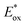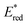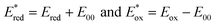Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Photoredox radical conjugate addition of dithiane-2-carboxylate promoted by an iridium(III) phenyl-tetrazole complex: a formal radical methylation of Michael acceptors†
Andrea
Gualandi
 a,
Elia
Matteucci
a,
Elia
Matteucci
 b,
Filippo
Monti
b,
Filippo
Monti
 c,
Andrea
Baschieri
b,
Nicola
Armaroli
c,
Andrea
Baschieri
b,
Nicola
Armaroli
 *c,
Letizia
Sambri
*c,
Letizia
Sambri
 *b and
Pier Giorgio
Cozzi
*b and
Pier Giorgio
Cozzi
 *a
*a
aDipartimento di Chimica “G. Ciamician”, ALMA MATER STUDIORUM Università di Bologna, Via Selmi 2, 40126 Bologna, Italy. E-mail: piergiorgio.cozzi@unibo.it
bDipartimento di Chimica Industriale “Toso Montanari”, ALMA MATER STUDIORUM Università di Bologna, Viale Risorgimento 4, 40136, Bologna, Italy. E-mail: letizia.sambri@unibo.it
cIstituto per la Sintesi Organica e la Fotoreattività, Consiglio Nazionale delle Ricerche, Via P. Gobetti 101, 40129 Bologna, Italy. E-mail: nicola.armaroli@isof.cnr.it
First published on 3rd November 2016
Abstract
A readily accessible iridium(III) phenyl-tetrazole complex ([Ir(ptrz)2(tBu-bpy)]+, 2; Hptrz = 2-methyl-5-phenyl-tetrazole; tBu-bpy = 4,4′-di-tert-butyl-2,2′-bipyridine) is shown to be a versatile catalyst for a new photocatalytic Michael reaction. Under light irradiation in the presence of 2, a dithiane 2-carboxylic acid, obtained by simple hydrolysis of a commercially available ethyl ester, generates a 1,3-dithiane radical capable of performing addition to a variety of Michael acceptors (e.g., unsaturated ketones, esters, amides and malonates). This broad scope reaction with high yields is a formal photo-redox addition of the elusive methyl radical and the adducts obtained can be starting materials for a variety of functionalized products. The excited-state oxidation potential of catalyst 2 allows selective formation of radicals only from α-heterosubstituted carboxylates. Chemical modification of this metal complex can tune the electrochemical properties, opening a route to new highly selective catalytic photo-oxidation reactions.
Introduction
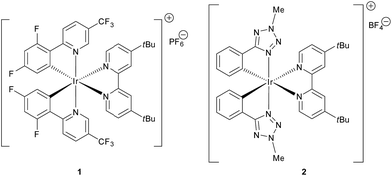
Photoredox catalysis has recently emerged as a mild and efficient method for the generation of radicals, as it is possible to take advantage of the photophysical properties1 of suitable organic dyes2 and transition metal complexes exhibiting tailored electrochemical and photophysical properties.3,4 In particular, MacMillan et al. investigated 1,4-conjugate addition (Michael reaction)5 of radicals6 in connection with a series of electrophilic olefins. In these reactions a photoredox-mediated CO2-extrusion mechanism is operative and a broad array of Michael acceptors have been used. Simple or α-functionalized (N; O) carboxylic acids are employed as Michael donors without the need for organometallic mediated activation or propagation. Recently, Overman et al. have also explored N-phthalimidoyl oxalate derivatives of tertiary alcohols for reductive coupling of tertiary radicals with Michael acceptors, using visible light and [Ru(bpy)3][PF6]2.7 The key relevant characteristics of both the Overman and MacMillan radical generation methodologies were recently merged in a new powerful protocol.8 A typical procedure entails the addition of 1–2 mol% of the photocatalyst Ir[dF(CF3)ppy]2(tBu-bpy)[PF6] (1, Fig. 1), which acts as a strong oxidant in the first step of the photocatalytic cycle.Although these methodologies are extremely effective and use cheap and abundant starting materials, there are still some inherent limitations. Primarily, the commercially available iridium photocatalyst 1 is expensive and a Suzuki coupling is necessary for its preparation.9 Furthermore, the generation of primary radicals under photocatalytic conditions after extrusion of CO2 is challenging, and just one example where primary radicals are intercepted by fluorinating agents is reported in the literature.10 However, to the best of our knowledge, the generation and reaction of primary radicals is still an open issue in photocatalytic Michael reactions. Furthermore, addition of methyl radicals, recently reported using an ambitious methodology based on a Minisci type reaction,11,12 has still not been developed in the area of photocatalysis. In the above described context, the present study has two main goals: (i) to demonstrate the use of an alternative, simply prepared and tunable iridium(III) photocatalyst (2, Fig. 1) for radical reactions, and (ii) to propose a new methodology for effective photocatalytic methylation.
Notably, Baran has recently shown that zinc bis[(phenylsulfonyl)methanesulfinate] can be effectively used for introducing a methyl group through a two-step synthetic procedure, in which a radical mediated process is involved in the first step.13 As a possible synthetic equivalent for a methyl group, we propose herein the chameleonic dithiane group, opening a route to the use of dithianecarboxylate as a Michael donor in photoredox catalysis. A secondary radical is formed, which is stabilized by the presence of two sulfur atoms, and can be used as a versatile synthetic equivalent. Remarkably, the dithiane can be not only replaced by a methyl group by treatment with RANEY®-nickel, but it is also possible to take advantage of its flexibility, allowing the installation of different functional groups such as aldehydes and ketones, upon the radical reaction.
Optimization of the photocatalytic reaction and scope
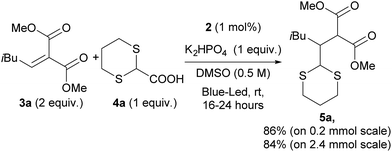
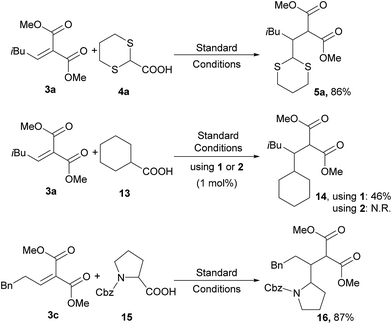
Dithianes, introduced by Corey and Seebach14 are widely used in the synthesis of natural products.15 For instance, they were exploited by Smith for the application of linchpins in the synthesis of complex natural molecules.16 However, the radical reactivity of dithianes has been only rarely reported in the literature, mainly through the installation of a radical initiator group (phenylseleno, xanthate, TEMPO or chloro) at the 2-position for the generation of C-2 centred radicals.17 Direct radical addition of 1,3-dithianes to alkenes was shown to occur in an intramolecular fashion. Quite interestingly, Nishida and co-workers reported an intramolecular photocatalytic addition for the 1,3-dithiane, and this reaction has also been reported with other radical initiators.18 Recently, Leow and co-workers reported the photocatalytic addition of 1,3-benzodithioles to several Michael acceptors.19 However, only aryl or alkyl C2-substituted benzodithiole could be used, while aryl dithiane was found to be completely unreactive. On the other hand, Koike and Akita reported the reaction of potassium 1,3-dithian-2-yl trifluoroborate with terminal olefins bearing electron withdrawing groups20 which act as a synthetic equivalent of carbonyl groups.Based on the previous work by MacMillan5 and on the basis of the recent report on the practical use of commercially available dithiane carboxylate in organocatalytic reactions,21 we used this cheap and commercially available starting material as a suitable reagent for installing various unsubstituted or substituted dithianes by photocatalytic reactions. Accordingly, we have carefully investigated the addition of dithiane carboxylate (4a) to alkylidene malonate (3a) (Scheme 1) in the presence of different photocatalysts, by varying the solvent and other reaction conditions (see ESI for all complexes tested and full details†). Although the commercially available iridium catalyst 1 was found to be effective, we have investigated the catalytic activity of alternative iridium complexes, which can be more easily prepared, such as phenyl-tetrazole and pyridyl-triazole derivatives (see ESI†), without any Suzuki coupling. Only complexes containing phenyl tetrazoles as cyclometalating ligands showed an efficiency comparable to catalyst 1. These compounds were recently reported by some of us22 as emitting materials in electroluminescent devices and here we show that they can also be successfully utilized to promote photocatalytic reactions.
Notably, by careful tuning of the pristine ligand structure, this class of complexes may provide remarkable opportunities to enhance reaction selectivity (vide infra). The photoredox radical conjugate addition of dithiane-2-carboxylate has been used to test our approach, giving full conversions and high isolated yields when the reaction is conducted in DMSO and in the presence of K2HPO4 as an inorganic base (see ESI for details†). MacMillan has shown that the presence of the latter is crucial to form the carboxylate, allowing oxidation and affording the release of CO2; the use of organic bases gave poorer results. Among all of the iridium catalysts tested (see ESI for full details,† and for the results obtained with catalyst 1), complex [Ir(ptrz)2(tBu-bpy)][BF4] (2, Fig. 1) gave the best results under the optimized reaction conditions (Scheme 1), also proving to be highly photostable in DMSO after prolonged irradiation (see ESI†). Specifically, complex 2 is easily obtained through a two-step synthesis22 involving a facile silver-assisted cyclometalation reaction of 2-methyl-5-phenyl-2H-tetrazole with IrCl3 and, notably, the solvato complex intermediate can be a useful precursor for other appropriately designed iridium(III) complexes.
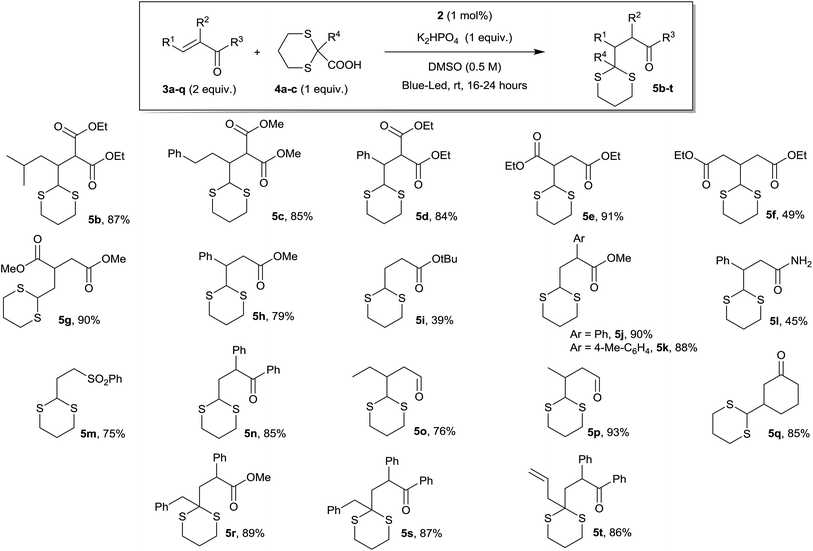
To test the general validity of our approach, we investigated a variety of Michael acceptors and the results obtained are summarized in Scheme 2. Differently substituted Michael acceptors can participate in the dithiane conjugate addition protocol. The mild reaction conditions reported in Scheme 2 are compatible with a wide range of functional groups (e.g., malonates, esters, amides, ketones, aldehydes, sulfones, etc.), which together provide a versatile group for further functionalization and transformation. Unsaturated ketones and aldehydes are well tolerated in both cyclic and acyclic forms (e.g., products 5n–q). In addition, this protocol could be further applied to other electrophilic alkenes, including α,β-unsaturated amides, sulfones, and malonates, as well as acrylates and fumarates, to afford a variety of alkylated dithianes in good to excellent yields. In terms of substituents present in the Michael acceptors, β-substituents are tolerated, as well as α-aryl and α-alkyl groups. It is also worth noting that the tailored synthesis of 2-substituted dithiane-2-carboxylates is possible (see ESI for details†) with a wide range of products (e.g., products 5r–t). In addition, it was possible to scale up the reaction without any problem from 0.2 mmol to 2.4 mmol.
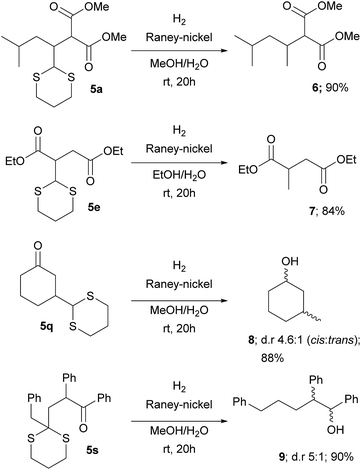
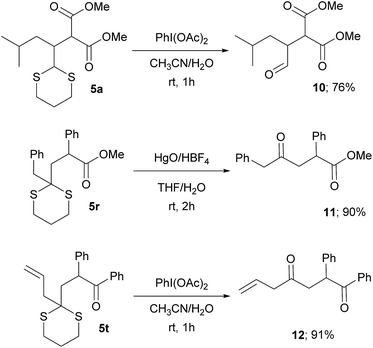
This protocol avoids the use of strong bases and allows the direct addition of a versatile dithiane under very mild reaction conditions. The methodology, by coupling the reaction with RANEY®-nickel desulfurization realized on the crude reaction mixture, gave direct access to the corresponding methyl group in a straightforward way (Scheme 3). When ketones are present, the direct treatment can lead to their reduction, as observed for 5q and 5s. Moderate to good diastereoselection is observed for the reaction. On the other hand, it is possible to transform the dithiane with the corresponding carbonyl group by an easy deprotection reaction under well-established conditions23 as is reported with selected examples, giving the desired products in high yields (Scheme 4). Eventually, substituted or unsubstituted dithiane can be used for further modifications.
Photocatalyst selectivity
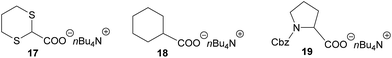
An interesting feature of the catalyst 2 is its selectivity towards oxidation of substrates. In fact, while the photocatalyst 1 is not able to discriminate between functionalized or unfunctionalized carboxylic acids, the complex 2 is able to selectively oxidize only α-functionalized acids (e.g., 4a and 15 in Scheme 5). Accordingly, no photo-generated radicals can be formed from unfunctionalized derivatives like, for instance, cyclohexanecarboxylic acid (13 in Scheme 5).24Electrochemical and photophysical studies of the catalytic system
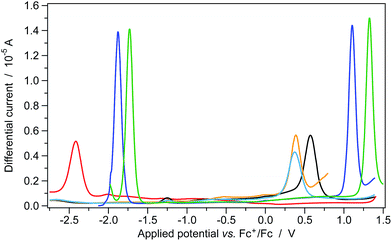
The photo-oxidation selectivity of the complex 2, compared to the iridium catalyst 1, can be easily understood by comparing the excited-state redox potentials, estimated by combining photophysical and electrochemical data (see ESI for details†).The redox potentials of the two above mentioned iridium photocatalysts (i.e., 1 and 2), together with those of the tetrabutylammonium carboxylates of selected carboxylic acids (Fig. 2) are gathered in Fig. 2, Fig. S3† and Table 1.
| E ox [V] | E red [V] | ΔEredoxc [V] | [V] | [V] | |
|---|---|---|---|---|---|
a All of the measurements were performed in room-temperature acetonitrile solution + 0.1 M TBAPF6. All of the redox potentials are referenced to the ferrocene/ferrocenium couple, which was used as an internal standard.
b An irreversible redox process with an estimated error of ±0.05 V.
c ΔEredox = Eox − ΔEred.
d
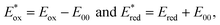 where E00 is the energy gap between the ground and excited states determined spectroscopically using the data reported in Fig. S4. Estimated error: ±0.1 V. where E00 is the energy gap between the ground and excited states determined spectroscopically using the data reported in Fig. S4. Estimated error: ±0.1 V.
|
|||||
| 17 | +0.39a | — | — | — | — |
| 18 | +0.58a | — | — | — | — |
| 19 | +0.37a | — | — | — | — |
| 3a | — | −2.42b | — | — | — |
| 1 | +1.32 | −1.73 | 3.05 | ≈−1.1 | ≈+0.7 |
| 2 | +1.11 | −1.89 | 3.00 | ≈−1.2 | ≈+0.4 |
The electrochemical and photophysical experiments were carried out on carboxylates – instead of pristine carboxylic acids – because only after deprotonation by K2HPO4 are the acid derivatives oxidized by the iridium photocatalyst, leading to CO2 release and their subsequent radical addition of Michael acceptors.5–7 Tetrabutylammonium was selected as the counterion to increase the solubility of the carboxylates in acetonitrile and in order to have the same cation as that of the supporting electrolyte used for the electrochemical experiments.25
The oxidation potential of the unfunctionalized cyclohexanecarboxylate 18 is approx. 0.2 V higher when compared to that of the more electron-rich heterocyclic compounds 17 and 19 (see Table 1). Therefore, a stronger oxidant is required to activate 18, while the two functionalized derivatives 17 and 19 are more prone to oxidation.
It is worth noting that, despite both iridium complexes having virtually the same redox gap (of around 3 eV), the oxidation and reduction processes of 2 occur at more positive potentials (approx. +0.2 V) when compared to the polyfluorinated 1 complex. This is due to the lack of electron-withdrawing fluorine substituents in the new tetrazole-based complex. It is well-known that iridium-based photocatalysts (e.g., 1 and 2) are strongly oxidizing species once excited to their lowest excited state.5–7 The excited-state redox potential of these molecules 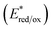 can be roughly estimated using a simplified version of the so-called Rehm–Weller equation:26
can be roughly estimated using a simplified version of the so-called Rehm–Weller equation:26
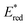 values of 1 and 2 (Table 1) do provide an explanation for the photo-oxidation selectivity of the complex 2, compared to the commercially available iridium catalyst 1. In fact, while the excited-state reduction potential of 2 is not high enough to oxidize 18 (i.e., +0.4 V < +0.58 V), this is not the case for the complex 1, having
values of 1 and 2 (Table 1) do provide an explanation for the photo-oxidation selectivity of the complex 2, compared to the commercially available iridium catalyst 1. In fact, while the excited-state reduction potential of 2 is not high enough to oxidize 18 (i.e., +0.4 V < +0.58 V), this is not the case for the complex 1, having 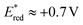 (i.e., well above the oxidation potential of 18; see Table 1).
(i.e., well above the oxidation potential of 18; see Table 1).
Stern–Volmer experiments
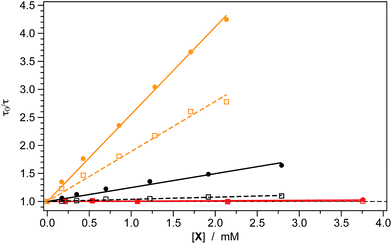
This scenario is also corroborated by the Stern–Volmer quenching experiments which were carried out to explore in more detail the intramolecular reductive quenching of the photocatalyst phosphorescence operated by the carboxylate substrates. The results are summarized in Table 2 and depicted in Fig. 3. As expected, both 1 and 2 are quenched by the presence of 17 due to a bimolecular quenching process having approximately the same rate constant for both photocatalysts (kq ≈ 7 × 108 M−1 s−1, see Table 2).| Photocatalyst 1a | Photocatalyst 2a | |||
|---|---|---|---|---|
| K SV [mM−1] | k q [108 M−1 s−1] | K SV [M−1] | k q [108 M−1 s−1] | |
| a All of the experiments were carried out in oxygen-free acetonitrile at 298 K with a photocatalyst concentration of 0.015 mM, with excitation at 330 nm. Data are reported with a ±95% confidence interval. In all cases, the quality of the fitting is assured by a R2 > 0.98. b k q = KSV/τ0, where τ0 is the unquenched excited-state lifetime of the photocatalyst. c There is no evidence of there being correlation between the excited-state quenching of the photocatalyst and the increasing amounts of 3a up to a concentration of 4 mM. | ||||
| 17 | 1.56 ± 0.05 | 6.4 ± 0.2 | 0.89 ± 0.07 | 7.6 ± 0.6 |
| 18 | 0.24 ± 0.03 | 1.0 ± 0.1 | 0.039 ± 0.006 | 0.32 ± 0.05 |
| 3a | Quenching not observedc | Quenching not observedc | ||
On the other hand, the unsubstituted carboxylate 18 is able to quench the excited state of 1 with a kq that is more than three times higher than in the case of 2 (i.e., 1.0 vs. 0.3 × 108 M−1 s−1, see Table 2).
As shown in Fig. 4, the excited-state lifetime of both 1 and 2 is virtually unaffected by the presence of the Michael acceptor 3a (at least for concentrations up to 3.75 mM). This evidence is in accordance with the electrochemical data reported in Table 1, showing that the excited-state redox potentials of both of the iridium photocatalysts cannot promote any redox process on 3a.
Determination of quantum yield
In order to estimate the efficiency of our photocatalyst 2 and to assess the potential presence of an important radical chain contribution to the catalytic cycle, we evaluated the quantum yield of the reaction between the Michael acceptor 3a and the carboxylate 17, under the optimized conditions reported in the ESI.† The reaction was irradiated at 334 nm with a 100 W Hg lamp; see the Experimental section for further details. The choice of such an excitation wavelength was dictated by: (i) the higher molar absorptivity of the photocatalyst compared to 450 nm blue LED excitation;22 (ii) the high reliability of the potassium ferrioxalate actinometer in this spectral region, which is not the case for λ > 450 nm;28 (iii) the still high selectivity of excitation, since all the reagents are optically transparent for λ > 300 nm (see Fig. S6†).The determined quantum yield of the reaction is 0.28 ± 0.05. This value indicates that a radical chain mechanism is unlikely in our reaction; however, it could not be totally ruled out.29
Proposed reaction mechanism
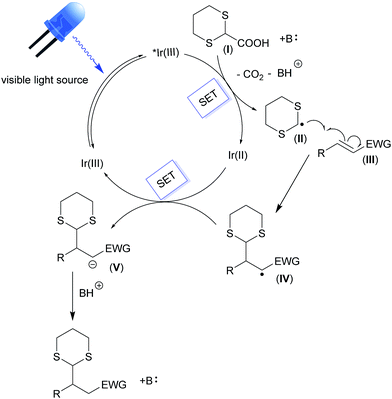
The above illustrated experimental findings corroborate the reaction mechanism depicted in Fig. 5. Upon light absorption in the visible part of the electromagnetic spectrum, the iridium photocatalyst (IrIII) is excited to its lowest electronic excited state (*IrIII). This initial event is the only one possible, since all of the other molecules are transparent to visible light (see Fig. S5 and S6†). Next, the photoexcited complex is able to oxidize a suitable carboxylate derivative (I) inducing its decarboxylation and the formation of the corresponding radical species (II). Subsequently, the addition of this radical to the Michael acceptor (III) affords the radical intermediate IV, which undergoes a second SET event from the reduced iridium complex (IrII). The photocatalyst is then restored and IV is converted into the carbanionic intermediate V. The latter can be easily protonated, leading to the final reaction products.Conclusions
In conclusion, we have developed a practical and effective photocatalytic addition of dithiane-2-carboxylates to Michael-type acceptors promoted by the iridium(III) complex 2, which is used for the first time as a powerful photocatalyst. The reaction has broad scope and allows the introduction of dithiane in a variety of Michael acceptors (unsaturated ketones, esters, amides, malonates, etc.) in high yields. The adducts can be further functionalized with established chemistry by oxidative deprotection or alkylation. In particular, this photocatalytic reaction can be used to introduce a methyl group to unsaturated compounds, after elimination of dithiane by RANEY®-nickel. The photocatalyst 2 is easily accessible and its redox properties can be finely tuned by chemical modification of the ligands, affording a brand new class of new Ir(III) photocatalysts. The electrochemical potential of 2 allows tailored oxidation and this opens the way to its selective use in photocatalysis to activate substrates. Studies on a stereoselective variant of the reaction proposed here are under active investigation in our laboratories.Experimental section
General procedure
In a Schlenk tube with a rotaflo® stopcock under an argon atmosphere at r.t., catalyst 2 (1.7 mg, 0.002 mmol), 1,3-dithiane-2-carboxylic acid 4a (0.2 mmol, 0.032 g) and K2HPO4 (0.2 mmol, 0.034 g) were dissolved in 400 μL of DMSO. After 2 min, the Michael acceptor (0.4 mmol) was added. The reaction mixture was carefully degassed via freeze–pump–thaw (three times), and the vessel was refilled with argon. The Schlenk tube was stirred and irradiated with a blue LED positioned approximately at a 10 cm distance from the reaction vessel. After 16 h of irradiation, NaHCO3 sat. solution (2 mL) was added and the mixture was extracted with ethyl acetate (4 × 5 mL). The collected organic layers were dried over Na2SO4, filtered and concentrated under reduced pressure to give the crude products. Column chromatography on silica (cyclohexane:ethyl acetate or cyclohexane:Et2O) afforded pure compounds.Electrochemistry
Voltammetric experiments were performed using a Metrohm AutoLab PGSTAT 302 electrochemical workstation in combination with the NOVA software package. All of the measurements were carried out at room temperature in acetonitrile solutions with a sample concentration of approx. 1 mM using 0.1 M tetrabutylammonium hexafluorophosphate (electrochemical grade, TBAPF6) as the supporting electrolyte. Oxygen was removed from the solutions by bubbling them with argon for 20 minutes. All of the experiments were carried out using a three-electrode setup (BioLogic VC-4 cell, with a cell volume of 5 mL) with a glassy-carbon working electrode (1.6 mm diameter), the Ag/AgNO3 redox couple (0.01 M in acetonitrile with 0.1 M TBAClO4 supporting electrolyte) as the reference electrode and a platinum wire as the counter electrode. At the end of each measurement, ferrocene was added as the internal reference. Cyclic voltammograms (CV) were typically recorded at a scan rate of 200 mV s−1, but several rates were used to check reversibility (in the range between 50 and 2000 mV s−1). Osteryoung square-wave voltammograms (OSWV) were recorded with a scan rate of 100 mV s−1, a SW amplitude of ±20 mV and a frequency of 25 Hz.Photophysical measurements
All of the spectroscopic investigations were carried out in spectrofluorimetric grade acetonitrile using fluorimetric Suprasil® quartz cuvettes with a 10.00 mm path length. Absorption spectra were recorded with a Perkin-Elmer Lambda 950 spectrophotometer. All photoluminescence experiments were performed in oxygen-free solution, by removing oxygen through argon bubbling for 20 minutes. The uncorrected emission spectra were obtained with an Edinburgh Instruments FLS920 spectrometer equipped with a Peltier-cooled Hamamatsu R928 photomultiplier tube (PMT) (185–850 nm). An Edinburgh Xe 900 (450 W xenon arc lamp) was used as the excitation light source. The corrected spectra were obtained via a calibration curve supplied with the instrument. The photoluminescence quantum yields (PLQY) in solution were obtained from the corrected spectra on a wavelength scale (nm) and measured according to the approach described by Demas and Crosby30 using an air-equilibrated water solution of quinine sulfate in 1 N H2SO4 as the reference (PLQY = 0.546).31 The emission lifetimes (τ) were measured through the time-correlated single photon counting (TCSPC) technique using an HORIBA Jobin Yvon IBH FluoroHub controlling a spectrometer equipped with a pulsed NanoLED (λexc = 330 nm; FWHM = 11 nm) as the excitation source and a red-sensitive Hamamatsu R-3237-01 PMT (185–850 nm) as the detector. Analysis of the luminescence decay profiles was accomplished with DAS6 Decay Analysis Software provided by the manufacturer, and the quality of the fitting was assessed with the χ2 value close to unity and with the residuals regularly distributed along the time axis. Samples were excited at 340 nm for the evaluation of PLQYs and at 330 nm for τ determination. Experimental uncertainties are estimated to be ±10% for τ determinations, ±20% for PLQY, and ±2 nm and ±5 nm for absorption and emission peaks, respectively. Stern–Volmer quenching experiments were performed at room-temperature under oxygen-free conditions (in an argon-saturated environment) using 3 mL of acetonitrile solution containing the appropriate iridium photocatalyst (with a concentration of 1.5 × 10−5 M) and increasing amounts of quencher. For determination of the photocatalytic quantum yield, the reaction was carried out in spectrofluorimetric grade DMSO and placed in a Suprasil® quartz cuvette with a 2.00 mm path length. The reaction mixture was excited at 334 nm, using a 100 W Hg lamp equipped with an appropriate dichroic filter. The photon flux was estimated using a ferrioxalate actinometer, following the procedure reported by Montalti et al.28 The conversion of the reaction was determined by 1H-NMR.Acknowledgements
A. G. and P. G. C. are grateful to Fondazione Del Monte, Farb funds University of Bologna (project SLAMM to A. G.) and EU-Foundation through the TEC FP7 ICT-Molarnet project (318516) for partial financial support of this research. N. A. and F. M. thank the CNR for financial support through the projects PHEEL, N-CHEM, and bilateral CNR-CONICET. We are grateful to Giandomenico Magagnano for preliminary results and Daniele Mazzarella for the preparation of tetrabutylammonium salts.Notes and references
- For reviews, see: (a) J. Svoboda and B. König, Chem. Rev., 2006, 106, 5413–5430 CrossRef CAS PubMed; (b) C. K. Prier, D. A. Rankic and D. W. C. MacMillan, Chem. Rev., 2013, 113, 5322–5363 CrossRef CAS PubMed; (c) J. M. R. Narayanam and C. R. J. Stephenson, Chem. Soc. Rev., 2011, 40, 102–113 RSC; (d) K. L. Skubi and T. P. Yoon, Nature, 2014, 515, 45–46 CrossRef CAS PubMed; (e) R. Brimioulle, D. Lenhart, M. M. Maturi and T. Bach, Angew. Chem., Int. Ed., 2015, 54, 3872–3890 CrossRef CAS PubMed; (f) E. Meggers, Chem. Commun., 2015, 51, 3290–3301 RSC.
- M. Neumann, S. Füldner, B. König and K. Zeitler, Angew. Chem., Int. Ed., 2011, 50, 951–954 CrossRef CAS PubMed.
- (a) D. A. Nicewicz and D. W. C. MacMillan, Science, 2008, 322, 77–80 CrossRef CAS PubMed; (b) D. A. Nagib, M. E. Scott and D. W. C. MacMillan, J. Am. Chem. Soc., 2009, 131, 10875–10877 CrossRef CAS PubMed; (c) H.-W. Shih, M. N. Vander Wal, R. L. Grange and D. W. C. MacMillan, J. Am. Chem. Soc., 2010, 132, 13600–13603 CrossRef CAS PubMed; (d) D. A. DiRocco and T. Rovis, J. Am. Chem. Soc., 2012, 134, 8094–8097 CrossRef CAS PubMed; (e) K. T. Tarantino, P. Liu and R. R. Knowles, J. Am. Chem. Soc., 2013, 135, 10022–10025 CrossRef CAS PubMed; (f) J. Du, K. L. Skubi, D. M. Schultz and T. P. Yoon, Science, 2014, 344, 392–396 CrossRef CAS PubMed.
- (a) M. Cherevatskaya, M. Neumann, S. Füldner, C. Harlander, S. Kümmel, S. Dankesreiter, A. Pfitzner, K. Zeitler and B. König, Angew. Chem., Int. Ed., 2012, 51, 4062–4066 CrossRef CAS PubMed; (b) P. Riente, A. Matas Adams, J. Albero, E. Palomares and M. A. Pericás, Angew. Chem., Int. Ed., 2014, 53, 9613–9616 CrossRef CAS PubMed; (c) H. Huo, X. Shen, C. Wang, L. Zhang, P. Röse, L.-A. Chen, K. Harms, M. Marsch, G. Hilt and E. Meggers, Nature, 2014, 515, 100–103 CrossRef CAS PubMed.
- L. Chu, C. Ohta, Z. Zuo and D. W. C. MacMillan, J. Am. Chem. Soc., 2014, 136, 10886–10889 CrossRef CAS PubMed For seminal work involving the addition of alkyl radicals to Michael acceptors using a photoactive metal complex see: K. Okada, K. Okamoto, N. Morita, K. Okubo and M. Oda, J. Am. Chem. Soc., 1991, 113, 9401–9402 CrossRef For selected reviews on 1,4-conjugate additions, see: (a) P. Perlmutter, Conjugate Addition Reactions in Organic Synthesis, Pergamon, Oxford, 1992 Search PubMed; (b) H.-G. Schmalz, in Comprehensive Organic Synthesis, ed. B. M. Trost and I. Fleming, Pergamon Press, Oxford, 1991, ch. 1.5, vol. 4 Search PubMed.
- For selected reviews on radical conjugate additions, see: (a) P. Renaud and M. Gerster, Angew. Chem., Int. Ed., 1998, 37, 2562–2579 CrossRef CAS; (b) M. P. Sibi and N. A. Porter, Acc. Chem. Res., 1999, 32, 163–171 CrossRef CAS; (c) M. P. Sibi, S. Manyem and J. Zimmerman, Chem. Rev., 2003, 103, 3263–3329 CrossRef CAS PubMed; (d) G. S. C. Srikanth and S. L. Castle, Tetrahedron, 2005, 61, 10377–10441 CrossRef CAS.
- (a) G. L. Lackner, K. W. Quasdorf and L. E. Overman, J. Am. Chem. Soc., 2013, 135, 15342–15345 CrossRef CAS PubMed; (b) G. L. Lackner, K. W. Quasdorf, G. Pratsch and L. E. Overman, J. Org. Chem., 2015, 80, 6012–6024 CrossRef CAS PubMed.
- C. C. Nawrat, C. R. Jamison, Y. Slutskyy, D. W. C. MacMillan and L. E. Overman, J. Am. Chem. Soc., 2015, 137, 11270–11273 CrossRef CAS PubMed.
- M. S. Lowry, J. I. Goldsmith, J. D. Slinker, R. Rohl, R. A. Pascal Jr, G. G. Malliaras and S. Bernhard, Chem. Mater., 2005, 17, 5712–5719 CrossRef CAS.
- S. Ventre, F. R. Petronijevic and D. W. C. MacMillan, J. Am. Chem. Soc., 2015, 137, 5654–5657 CrossRef CAS PubMed.
- D. A. DiRocco, K. Dykstra, S. Krska, P. Vachal, D. V. Conway and M. Tudge, Angew. Chem., Int. Ed., 2014, 53, 4802–4806 CrossRef CAS PubMed.
- For a review of the Minisci reaction, see: C. Punta and F. Minisci, Trends Heterocycl. Chem., 2008, 13, 1–68 CAS.
- J. Gui, Q. Zhou, C.-M. Pan, Y. Yabe, A. C. Burns, M. R. Collins, M. A. Ornelast, Y. Hishihara and P. S. Baran, J. Am. Chem. Soc., 2014, 136, 4853–4856 CrossRef CAS PubMed See also: (a) Y. Ji, T. Brueckl, R. D. Baxter, Y. Fujiwara, I. B. Seiple, S. Su, D. G. Blackmond and P. S. Baran, Proc. Natl. Acad. Sci. U. S. A., 2011, 108, 14411–14415 CrossRef CAS PubMed; (b) I. B. Seiple, S. Su, R. A. Rodriguez, R. Gianatassio, Y. Fujiwara, A. L. Sobel and P. S. Baran, J. Am. Chem. Soc., 2010, 132, 13194–13196 CrossRef CAS PubMed; (c) Y. Fujiwara, J. A. Dixon, F. O'Hara, E. D. Funder, D. D. Dixon, R. A. Rodriguez, R. D. Baxter, B. Herl, N. Sach, M. R. Collins, Y. Ishihara and P. S. Baran, Nature, 2012, 492, 95–99 CrossRef CAS PubMed.
- (a) E. J. Corey and D. Seebach, Angew. Chem., Int. Ed. Engl., 1965, 4, 1075–1077 CrossRef CAS; for reviews, see: (b) B. T. Gröbel and D. Seebach, Synthesis, 1977, 357–402 CrossRef; (c) D. Seebach, Angew. Chem., Int. Ed. Engl., 1979, 18, 239–258 CrossRef.
- (a) M. Yus, C. Nájera and F. Foubelo, Tetrahedron, 2003, 59, 6147–6212 CrossRef CAS; (b) A. B. Smith III and C. M. Adams, Acc. Chem. Res., 2004, 37, 365–377 CrossRef PubMed.
- M. Farrell, B. Melillo and A. B. Smith III, Angew. Chem., Int. Ed., 2016, 55, 232–235 CrossRef CAS PubMed.
- (a) J. H. Byers, C. C. Whitehead and M. E. Duff, Tetrahedron Lett., 1996, 37, 2743–2744 CrossRef CAS; (b) M. de Gref and S. Z. Zard, Tetrahedron, 2004, 60, 7781–7791 CrossRef; (c) A. J. Herrera and A. Studer, Synthesis, 2005, 1389–1396 CAS; (d) W. Du, L. Tian, J. Lai, X. Huo, X. Xie, X. She and S. Tang, Org. Lett., 2014, 16, 2470–2473 CrossRef CAS PubMed; (e) W. Du, J. Lai, L. Tian, X. Xie, X. She and S. Tang, Chem. Commun., 2014, 50, 14017–14020 RSC.
- A. Nishida, M. Nishida and O. Yonemitsu, Tetrahedron Lett., 1990, 31, 7035–7048 CrossRef CAS See also: (a) D. P. Curran and W. Shen, J. Am. Chem. Soc., 1993, 115, 6051–6059 CrossRef CAS; (b) B. P. Roberts, Chem. Soc. Rev., 1999, 28, 25–35 RSC; (c) H.-S. Dang and B. P. Roberts, Tetrahedron Lett., 1999, 40, 8929–8933 CrossRef CAS.
- M. Lee, Y.-H. Chen, T.-H. Hung, W. Chang, W.-C. Yan and D. Leow, RSC Adv., 2015, 5, 86402–86406 RSC.
- 1,3-Dithian-2-yltrifluoroborate is not commercially available and is prepared with carcinogenic HMPA; see: Y. Li, K. Miyazawa, T. Koike and M. Akita, Org. Chem. Front., 2015, 2, 319–323 RSC . In addition, only selected Michael-acceptors can be employed in this reaction.
- (a) A. Kondoh, M. Oishi, T. Takeda and M. Terada, Angew. Chem., Int. Ed., 2015, 54, 15836–15839 CrossRef CAS PubMed; (b) E. Massolo, M. Benaglia, A. Genoni, R. Annunziata, G. Celentano and N. Gaggero, Org. Biomol. Chem., 2015, 13, 5591–5596 RSC.
- F. Monti, A. Baschieri, I. Gualandi, J. J. Serrano-Pérez, J. M. Junquera-Hernández, D. Tonelli, A. Mazzanti, S. Muzzioli, S. Stagni, C. Roldan-Carmona, A. Pertegás, H. J. Bolink, E. Ortí, L. Sambri and N. Armaroli, Inorg. Chem., 2014, 53, 7709–7721 CrossRef CAS PubMed.
- (a) Z. Meng and S. Danishefsky, Angew. Chem., Int. Ed., 2005, 44, 1511–1513 CrossRef CAS PubMed; (b) A. Gualandi, E. Emer, M. G. Capdevila and P. G. Cozzi, Angew. Chem., Int. Ed., 2011, 50, 7842–7846 CrossRef CAS PubMed.
- Compound 13 was completely recovered unchanged after the work-up, in quantitative yields. No formation of di-cyclohexyl products or other byproducts due to reactions of the cyclohexyl radical were observed. Using standard conditions and replacing photocatalyst 2 with 1, the corresponding product (14) was obtained in 46% yield. MacMillan reported the use of cyclohexylcarboxylic acid (13) in photocatalytic radical Michael addition using a similar acceptor: the corresponding product was obtained in 75% yield using DMF as solvent and a 34 W blue LED (see ref. 5).
- The tetrabutylammonium carboxylate 17 in the reaction with 3a gave the desired product 5a in 72% yield, using standard reaction conditions, without the presence of K2HPO4; see ESI† for full details.
- V. Balzani, P. Ceroni and A. Juris, Photochemistry and Photophysics: Concept, Research, Applications, Wiley-VCH, Weinheim, 2014, ch. 4 Search PubMed.
- W. E. Jones Jr and M. A. Fox, J. Phys. Chem., 1994, 98, 5095–5099 CrossRef.
- M. Montalti, A. Credi, L. Prodi and M. T. Gandolfi, Handbook of Photochemistry, CRC Press, 3rd edn, 2006 Search PubMed.
- The value of the quantum yield obtained is similar to the value found by Nishibayashi in a photoredox radical decarboxylation reaction: Y. Miyake, K. Nakajima and Y. Nishibayashi, Chem. Commun., 2013, 49, 7854–7856 RSC.
- G. A. Crosby and J. N. Demas, J. Phys. Chem., 1971, 75, 991–1024 CrossRef CAS.
- S. R. Meech and D. Phillips, J. Photochem., 1983, 23, 193–217 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures, product characterizations, NMR spectra, compound preparation procedures, screening tests, and photophysical and electrochemical data. See DOI: 10.1039/c6sc03374a |
| This journal is © The Royal Society of Chemistry 2017 |