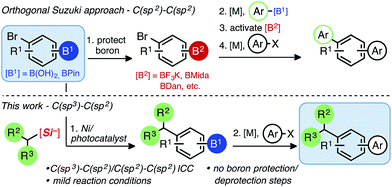
Iterative cross-coupling strategies in the presence of reactive handles for the rapid assembly of multifunctionalized small molecules have seen numerous advances in recent years,1 capitalizing on decades of increasingly reliable C(sp2)–C(sp2) cross-coupling approaches. Arenes and heteroarenes constitute the core structural feature in many materials (pharmaceuticals or agrochemicals), and polysubstituted arenes allow multidirectional syntheses, greatly extending accessible chemical space. Although significant improvements have been realized, primarily in the context of iterative Suzuki–Miyaura cross-coupling reactions,2 other traditional C–C cross-coupling methods have been associated with various constraints, such as high temperatures, long reaction times, and the need for strong bases and/or protecting groups.N-Methyliminodiacetic boronates (BMIDA),3 1,8-diaminonaphthalenes (BDAN),4 and trifluoroborates (BF3K)5 are the most popular modes of boron protection ([B2], Scheme 1) in Pd-based iterative cross-coupling, unmasking the latent functional group through solvent swaps/hydrolysis sequences to unveil the reactive boronic acid or ester ([B1]). Burke has been instrumental in employing MIDA boronate esters in an iterative and recently automated context.6 However, boronic acids and pinacol boronates remain the most widely employed and versatile partners because of their high and predictable reactivity using transition metal catalysis, even though protection/functionalization from these commercially available species is often non-ideal in terms of atom- and step economy.7
 | ||
| Scheme 1 Comparison between traditional orthogonal cross-coupling reactions and this work. ICC = iterative cross-coupling. | ||
In a separate vein, the general union of C(sp3) pronucleophiles with C(sp2) partners [aryl- or alkenyl(pseudo)halides] in the presence of reactive handles (e.g., boronic acids or boronates) has been a longstanding challenge under traditional Pd-based catalysis and still represents a formidable transformation for which a direct solution remains elusive.8 A powerful synthetic paradigm was recently unveiled by several groups, including our own, combining mild photoredox catalysis with transition metal cross-coupling, granting access to new C(sp3)–C(sp2) bond-forming reactions of alkyl radicals.9 This process takes advantage of a facile single electron transmetalation step, circumventing the high-energy and difficult two-electron transmetalation required by traditional alkyl pronucleophiles (e.g., alkylboron reagents).
Most recently, we reported the use of ammonium alkylsilicates that smoothly react with aryl bromides, generating new sp3–sp2 C–C bonds.10 Under this photoredox/Ni dual cross-coupling protocol, a variety of unactivated ammonium alkylsilicates were cross-coupled with various (hetero)aryl and alkenyl halides10e or sulfonates in high yields. In comparison to previous reports using potassium alkyltrifluoroborates9b,11,14 and carboxylic acid derivatives9e,12 in photoredox/nickel-mediated C(sp3)–C(sp2) cross-couplings, reactions involving ammonium alkylsilicate radical precursors do not require any additives or additional base, allowing exceptional functional group tolerance, especially for protic functional groups. The relatively low oxidation potential of the ammonium silicates (E0 = +0.75 V vs. SCE for 1° alkylsilicates, on average)13 allows the use of the less expensive and readily available [Ru(bpy)3](PF6)2 photocatalyst. Collectively, the extremely mild reaction parameters used for alkylsilicates and the virtually barrierless nature of alkyl-to-nickel transfer should allow the general inclusion of diverse, reactive handles under this odd-electron manifold.
We recently unveiled the first general and mechanistically orthogonal union of alkyl-BF3K nucleophiles with borylated bromoarenes.14 This departure from strictly C(sp2) coupling partners in an iterative fashion offers a potentially powerful tool to access structurally more elaborate molecules in short order. Unlike conventional, orthogonal Suzuki reactions between sp2-hybridized bromides and sp2-hybridized boronic acids, where orthogonality is based solely on boron speciation (Scheme 1),15 the applicability of mildly-generated sp3-carbon nucleophiles is a feature that not only complements Pd-based iterative cross-coupling protocols, but one that is mechanistically distinct from preexisting cross-coupling methods, suggesting broad impact.
Though successful in C(sp3)–C(sp2)/C(sp2)–C(sp2) cross-couplings, alkyl-BF3Ks possess several drawbacks, notably the release of BF3 upon oxidative fragmentation. This byproduct is corrosive and can inhibit reaction progression. Additionally, BF3 can be problematic for the tolerance of some functional groups, and its generation requires the addition of exogenous base. In the presence of boronate esters, these inherent limitations prevented the straightforward isolation of the intermediate boron-containing compounds.14 Therefore, we envisioned a mild C(sp3)–C(sp2) dual catalytic cross-coupling of alkylsilicates with borylated aryl bromides, complementing the paucity of Pd- and Ni-based C(sp3)–C(sp2) orthogonal Suzuki–Miyaura strategies. Successful execution would be a welcomed advance to the field by accelerating the modular synthesis of complex small molecules.16
Results and discussion
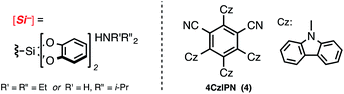
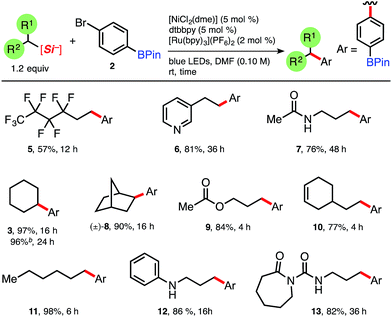
We began by gauging the reactivity and tolerability of cyclohexylsilicate 1 with a borylated bromoarene, 4-bromophenyl pinacolboronate (2). The combination of photocatalyst [Ru(bpy)3](PF6)2, (2 mol%), alongside [NiCl2(dme)]/dtbbpy (5 mol% of each; dtbbpy = 4,4′-di-tert-butyl-2,2′-bipyridine) in the presence of blue LEDs was previously shown to be suitable for cross-coupling and was attempted first.10a After 16 h we observed full conversion (99%, entry 1, Table 1) to the desired C(sp3)–C(sp2) coupled pinacol ester 3 by HPLC analysis, without any byproducts (e.g., protodeboronation or oxidation) traditionally associated with Pd-catalysis or more harsh coupling conditions.To verify that the proposed mechanistic events are transpiring as hypothesized, control experiments in the absence of blue LEDs, photocatalyst, and nickel (entries 2–4, Table 1, respectively) afforded none of the desired coupled product, only starting material (2). Fortunately, the organic photocatalyst 4CzIPN174 provided 3 in comparable yield to the ruthenium photocatalyst (entry 6, Table 1), as did the more strongly oxidizing iridium photocatalyst (entry 5, Table 1). Additionally, organic photocatalyst 4 was compatible with a standard 26 W compact fluorescent lightbulb (CFL) and white LEDs (95% and 94% yield, entries 9 and 10, respectively).
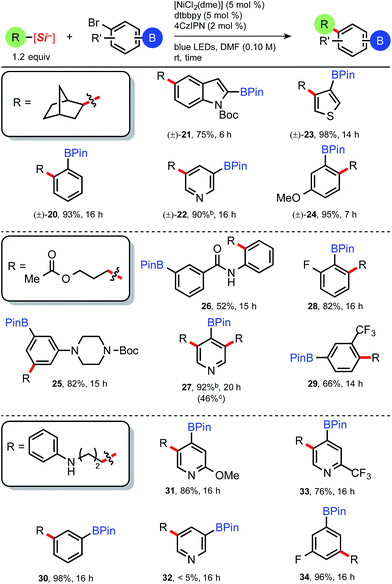
With suitable reaction conditions in hand for the photoredox/Ni dual-catalyzed cross-coupling of 4-bromophenyl pinacolboronate (2), we set out to determine the generality of the developed protocol using a library of ammonium alkylsilicates. A range of ammonium alkylsilicates were well tolerated, and various 2° and even 1° alkylsilicates were effectively cross-coupled in good to excellent yields without compromising the integrity of the boronate ester (Table 2). Brønsted and Lewis basic 3-pyridyl (6) and aniline (12) adducts can be generated and isolated from the corresponding silicates without issue. As the developed protocol requires no additives or base, protic amide 7 and urea 13 can be suitably cross-coupled with 2. To our knowledge, these often problematic moieties have never been cross-coupled in the presence of pinacolboronates, notably expanding the alkyl partners and functional groups that can be integrated under this C(sp3)–C(sp2) cross-coupling manifold. To demonstrate the scalability of this photocatalyzed reaction, 1.0 g of 4-bromophenyl pinacolboronate 2 was coupled in a simple round bottom flask (see ESI†) and also afforded adduct 3 in excellent yield (96%, Table 2), moreover with reduced catalyst loading {1.5 mol% 4CzIPN, 3 mol% [NiCl2(dme)] and [dtbbpy]}. It is also worth noting that the crude reaction profiles in nearly all cases are exceptionally clean following a simple basic workup and extraction.18
| a Isolated yields. Ar = 4-phenyl pinacol boronate. [NiCl2(dme)] was complexed with dtbbpy prior to reaction setup, although pre-complexation was shown to not always be necessary, see ESI. Reactions run on 0.5 mmol of bromide 2 for the allotted time. b 1.00 gram (3.5 mmol) of 2, dtbbpy (3 mol%), [NiCl2(dme)] (3 mol%) and 4CzIPN (1.5 mol%). |
|---|
|
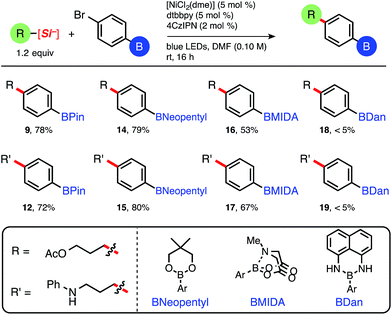
Satisfied with the functional group tolerance of primary and secondary ammonium alkylsilicates, we next explored other common boronate esters (Table 3). Although this mild C(sp3)–C(sp2) cross-coupling is tolerant of relatively reactive pinacolboronate esters and does not necessitate the use of more robust boron protecting groups as required in other iterative methods using Pd,15 diverse masked boronate esters are nonetheless useful reagents in cross-coupling chemistry. Having established the effectiveness of the 4CzIPN organic photocatalyst (4), we decided to use the latter because it is free of costly transition metals (i.e., Ir or Ru), and therefore inherently more sustainable and cost efficient (Table 3).19 Pinacolboronates 9 and 12 and the neopentyl boronate ester adducts (14 and 15) were isolated in good yields following standard column chromatography.
| a Isolated yields. [NiCl2(dme)] was complexed with dtbbpy ligand prior to reaction setup, see ESI. Reactions run on 0.5 mmol of aryl bromide. |
|---|
|
Interestingly, yields obtained for 9 and 12 while using 4CzIPN (Table 3) are in the same range as those obtained using [Ru(bpy)3](PF6)2 (Table 2), proving the efficiency of the organic photocatalyst under this reaction manifold. The reactions employing BMIDA aryl bromide proceeded to nearly full, clean conversion, although yields of 16 and 17 were moderate (16 isolated via crystallization) because of difficulties in purification. Nevertheless, the prepared BMIDA boronate esters can be useful intermediates in the automated, iterative assembly of complex molecules.6 The 1,8-diaminonaphthalene (BDan) adducts (18 and 19) uniformly provided trace desired product.
The attractiveness of a simplified assembly of alkylated arenes bearing reactive, borylated handles may perhaps be most relevant in pharmaceutical discovery chemistry. Although successive, bidirectional reactions at both boron and the halogen are attractive processes, we were initially interested in isolating the alkylated BPin intermediates, as we surmised these may serve as more useful synthetic intermediates toward discretionary tandem reactions.15d From this viewpoint, we set out to demonstrate the breadth of structural diversity by examining three distinct ammonium alkylsilicates (Table 4) with various bromo-substituted aryl- and heteroaryl pinacol boronates. To our knowledge, heteroaromatic BPins, which are known to be particularly sensitive because of their reactivity, have never been successfully alkylated under a transition metal-mediated process. The bicycloheptylsilicate (35) coupled smoothly with hindered benzene-derived bromo BPins to afford 20 and 24 (Table 4), as well as several heteroarenes (21–23) for the first time. Boronate esters adjacent to heteroatoms as in 21 are notoriously challenging to couple under Pd-catalysis (owing to competitive protodeboronation),20 but prove to be robust under these Ni-catalyzed conditions. 3-Bromopyridyl BPin (precursor to 22) was an intriguing case, as the crude reaction mixture contained only 22 and residual catechol, yet 22 largely decomposed upon purification attempts (90% crude yield, see ESI†). We conjectured 22 could be carried on crude though subsequent transformations (vide infra).
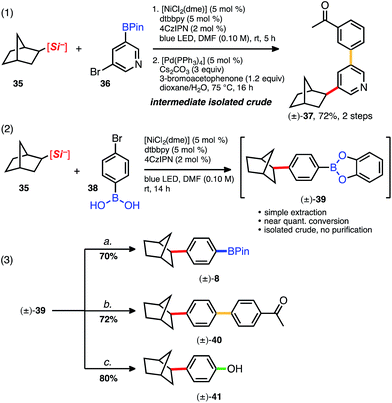
The 3-acetoxypropylsilicate was found to be a suitable substrate when paired with various borylated aryl- and heteroaryl bromides. The more structurally complex bromo piperazinylarene and 2-bromophenylbenzamide BPins afforded 25 and 26, respectively, in acceptable yields (82% and 52%; Table 4). The dialkylated pyridine 27 suffered from higher than normal instability on silica, yet in this case could be isolated pure in a modest 46% yield. Protic and even mildly Brønsted acidic substrates are traditionally incompatible under basic Suzuki cross-coupling conditions, and in this context, the aniline-derived silicate (3rd box, Table 4) together with various borylated aryl bromides was next examined. Collectively, alkylation of various bromoarenes proceeded in generally high yields, including the electron-rich 2-methoxypyridyl BPin 31 and the electron-poor 2-trifluoromethylpyridyl BPin 33. 5-Bromopyridyl-3-pinacol boronate 32 was not compatible with this particular silicate. Observing clean crude reaction profiles from the C(sp3)–C(sp2) cross-coupling, we surmised purification limitations (i.e., for compounds 22 and 27, Table 4) could be overcome by directly reacting the crude cross-coupled BPin in a following transformation. Alkylated pyridyl BPin 22 expressed uncharacteristic instability on silica gel and thus provided an opportunity to investigate this notion. Silicate 35 and bromopyridyl BPin 36 were subjected to the standard reaction conditions using 4CzIPN (4), and following a basic aqueous workup and isolation, the alkylated crude material (22) was next subjected to standard Suzuki conditions with 3-bromoacetophenone (Scheme 2, eqn (1)). The reaction proceeded smoothly (even in the presence of trace residual catechol and 4CzIPN), and difunctionalized pyridine 37 was isolated in 72% yield over 2 steps. In direct comparison to other orthogonal cross-coupling reactions, it is evident that this iterative C(sp3)–C(sp2), C(sp2)–C(sp2) coupling does not require boron speciation control,1b,15a activation, deprotection, or neighboring group control3 of any kind, streamlining accessibility to multifunctionalized molecules.
We next questioned whether these mild and base-free reaction conditions were tolerant of bromoarylboronic acids. Arylboronic acids arguably represent the most accessible and convenient modular building blocks in cross-coupling chemistry, yet notoriously require protection in multistep syntheses because of their high reactivity under various modes of catalysis. Bicycloheptylsilicate 35 was subjected to a standard C(sp3)–C(sp2) cross-coupling with 4-bromophenyl-boronic acid 38 using 4 as the photocatalyst (Scheme 2, eqn (2)). Full conversion was accomplished after 14 h, but esterification of the boronic acid had occurred, generating the catechol boronate ester (39, Scheme 2).
Even though catecholboronate esters are among the most sensitive of all the boronate functional groups, 39 nonetheless proved synthetically useful if promptly reacted. Thus, catechol 39 was subsequently transformed in several different ways. Alkylation and subsequent protection with pinacol afforded BPin 8 in good yield (70%, 2 steps; Scheme 2, eqn (3)). Subjection of 39 to Suzuki–Miyaura conditions afforded arylated 40 in 72% yield over two steps, and straightforward oxidation of 39 also proceeded smoothly, generating phenol 41 in 80% yield over 2 steps. To our knowledge, these tandem 2-step syntheses beginning from unadulterated arylboronic acids are the first examples of C(sp3)–C(sp2) cross-coupling with such general tolerance of these abundant building blocks. Collectively, reactions using bromoboronic acids are comparable in yield and setup to those employing more robust bromoaryl BPins.
Conclusions
In summary, secondary and primary ammonium alkylsilicates were found to be exceptional alkylating agents in the presence of various brominated aryl- and heteroaryl boronate esters, and for the first time, ubiquitous boronic acids prove to be generally tolerable substrates to more elaborate compounds. Indeed, because of the extremely mild reaction conditions and single-electron regime used for the cross-coupling of ammonium alkylbis(catecholato)silicates with (hetero)aryl bromides, various boronate ester functional groups were largely allowable while forging C(sp3)–C(sp2) bonds. Moreover, unlike most of the methods reported so far, the versatile, alkylated (hetero)aryl boronates can be isolated in high yields or carried through in crude form. Under this photoredox/Ni dual-catalysis manifold, the implementation of readily available Ni(II), light, and a cost-effective organic photocatalyst in place of late transition metal-based photocatalysts constitutes an unparalleled method toward multifunctionalized, alkylated arenes.Acknowledgements
We thank Kingson Lin (University of Pennsylvania) for the preparation of alkylsilicates. We thank NIGMS (RO1 GM-113878) for financial support of this research.Notes and references
- (a) C. Wang and F. Glorius, Angew. Chem., Int. Ed., 2009, 48, 2 CrossRef; (b) L. Xu, S. Zhang and P. Li, Chem. Soc. Rev., 2015, 44, 8848 RSC.
- (a) N. Miyaura, Bull. Chem. Soc. Jpn., 2008, 81, 1535 CrossRef CAS; (b) A. Suzuki, Angew. Chem., Int. Ed., 2011, 50, 6722 CrossRef CAS PubMed.
- For examples of the use of BMIDA protecting groups: (a) E. P. Gillis and M. D. Burke, J. Am. Chem. Soc., 2007, 129, 6716 CrossRef CAS PubMed; (b) S. J. Lee, K. C. Gray, J. S. Paek and M. D. Burke, J. Am. Chem. Soc., 2008, 130, 466 CrossRef CAS PubMed; (c) Z. He, A. Zajdlik, J. D. S. Denis, N. Assem and A. K. Yudin, J. Am. Chem. Soc., 2012, 134, 9926 CrossRef CAS PubMed; (d) N. A. Isley, F. Gallou and B. H. Lipshutz, J. Am. Chem. Soc., 2013, 135, 17707 CrossRef CAS PubMed; (e) E. M. Woerly, J. Roy and M. D. Burke, Nat. Chem., 2014, 6, 484 CrossRef CAS PubMed.
- For examples of the use of BDan protecting groups: (a) H. Noguchi, K. Hojo and M. Suginome, J. Am. Chem. Soc., 2007, 129, 758 CrossRef CAS PubMed; (b) H. Noguchi, T. Shioda, C.-M. Chou and M. Suginome, Org. Lett., 2008, 10, 377 CrossRef CAS PubMed; (c) N. Iwadate and M. Suginome, Org. Lett., 2009, 11, 1899 CrossRef CAS PubMed; (d) N. Iwadate and M. Suginome, Chem. Lett., 2010, 39, 558 CrossRef CAS.
- (a) G. A. Molander and B. Canturk, Angew. Chem., Int. Ed., 2009, 48, 9240 CrossRef CAS PubMed; (b) G. A. Molander and D. L. Sandrock, J. Am. Chem. Soc., 2008, 130, 15792 CrossRef CAS PubMed.
- J. Li, S. G. Ballmer, E. P. Gillis, S. Fujii, M. J. Schmidt, A. M. E. Palazzolo, J. W. Lehmann, G. F. Morehouse and M. D. Burke, Science, 2015, 347, 1221 CrossRef CAS PubMed.
- T. Newhouse, P. S. Baran and R. W. Hoffmann, Chem. Soc. Rev., 2009, 38, 3010 RSC.
- (a) K. Endo, T. Ohkubo, M. Hirokami and T. Shibata, J. Am. Chem. Soc., 2010, 132, 11033 CrossRef CAS PubMed; (b) S. N. Mlynarski, C. H. Schuster and J. P. Morken, Nature, 2014, 505, 386 CrossRef CAS PubMed.
- Seminal reports: (a) D. Kalyani, K. B. McMurtrey, S. R. Neufeldt and M. S. Sanford, J. Am. Chem. Soc., 2011, 133, 18566 CrossRef CAS PubMed; (b) Y. Ye and M. S. Sanford, J. Am. Chem. Soc., 2012, 134, 9034 CrossRef CAS PubMed; (c) B. Sahoo, M. N. Hopkinson and F. Glorius, J. Am. Chem. Soc., 2013, 135, 5505 CrossRef CAS PubMed; (d) J. C. Tellis, D. N. Primer and G. A. Molander, Science, 2014, 345, 433 CrossRef CAS PubMed; (e) Z. W. Zuo, D. T. Ahneman, L. L. Chu, J. A. Terrett, A. G. Doyle and D. W. C. MacMillan, Science, 2014, 345, 437 CrossRef CAS PubMed.
- (a) M. Jouffroy, D. N. Primer and G. A. Molander, J. Am. Chem. Soc., 2016, 138, 475 CrossRef CAS PubMed; (b) V. Corcé, L.-M. Chamoreau, E. Derat, J.-P. Goddard, C. Ollivier and L. Fensterbank, Angew. Chem., Int. Ed., 2015, 54, 11414 CrossRef PubMed; (c) M. Jouffroy, C. B. Kelly and G. A. Molander, Org. Lett., 2016, 18, 876 CrossRef CAS PubMed; (d) M. Jouffroy, G. H. M. Davies and G. A. Molander, Org. Lett., 2016, 18, 1606 CrossRef CAS PubMed; (e) N. R. Patel, C. B. Kelly, M. Jouffroy and G. A. Molander, Org. Lett., 2016, 18, 746 Search PubMed.
- (a) D. Ryu, D. N. Primer, J. C. Tellis and G. A. Molander, Chem.–Eur. J., 2016, 22, 120 CrossRef CAS PubMed; (b) M. El Khatib, R. A. M. Serafim and G. A. Molander, Angew. Chem., Int. Ed., 2016, 55, 254 CrossRef CAS PubMed; (c) I. Karakaya, D. N. Primer and G. A. Molander, Org. Lett., 2015, 17, 3294 CrossRef CAS PubMed; (d) D. N. Primer, I. Karakaya, J. C. Tellis and G. A. Molander, J. Am. Chem. Soc., 2015, 137, 2195 CrossRef CAS PubMed.
- (a) L. L. Chu, J. M. Lipshultz and D. W. C. MacMillan, Angew. Chem., Int. Ed., 2015, 54, 7929 CrossRef CAS PubMed; (b) C. C. Nawrat, C. R. Jamison, Y. Slutskyy, D. W. C. MacMillan and L. E. Overman, J. Am. Chem. Soc., 2015, 137, 11270 CrossRef CAS PubMed.
- Y. Nishigaichi, A. Suzuki and A. Takuwa, Tetrahedron Lett., 2007, 48, 211 CrossRef CAS.
- Y. Yamashita, J. C. Tellis and G. A. Molander, Proc. Natl. Acad. Sci. U. S. A., 2015, 112, 12026 CrossRef CAS PubMed.
- (a) A. J. J. Lennox and G. C. Lloyd-Jones, Chem. Soc. Rev., 2014, 43, 412 RSC; (b) C. P. Seath, J. W. B. Fyfe, J. J. Molloy and A. J. B. Watson, Angew. Chem., Int. Ed., 2015, 54, 9976 CrossRef CAS PubMed; (c) C. W. Muir, J. C. Vantourout, A. Isidro-Llobet, S. J. F. Macdonald and A. J. B. Watson, Org. Lett., 2015, 17, 6030 CrossRef CAS PubMed; (d) E. P. Gillis and M. D. Burke, Aldrichimica Acta, 2009, 1, 17 Search PubMed.
- A single example of the cross-coupling of 4-bromophenyl pinacolboronate with a potassium alkylbis(catecholato)silicate was reported: C. Lévêque, L. Chenneberg, V. Corcé, J.-P. Goddard, C. Olivier and L. Fensterbank, Org. Chem. Front., 2016, 3, 462 RSC.
- J. Luo and J. Zhang, ACS Catal., 2016, 6, 873 CrossRef CAS.
- Upon consumption of the aryl bromide and subsequent workup, the crude reaction mixture contains trace catechol and ligand. In many cases, this crude material can be carried forward without the need for purification. See ESI.†.
- H. Uoyama, K. Goushi, K. Shizu, H. Nomura and C. Adachi, Nature, 2012, 492, 234 CrossRef CAS PubMed.
- (a) G. A. Molander and B. Biolatto, J. Org. Chem., 2003, 68, 4302 CrossRef CAS PubMed; (b) J. Z. Deng, D. V. Paone, A. T. Ginnetti, H. Kurihara, S. D. Dreher, S. A. Weissman, S. R. Stauffer and C. S. Burgey, Org. Lett., 2009, 11, 345 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental details and spectral data. See DOI: 10.1039/c6sc03236b |
| ‡ These authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2017 |