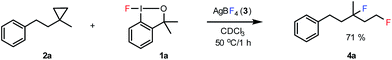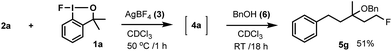Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Fluorinative ring-opening of cyclopropanes by hypervalent iodine reagents. An efficient method for 1,3-oxyfluorination and 1,3-difluorination†
Nadia O.
Ilchenko
,
Martin
Hedberg
and
Kálmán J.
Szabó
*
Stockholm University, Arrhenius Laboratory, Department of Organic Chemistry, SE-106 91 Stockholm, Sweden. E-mail: kalman.j.szabo@su.se
First published on 16th September 2016
Abstract
A new method is presented for 1,3-difluorination and 1,3-oxyfluorination reactions. The process is based on iodonium mediated opening of 1,1-disubstituted cyclopropanes. The reaction proceeds with high chemo- and regioselectivity under mild reaction conditions typically at room temperature in a couple of hours. The reaction probably occurs via electrophilic ring-opening of cyclopropanes.
Fluorinated organic compounds have found broad application in the pharmaceutical,1 and agrochemical industries2 as well as in medical diagnostics.3 The impetus for the application of organofluorine compounds in agrochemical and pharmaceutical products is their beneficial pharmacokinetic properties, such as high metabolic stability and lipophilicity.1a–d The useful radionuclear properties of the unnatural isotope 18F makes 18F labelled organofluoro compounds indispensable for positron emission tomography (PET).3a The short half-life of 18F requires development of a rapid late stage introduction of the fluorine atom,3 which is a challenging task in synthetic organic chemistry.4 In the last decade, many new fluorinating reagents have appeared, which in combination of catalysts allowed development of new selective methodologies to access a broad variety of bioactive organofluorines.4a,5
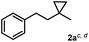
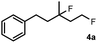
The most efficient methods are even suitable for fluorination based difunctionalization reactions.5b–d The most studied approach involves vicinal difunctionalization reactions, such as 1,2-oxyfluorination,6 1,2-aminofluorination,6a,7 1,2-carbofluorination6a,8 and related methods.9 Recently a number of interesting geminal fluorination methods were also reported, such as 1,1-difluorination,10 1,1-oxyfluorination11 and 1,1-aminofluorination.12 The 1,2-difunctionalization methods are usually based on alkene substrates, while the 1,1-difunctionalizations are often realized using diazo compounds, as substrates. However, the analogue methodology is much less developed for 1,3-difunctionalization based fluorination methods. Considering the typical synthetic methodologies for 1,3-difunctionalization reactions,13 a related fluorination reaction can probably be achieved by ring opening of cyclopropane substrates. Recently, we have shown that hypervalent iodine based14 benziodoxol(on) derivatives are excellent reagents for 1,1- and 1,2-difunctionalization for synthesis of organic trifluoromethyl and fluoro compounds.6a,9a,10a,11,15 As a part of our concept driven fluorine chemistry program, we sought to employ fluoro-benziodoxol reagent 1a for a fluorinative ring opening of cyclopropane derivatives. To our delight, 1a reacted smoothly with cyclopropane derivative 2a in the presence of AgBF4 affording 1,3-difluoro substituted compound 4a with 71% yield (Scheme 1).
As we employed 1a and 2a in equimolecular ratio in this reaction, one of the fluorine atoms originated from 1a, while the other one is from the BF4− counter ion of the Ag-mediator. We have previously reported10a a similar 1,1-difluorination method of styrenes. Although, several chlorination and bromination methods of cyclopropane are reported in the literature,16 synthetically useful cyclopropane opening is a very unusual methodology for fluorination reactions. As far as we know the above process is the first 1,3-difluorination reaction. In addition, we have found only a single fluorination based 1,3-difunctionalization reaction in the literature. Very recently, Lectka and co-workers17 reported an aminofluorination method based on cyclopropane substrates.
As mentioned above the 1,3-difluorination of cyclopropane 2a could be carried out selectively and in high yield using 1a and a stoichiometric amount of AgBF4 (Table 1, entry 1) in CDCl3. We used CDCl3 as the solvent to directly monitor the possible formation of the volatile fluorinated (and other) by-products in the reactions. Replacing AgBF4 with AgPF6 as a secondary fluorine source led to formation of 4a, but the yield dropped to 34% (entry 2). Cu(MeCN)4BF4 can also be used instead of AgBF4. The yield was lowered indicating that silver is a better mediator than copper for this transformation (entry 3). However, simple silver sources such as AgF showed to be inactive in 1,3-difluorination reaction (entry 4). Zinc salts have proved to be efficient activators of benziodoxole reagents.6a,18 Therefore, we attempted to replace AgBF4 with Zn(BF4)2 but the corresponding reaction did not result 4a (entry 4). Other silver salts without transferable fluoride in the counter ion, such as AgCN or AgTFA, did not show any activity (entry 5). When a sub-stoichiometric amount (30 mol%) of Ag-salt was used, the yields sharply decreased (entries 6–7). Pd(BF4)2(MeCN)4 (30 mol%) was also inefficient as catalyst (entry 7). Interestingly, Cu(MeCN)2BF4 showed some catalytic activity but the yield was very low (entry 8). Only traces of product 4a (<5%) could be obtained with 30 mol% of AgBF4 and stoichiometric amount of NaBF4 (entry 9). The reaction was completely shut down when KF was employed instead of NaBF4 (entry 10). This indicates that the most efficient secondary fluorine source is AgBF4. We could not observe any reaction without application of AgBF4 or 1a (entry 11).
| Entry | Deviation from the standard conditionsa | Yield 4a (%) |
|---|---|---|
| a Reagent 1a (0.1 mmol) cyclopropane 2a (0.1 mmol) and AgBF4 (3) (0.1 mmol) were mixed in CDCl3 (0.5 ml). This mixture was stirred at 50 °C for 1 h. | ||
| 1 | 1 equiv. of 3 | 71 |
| 2 | 1 equiv. AgPF6 instead of 3 | 34 |
| 3 | 1 equiv. Cu(MeCN)4BF4 instead of 3 | 30 |
| 4 | 1 equiv. AgF or Zn(BF4)2 × H2O instead of 3 | <5 |
| 5 | 1 equiv. AgCN or AgTFA instead of 3 | 0 |
| 6 | 30 mol% of 3 | 9 |
| 7 | 30 mol% AgPF6 or Pd(BF4)2(MeCN)4 instead of 3 | <5 |
| 8 | 30 mol% Cu(MeCN)4BF4 instead of 3 | 15 |
| 9 | 30 mol% 3 and 1 equiv. NaBF4 | <5 |
| 10 | 30 mol% 3 and 1 equiv. KF | 0 |
| 11 | Without 3 or 1a | 0 |
| 12 | 1 equiv. Selectfluor or NFSI instead of 1a | 0 |
| 13 | Without 3, 1 equiv. of Tol-IF2 instead of 1a | 24 |
| 14 | DCM instead of CDCl3 | 10 |
| 15 | MeCN or MeOH instead of CDCl3 | 0 |
Neither Selectfluor nor NFSI could replace fluoroiodoxol 1a as the electrophilic fluorination reagent (entry 12). When benziodoxole based 1a was replaced by 4-iodotoluene difluoride (Tol-IF2), a related hypervalent iodine reagent,14a product 4a did not form at all. Unlike 1a, Tol-IF2 underwent rapid decomposition in the presence of AgBF4. When the reaction was performed in the absence of AgBF4 (3) with Tol-IF2 a complex reaction mixture was obtained, from which compound 4a could be isolated in 24% yield (entry 13). In general, we found Tol-IF2 much less bench-stable than 1a and more prone to providing complex product mixtures.
A brief solvent screen has shown that dichloromethane is a less suitable solvent providing the product in 10% yield (entry 14). However formation of product 4a was not observed when chloroform was replaced by acetonitrile or methanol (entry 15).
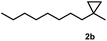
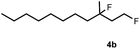
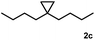
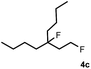
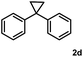
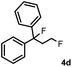
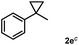
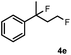
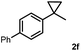
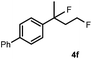
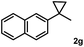
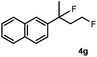
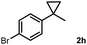
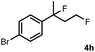
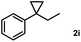
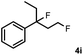
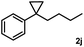
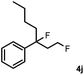
Subsequently, we investigated the synthetic scope of the silver mediated 1,3-difluorination reaction (Table 2). We found that several substrates required longer reaction times for full conversion relative to 2a (Table 2, entry 1). Under an elongated reaction time 1a underwent partial decomposition (see below). Therefore, in most reactions we employed two equivalents of 1a to obtain a full conversion of 2 and, thus optimal yields of 4. Aliphatic substrate 2b reacted for 4 h at room temperature affording 4b. Dialkyl cyclopropanes such as, 1,1-dibutyl cyclopropane 2c also reacted affording 4c (entry 3). In this case the yield was lower than for difluorination of 2b indicating that the reaction is fairly sensitive to the steric factors of the cyclopropane substituents. We have studied the reactivity of aryl substituted cyclopropanes as well. 1,1-Dipenyl cyclopropane 2d is a particularly challenging substrate. It is sterically hindered and the fluorine expected to enter to a dibenzylic position. We found that 2d reacted relatively quickly (2 hours) with 1a resulting in 4d (entry 4) in 47% yield. As expected 4d had a limited stability, which could explain the relatively low yield. A possible reason for the poor stability is the easy dissociation of the fluoride from the dibenzylic position. When one of the phenyl groups in 2d was changed to a methyl group, 2e, the reaction required a longer reaction time (6 hours), however the yield of the corresponding product 4e was higher, 59% (entry 5). Product 4e was also more stable than 4d probably because of the stronger quaternary C–F bond. In the presence of electron donating group in the para position of the aromatic substituent, 2f, we obtained a fast fluorination reaction (only 1 hour at room temperature) affording 4f in 55% yield (entry 6). Apparently electron donating groups accelerate the reaction. Naphthyl substituted substrate 2g also reacted smoothly to give 4g in 65% yield (entry 7). The rate of the reaction was much slower in the presence of an electron withdrawing group (e.g.2h) than for electron donating group (e.g.2f) in the para position of the aryl substituent. Thus, para-bromo substituted 2h had to be reacted 24 hours to provide 4h (entry 8), while the reaction of para phenyl substituted substrate 2f was compete in 1 hour (entry 6). Similarly to the aliphatic substrates (e.g.2c) the difluorination reaction can be carried out for longer homologues of the methyl substituents. For example 2i–j reacted with high yields affording difluorinated products 4i–j (entries 9–10). The presented 1,3-difluorination method can be easily scaled up by five times without significant change in yield (entry 1).
| Entry | Substrate | t (h) | Product | Yieldb (%) |
|---|---|---|---|---|
| a Unless otherwise stated, substrate 2 (0.1 mmol), 1a (0.2 mmol) and AgBF4 (3) (0.1 mmol) in CDCl3 (0.5 ml) were stirred at room temperature. b Isolated yields. c (0.1 mmol) of 1a was used. d The reaction was performed at 50 °C. e The reaction was performed in 0.5 mmol scale. | ||||
| 1 |
|
1 |
|
71 (67%)e |
| 2 |
|
4 |
|
70 |
| 3 |
|
4 |
|
51 |
| 4 |
|
2 |
|
47 |
| 5 |
|
6 |
|
59 |
| 6 |
|
1 |
|
55 |
| 7 |
|
3 |
|
65 |
| 8 |
|
24 |
|
57 |
| 9 |
|
24 |
|
70 |
| 10 |
|
24 |
|
66 |
Table 2 shows that the above reaction is suitable for the synthesis of quaternary 1,3-difluoro compounds 4a–j from 1,1-disubstituted cyclopropanes 2a–j. However, when we attempted to react 1,2-disubstituted cyclopropanes, we obtained very complex, inseparable mixtures with several fluorinated products. The observation that this reaction proceeds faster in the presence of electron donating and/or aryl substituents on the cyclopropane moiety suggests an electrophilic fluorinative cyclopropane opening mechanism. As mentioned above (Scheme 1, Table 1) the overall reaction can be regarded as a formal introduction of an F2 molecule into the cyclopropane substrates. The electrophilic fluorine atom (formally F+) supposedly comes from reagent 1a, while the nucleophilic fluorine atom (formally F−) from the BF4− counter ion.19 Considering this hypothesis, we attempted to introduce fluorine and a different functionality to cyclopropanes applying this concept.
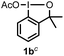
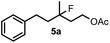
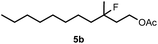
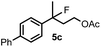
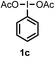
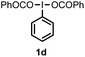
When we replaced fluoroiodoxole 1a with acetoxyiodoxole 1b, the reaction with 2a resulted in 1,3-oxyfluorinated product 5a (Table 3, entry 1) in 84% yield. In this reaction, we did not observe formation of difluorinated product 4a. In addition, the regioselectivity was also very high as we could not detect formation of the regioisomer of 5a. Aliphatic and aryl substrates 2b and 2f also reacted with the same chemo- and regioselectivity as 2a (entries 2 and 3). Products 5b–c had a limited stability, and decomposed within a couple of hours at room temperature. Instead of 1b, 1c (PIDA) could also be employed as acetoxy source. In this reaction, we also obtained 5a in good yield (entry 4) without formation of diacetoxy or difluoro (4a) analogues. Interestingly, 1c reacted much faster (20 min) than the iodoxole analogue 1b (4 hours). When benzoyl analogue 1d was used benzoyl product 5d formed instead of 5a (entry 5).
| Entry | Substrate | Hypervalent iodine | t (h) | Product | Yieldb (%) |
|---|---|---|---|---|---|
| a Unless otherwise stated, substrate 2 (0.1 mmol), 1 (0.1 mmol) and AgBF4 (3) (0.1 mmol) in CDCl3 (0.5 ml) were stirred at room temperature. b Isolated yields. c (0.2 mmol) of 1b was used. d Substrate 2a–b (0.1 mmol), 1a (0.1 mmol), AgBF4 (3) (0.1 mmol) and BnOH (6) (0.3 mmol) in CDCl3 (0.5 ml) were stirred at room temperature. e AgBF4 (3) (30 mol%). | |||||
| 1 | 2a |
|
4 |
|
84 |
| 2 | 2b | 1b | 2 |
|
50 |
| 3 | 2f | 1b | 2 |
|
51 |
| 4 | 2a |
|
20 min | 5a | 84 |
| 5 | 2a |
|
20 min |
|
80 |
| 6 | 2a | 1a/6d | 18 |
|
80 |
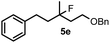
| 7 | 2a | 1a /6d | 18 | 5e | 17 |
| 8 | 2b | 1a/6d | 18 |
|
91 |
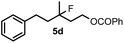
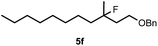
Cyclopropane derivatives 2a and 2b were also reacted with fluoroiodoxole 1a in the presence of benzyl alcohol (6) and AgBF4. In these reactions the final products were 1,3-oxyfluorinated species 5e–f (Table 3, entries 6–8) instead of 4a–b (Table 2, entries 1–2), which were formed in the absence of benzyl alcohol. Since in oxyfluorination only a single fluorine is introduced, we attempted to react 2a and 1a in the presence of benzyl alcohol and sub-stoichiometric amount of AgBF43 (entry 7). However, the yield of the oxyfluorinated product 5e substantially decreased (c.f. entries 6 and 7). Apparently, application of stoichiometric amount of AgBF4 is required, for both as a source for the secondary fluorine atom in the difluorination reaction (such as for formation of 4a) and also in the oxyfluorination reaction for efficient activation of 1a. In the oxyfluorination reactions the activated hypervalent iodine reagents proved to be more stable than in the difluorination reactions. Therefore, in most processes (entries 2–7) one equivalent of the iodine reagent was sufficient to obtain the reported isolated yields.
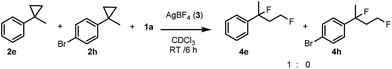
In order to obtain more insight into the electronic effects of the reactions and the role of the applied hypervalent iodine, we performed a couple of control experiments. When an equimolar ratio of 2e, 2h and 1a reacted in the presence of AgBF4, we obtained only 4e, while formation of 4h was not observed (Scheme 2). This competitive reaction indicates that cyclopropane substrates bearing an electron withdrawing group, such as 2h, react much slower than the parent compound 2e.
This confirms the suggestion of the electrophilic mechanism for the opening of the cyclopropane ring. When 2a was reacted with equimolar amounts of fluoro- (1a) and acetoxyiodoxoles (1b) products 4a and 5a were formed in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 ratio (Scheme 3) indicating that the oxidation power or the electrophilicity of the hypervalent iodine is an important factor for the reaction rate.
2 ratio (Scheme 3) indicating that the oxidation power or the electrophilicity of the hypervalent iodine is an important factor for the reaction rate.
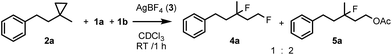 | ||
| Scheme 3 Competitive 1,3-difluorination vs. 1,3-oxyfluorination using equimolar amounts of 2a, 1a and 1b. | ||
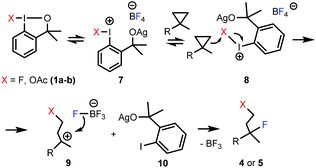
Considering the above and the literature data for related reactions,6a,9a,10a we propose a plausible mechanism for the fluorinative opening of cyclopropanes with hypervalent iodines (Scheme 4). Benziodoxole reagents 1a–b are stable6b under ambient conditions, and usually require activation in the substitution and addition reactions.6a,9a,10a We suggest that AgBF4 activates 1a–b by coordination of the oxygen atom of the benziodoxole ring to the silver cation affording intermediate 7. Similar, Lewis-acid type of activation of benziodoxoles was reported by Togni and co-workers.18 Unlike, 1a–b, activated benziodoxole 7 is very reactive, and besides the desired fluorination reaction it may undergo decomposition (or other side-reactions). This is the reason for application of two equivalents of 1a in some difunctionalization reactions where the substrate has a low reactivity or the rate of decomposition of intermediate is high. We suggest that 7 undergoes side-attack of the cyclopropane ring (8) to give carbocationic intermediate 9 and iodobenzene derivative 10. This mechanism is reminiscent of our proposal for the difluorination of styrenes with 1a.10a The high regioselectivity of the attack is an interesting feature of the process (Table 3). A possible explanation is that the regioselectivity is controlled by electronic effects, i.e. hyperconjugative stabilization of the tertiary carbocation center. The final step of the process could be a nucleophilic attack by fluorine from the BF4− counterion19 to obtain the final product (4 or 5).
In case of oxyfluorination with benzyl alcohol (Table 3, entries 6–8) 1a was probably reacted with 6 prior to the ring opening providing benzyloxy-benziodoxole (analogue to 1b). In this case intermediate 9 is a benzyl ether (X = OBn). This idea is supported by the control experiment (Scheme 5), in which, we first performed a difluorination affording 4a, then 6 was added. In this reaction we obtained 5g, which is the regioisomer of 5e (see Table 3, entry 6).
Accordingly, when 2a, 1a, 3 and 6 were mixed at the onset of the reaction (Table 3, entry 6) difluorination product 4a did not form. This reaction lead to the formation of 5e directly (according to the mechanism outlined in Scheme 4). If 4a formed first in the process, benzyl alcohol (6) would have displaced the tertiary fluorine affording 5g (Scheme 5).
Modelling and experimental studies are underway to explore the mechanistic details of the above and related6a,9a,10a metal mediated reactions of fluoro-benziodoxol reagent 1a.
In conclusion, we have shown that the air- and moisture stable fluoroiodine reagent 1a is suitable for the silver mediated 1,3-difluorination reaction of 1,1-disubstituted cyclopropanes. The reaction can be extended to 1,3-oxydifluorination by using hypervalent acetoxy and benzoyloxy iodines. The reaction probably proceeds via electrophilic ring opening of cyclopropanes. As the above process is the first 1,3-difluorination and 1,3-oxydifluorination reaction, it broaden the synthetic scope of the fluorination reactions, and the application area of hypervalent fluoroiodines.
Conflict of interest
The authors declare no competing financial interests.Acknowledgements
The authors thank the financial support of the Swedish Research Council (VR) and the Knut och Alice Wallenbergs Foundation.Notes and references
- (a) Y. Zhou, J. Wang, Z. Gu, S. Wang, W. Zhu, J. L. Aceña, V. A. Soloshonok, K. Izawa and H. Liu, Chem. Rev., 2016, 116, 422 CrossRef CAS PubMed; (b) P. A. Champagne, J. Desroches, J.-D. Hamel, M. Vandamme and J.-F. Paquin, Chem. Rev., 2015, 115, 9073 CrossRef CAS PubMed; (c) J. Wang, M. Sánchez-Roselló, J. L. Aceña, C. del Pozo, A. E. Sorochinsky, S. Fustero, V. A. Soloshonok and H. Liu, Chem. Rev., 2014, 114, 2432 CrossRef CAS PubMed; (d) K. Müller, C. Faeh and F. Diederich, Science, 2007, 317, 1881 CrossRef PubMed; (e) S. Purser, P. R. Moore, S. Swallow and V. Gouverneur, Chem. Soc. Rev., 2008, 37, 320 RSC.
- (a) P. Jeschke, R. Nauen and M. E. Beck, Angew. Chem., Int. Ed., 2013, 52, 9464 CrossRef CAS PubMed; (b) P. Jeschke, ChemBioChem, 2004, 5, 570 CrossRef CAS PubMed.
- (a) P. W. Miller, N. J. Long, R. Vilar and A. D. Gee, Angew. Chem., Int. Ed., 2008, 47, 8998 CrossRef CAS PubMed; (b) S. Preshlock, M. Tredwell and V. Gouverneur, Chem. Rev., 2016, 116, 719 CrossRef CAS PubMed; (c) M. Tredwell and V. Gouverneur, Angew. Chem., Int. Ed., 2012, 51, 11426 CrossRef CAS PubMed.
- (a) T. Liang, C. N. Neumann and T. Ritter, Angew. Chem., Int. Ed., 2013, 52, 8214 CrossRef CAS PubMed; (b) C. N. Neumann and T. Ritter, Angew. Chem., Int. Ed., 2015, 54, 3216 CrossRef CAS PubMed; (c) M. S. Sanford and P. J. H. Scott, ACS Cent. Sci., 2016, 2, 128–130 CrossRef CAS PubMed.
- (a) J. Charpentier, N. Früh and A. Togni, Chem. Rev., 2015, 115, 650 CrossRef CAS PubMed; (b) X. Yang, T. Wu, R. J. Phipps and F. D. Toste, Chem. Rev., 2015, 115, 826 CrossRef CAS PubMed; (c) H. Egami and M. Sodeoka, Angew. Chem., Int. Ed., 2014, 53, 8294 CrossRef CAS PubMed; (d) W. Kong, E. Merino and C. Nevado, Chimia, 2014, 68, 430 CrossRef CAS PubMed.
- (a) W. Yuan and K. J. Szabó, Angew. Chem., Int. Ed., 2015, 54, 8533 CrossRef CAS PubMed; (b) G. C. Geary, E. G. Hope and A. M. Stuart, Angew. Chem., Int. Ed., 2015, 54, 14911 CrossRef CAS PubMed; (c) A. Ulmer, C. Brunner, A. M. Arnold, A. Pöthig and T. Gulder, Chem.–Eur. J., 2016, 22, 3660 CrossRef CAS PubMed; (d) D. Parmar and M. Rueping, Chem. Commun., 2014, 50, 13928 RSC; (e) V. Rauniyar, A. D. Lackner, G. L. Hamilton and F. D. Toste, Science, 2011, 334, 1681 CrossRef CAS PubMed; (f) O. Lozano, G. Blessley, T. Martinez del Campo, A. L. Thompson, G. T. Giuffredi, M. Bettati, M. Walker, R. Borman and V. Gouverneur, Angew. Chem., Int. Ed., 2011, 50, 8105 CrossRef CAS PubMed.
- (a) T. Wu, G. Yin and G. Liu, J. Am. Chem. Soc., 2009, 131, 16354 CrossRef CAS PubMed; (b) W. Kong, P. Feige, T. de Haro and C. Nevado, Angew. Chem., Int. Ed., 2013, 52, 2469 CrossRef CAS PubMed; (c) H. P. Shunatona, N. Früh, Y.-M. Wang, V. Rauniyar and F. D. Toste, Angew. Chem., Int. Ed., 2013, 52, 7724 CrossRef CAS PubMed.
- (a) S. Kindt and M. R. Heinrich, Chem.–Eur. J., 2014, 20, 15344 CrossRef CAS PubMed; (b) L. Wang, W. Meng, C.-L. Zhu, Y. Zheng, J. Nie and J.-A. Ma, Angew. Chem., Int. Ed., 2011, 50, 9442 CrossRef CAS PubMed; (c) J. R. Wolstenhulme, J. Rosenqvist, O. Lozano, J. Ilupeju, N. Wurz, K. M. Engle, G. W. Pidgeon, P. R. Moore, G. Sandford and V. Gouverneur, Angew. Chem., Int. Ed., 2013, 52, 9796 CrossRef CAS PubMed.
- (a) N. O. Ilchenko, M. A. Cortés and K. J. Szabo, ACS Catal., 2016, 6, 447 CrossRef CAS; (b) Z. Yuan, H.-Y. Wang, X. Mu, P. Chen, Y.-L. Guo and G. Liu, J. Am. Chem. Soc., 2015, 137, 2468 CrossRef CAS PubMed.
- (a) N. O. Ilchenko, B. O. A. Tasch and K. J. Szabó, Angew. Chem., Int. Ed., 2014, 53, 12897 CrossRef CAS PubMed; (b) T. Kitamura, K. Muta and J. Oyamada, J. Org. Chem., 2015, 80, 10431 CrossRef CAS PubMed; (c) J. Tao, R. Tran and G. K. Murphy, J. Am. Chem. Soc., 2013, 135, 16312 CrossRef CAS PubMed; (d) E. Emer, J. Twilton, M. Tredwell, S. Calderwood, T. L. Collier, B. Liégault, M. Taillefer and V. Gouverneur, Org. Lett., 2014, 16, 6004 CrossRef CAS PubMed.
- W. Yuan, L. Eriksson and K. J. Szabó, Angew. Chem., Int. Ed., 2016, 55, 8410 CrossRef CAS PubMed.
- G. Chen, J. Song, Y. Yu, X. Luo, C. Li and X. Huang, Chem. Sci., 2016, 7, 1786 RSC.
- (a) M. Rubin, M. Rubina and V. Gevorgyan, Chem. Rev., 2007, 107, 3117 CrossRef CAS PubMed; (b) M. A. Cavitt, L. H. Phun and S. France, Chem. Soc. Rev., 2014, 43, 804 RSC; (c) T. F. Schneider, J. Kaschel and D. B. Werz, Angew. Chem., Int. Ed., 2014, 53, 5504 CrossRef CAS PubMed; (d) H. N. C. Wong, M. Y. Hon, C. W. Tse, Y. C. Yip, J. Tanko and T. Hudlicky, Chem. Rev., 1989, 89, 165 CrossRef CAS.
- (a) A. Yoshimura and V. V. Zhdankin, Chem. Rev., 2016, 116, 3328 CrossRef CAS PubMed; (b) V. V. Zhdankin and P. J. Stang, Chem. Rev., 2008, 108, 5299 CrossRef CAS PubMed.
- (a) P. G. Janson, I. Ghoneim, N. O. Ilchenko and K. J. Szab, Org. Lett., 2012, 14, 2882 CrossRef CAS PubMed; (b) N. O. Ilchenko, P. G. Janson and K. J. Szabo, J. Org. Chem., 2013, 78, 11087 CrossRef CAS PubMed; (c) N. O. Ilchenko, P. G. Janson and K. J. Szabó, Chem. Commun., 2013, 49, 6614 RSC.
- (a) L. K. B. Garve, P. Barkawitz, P. G. Jones and D. B. Werz, Org. Lett., 2014, 16, 5804 CrossRef CAS PubMed; (b) J. B. Lambert, W. J. Schulz, P. H. Mueller and K. Kobayashi, J. Am. Chem. Soc., 1984, 106, 792 CrossRef CAS; (c) J. B. Lambert and B. B. Iwanetz, J. Org. Chem., 1972, 37, 4082 CrossRef CAS; (d) N. V. Zyk, A. Y. Gavrilova, O. B. Bondarenko, O. A. Mukhina and V. N. Tikhanushkina, Russ. J. Org. Chem., 2011, 47, 340 CrossRef CAS.
- C. R. Pitts, B. Ling, J. A. Snyder, A. E. Bragg and T. Lectka, J. Am. Chem. Soc., 2016, 138, 6598 CrossRef CAS PubMed.
- R. Koller, K. Stanek, D. Stolz, R. Aardoom, K. Niedermann and A. Togni, Angew. Chem., Int. Ed., 2009, 48, 4332 CrossRef CAS PubMed.
- (a) A. J. Cresswell, S. G. Davies, P. M. Roberts and J. E. Thomson, Chem. Rev., 2015, 115, 566 CrossRef CAS PubMed; (b) J. Barluenga, J. M. González, P. J. Campos and G. Asensio, Angew. Chem., Int. Ed. Engl., 1985, 24, 319 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures, characterization and NMR spectra of the products. See DOI: 10.1039/c6sc03471c |
| This journal is © The Royal Society of Chemistry 2017 |