Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Highly enantioselective metallation–substitution alpha to a chiral nitrile†
Arghya
Sadhukhan
,
Melanie C.
Hobbs
,
Anthony J. H. M.
Meijer
 and
Iain
Coldham
and
Iain
Coldham
 *
*
Department of Chemistry, University of Sheffield, Brook Hill, Sheffield, S3 7HF, UK. E-mail: i.coldham@sheffield.ac.uk
First published on 25th October 2016
Abstract
We report the deprotonation of a chiral nitrile and reaction of the resulting chiral organometallic species with a variety of electrophiles to give highly enantiomerically enriched 2-substituted nitrile products. The nitrile was treated with TMPMgCl and the resulting anion, an asymmetric alpha cyano Grignard species, was found to be configurationally stable at low temperature for a short time (half-life several minutes at −104 °C).
Introduction
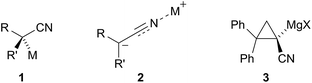
Metallated nitriles are well-used intermediates in synthetic chemistry due to their excellent reactivity as nucleophiles.1 The formation of the organometallic species and its reaction with an electrophile such as an alkyl halide or aldehyde allows a high-yielding preparation of the desired substituted nitrile that can then be converted readily to other functional groups. The most common method to prepare the metallated nitrile is to treat the nitrile with a base such as lithium diisopropylamide (LDA) and this is known to give the lithiated nitrile in which the lithium ion normally resides on the nitrogen atom.2–5 Although this gives a reactive nitrile anion, one of its drawbacks is that this provides an achiral organometallic species (e.g.Fig. 1),3,4 even starting from a chiral, enantiomerically enriched nitrile. Therefore it would be expected that achiral products would result from using chiral enantiomerically enriched nitrile starting materials and this is typically the case.6–8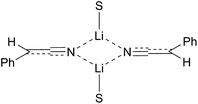 | ||
| Fig. 1 Structure of lithiatiated phenylacetonitrile, S = solvent.3,4 | ||
Remarkably, however, Takeda and co-workers reported recently that it is possible in certain cases at low temperature with in situ reactive electrophiles to trap the intermediate anions to give enantioenriched products.9,10 At about the same time, we began to explore this possibility but by using magnesiated nitriles.11 The idea that magnesiated nitriles may allow asymmetric reaction through a chiral organometallic species 1 rather than 2 (Fig. 2) was based on results from several groups including that of Carlier and co-workers, who reported the first metallated nitrile with macroscopic configurational stability, albeit a cyclopropyl derivative 3 (prepared by Br–Mg exchange).12 In addition, Fleming and co-workers had found opposing selectivities for reactions of lithiated and magnesiated nitriles and surmised that the magnesium cation has a preference for location on carbon.13,14
If the metal atom is located on the carbon atom, as illustrated by the contact ion pair 1 (or its related solvent-separated ion pair in which the metal cation is nearby), then the metallated nitrile is chiral and has the possibility to transfer its chirality to the product on reaction with an electrophile. However, very little is known about the rate of enantiomerisation of such nitrile anions.11,15 The importance of nitrogen-containing heterocycles in natural products and medicinal compounds led us to explore the metallation of nitrile 4 with the aim to determine whether the deprotonation–electrophilic quench would be feasible and how fast the intermediate magnesiated nitrile undergoes racemisation. Herein, we describe the first high yielding, highly enantioselective metallation–substitutions of a chiral α-amino-nitrile by using a simple magnesium base.
Results and discussion
The carboxylic acid N-Boc-pipecolic acid is commercially available as the (S) enantiomer and this was converted to (S)-N-Boc-2-cyanopiperidine 4 in two steps (see ESI†). This method involved simple amide formation with ethyl chloroformate and ammonia to give the primary amide, followed by dehydration to give the nitrile (S)-4. The corresponding racemic nitrile could be prepared in the same way starting from racemic pipecolic acid by initial N-Boc protection with Boc2O, Et3N, CH2Cl2 followed by using the same method.Initially we investigated the deprotonation of the nitrile (S)-4 with LDA in THF at −78 °C. After 10 min, the anion was quenched by addition of various electrophiles. Under these conditions we generally obtained good yields of racemic products (see ESI†), as determined by chiral stationary phase (CSP) GC or HPLC analysis. The formation of racemic products was expected under these conditions as lithiated nitriles are known to exist with the lithium cation on the nitrogen atom2–5 and therefore the stereochemistry at the carbon centre would be readily lost.
We then turned to the asymmetric reaction by deprotonation and electrophilic quench, particularly on using a softer metal counterion where we hoped that the metal would remain on the carbon centre for long enough to maintain the configuration. We had previously obtained some success in this approach by using magnesium bases,11 with one equivalent of iPrMgCl, TMPMgCl·LiCl, or TMPMgCl. All these bases resulted in significant enantioenrichment after leaving the magnesiated intermediate for 5 s at −107 °C prior to addition of acetone (enantiomer ratio, er, up to 96![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 of the substituted product 5e). However the conversion in these reactions was low and this product could not be isolated.
4 of the substituted product 5e). However the conversion in these reactions was low and this product could not be isolated.
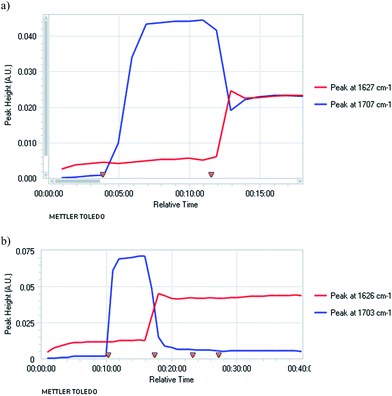
To optimise the reaction we carried out in situ IR studies and found that only partial conversion occurs with one equivalent of TMPMgCl (Fig. 3a). However, almost complete conversion occurs with two equivalents and full conversion with three equivalents of TMPMgCl (Fig. 3b).
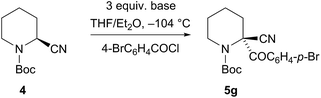
Several bases were tested under these optimised conditions (Scheme 1). The bases CuOtBu, mesityl copper, or TMPZnCl·MgCl2 were unsuccessful. The method developed by Takeda and co-workers10 with NaHMDS (and 4-BrC6H4COCl in situ) did give the product 5g but the enantioselectivity was poor (71% yield, er 53![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 47). With the magnesium base iPrMgCl the yield was low (10% yield of product 5a with PhSSO2Ph as the in situ electrophile, er not determined). However the base TMPMgCl was much more successful and a good yield and er of the product 5g was obtained (68% yield 5g, er 81
47). With the magnesium base iPrMgCl the yield was low (10% yield of product 5a with PhSSO2Ph as the in situ electrophile, er not determined). However the base TMPMgCl was much more successful and a good yield and er of the product 5g was obtained (68% yield 5g, er 81![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 19).
19).
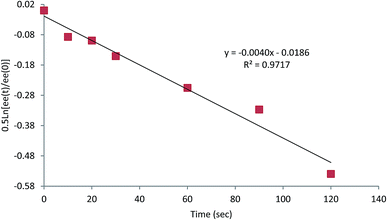
We therefore selected TMPMgCl as the most suitable base. The enantioselectivity was not optimal and we were aware that Carlier and co-workers had found that a magnesiated cyclopropylnitrile racemises more rapidly in THF than in Et2O.15 Therefore we conducted kinetic experiments to determine the rate of enantiomerisation of this organomagnesium species in THF/Et2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and in Et2O (see ESI† and Fig. 4). At −104 °C the intermediate organomagnesium compound was trapped after various time periods to give the product 5a or 5f and the er was measured by CSP HPLC or GC respectively. These gave good first order plots and revealed rates for inversion k ∼ 6.5 × 10−3 s−1 in THF/Et2O (see ESI†) and k ∼ 4 × 10−3 s−1 in Et2O (Fig. 4).
1) and in Et2O (see ESI† and Fig. 4). At −104 °C the intermediate organomagnesium compound was trapped after various time periods to give the product 5a or 5f and the er was measured by CSP HPLC or GC respectively. These gave good first order plots and revealed rates for inversion k ∼ 6.5 × 10−3 s−1 in THF/Et2O (see ESI†) and k ∼ 4 × 10−3 s−1 in Et2O (Fig. 4).
The kinetic data demonstrate a slightly slower rate for inversion of the intermediate organomagnesium species in Et2O. In the case of the magnesiated nitrile 4, the enantiomerisation half-life t1/2 ∼ 3 min in Et2O and only ∼2 min in THF/Et2O, presumably as THF helps to solvate the magnesium cation. Therefore, we carried out the deprotonation in pure Et2O and were pleased to find that this improved the enantioselectivity of the metallation–electrophilic quench reaction (Scheme 2). The optimised conditions involved rapid addition of three equivalents of TMPMgCl (prepared from i-PrMgCl and TMPH in Et2O) to the nitrile 4 in Et2O at −104 °C, either with the electrophile added in situ pre-mixed with the nitrile 4 (in the case of the S-aryl benzenesulfonates) or with the electrophile added after about 10 s (for the carbonyl electrophiles).
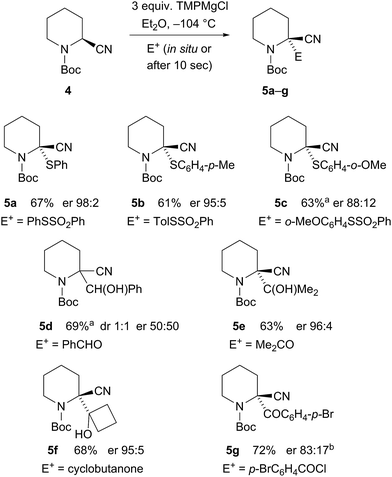 | ||
Scheme 2 Enantiospecific magnesiation-quench of nitrile 4. aReaction in Et2O–THF 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. bRecrystallization gave er 99 1. bRecrystallization gave er 99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. 1. | ||
High enantioselectivities of the arylthio derivatives 5a–c were obtained by the in situ method. With the ortho-methoxy compound 5c, the electrophile was only partially soluble in pure Et2O, so this reaction was carried out with some THF and this may account for the reduced selectivity. The organomagnesium intermediate has sufficient configurational stability to allow its formation followed by electrophilic quench without the need for an in situ electrophile. Benzaldehyde provided racemic product 5d, possibly due to single electron transfer. However, acetone gave highly enantioenriched alcohol 5e and cyclobutanone gave the alcohol 5f also with excellent er. The electrophile p-bromobenzoyl chloride gave the nitrile 5g with er 83![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 17 after 10 s quench and similar selectivity with in situ quench.
17 after 10 s quench and similar selectivity with in situ quench.
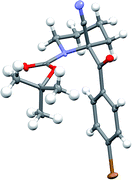
Recrystallisation of the nitrile 5g gave essentially enantiopure compound (er 99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
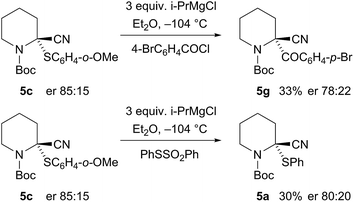
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 by CSP-HPLC) and the absolute configuration was determined by single crystal X-ray analysis (Fig. 5).† This demonstrated that the electrophilic quench occurred with retention of configuration. To determine the absolute configuration of the sulfides, we carried out sulfur–magnesium exchange with i-PrMgCl.16 This transformation has not to our knowledge been reported with an enantioenriched sulfide and it was intriguing to discover whether it would be possible to transfer chirality by this method. Addition of i-PrMgCl to the sulfide 5c in Et2O at −104 °C followed by addition of p-bromobenzoyl chloride gave the product 5g in moderate yield and only partial loss of enantioselectivity (Scheme 3). The major enantiomer of the product 5g had the same configuration as that obtained by the direct addition of p-bromobenzoyl chloride, thereby demonstrating that the sulfide 5c (and hence also likely the sulfides 5a and 5b) has the stereochemistry as shown, and was formed by reaction with retention of configuration. We have not been able to determine the absolute configurations of the alcohols 5e and 5f, but these are likely to be as shown with reaction by retention of configuration, and this would be in line with other known electrophilic quenches of metallated N-Boc-piperidines.17 A similar reaction was carried out, in which sulfur–magnesium exchange was followed, after 10 s, by addition of the electrophile PhSSO2Ph to give the product 5a (Scheme 3). This was formed in moderate yield without significant loss of enantiopurity, together with what appeared to be an alkene by-product from elimination.
1 by CSP-HPLC) and the absolute configuration was determined by single crystal X-ray analysis (Fig. 5).† This demonstrated that the electrophilic quench occurred with retention of configuration. To determine the absolute configuration of the sulfides, we carried out sulfur–magnesium exchange with i-PrMgCl.16 This transformation has not to our knowledge been reported with an enantioenriched sulfide and it was intriguing to discover whether it would be possible to transfer chirality by this method. Addition of i-PrMgCl to the sulfide 5c in Et2O at −104 °C followed by addition of p-bromobenzoyl chloride gave the product 5g in moderate yield and only partial loss of enantioselectivity (Scheme 3). The major enantiomer of the product 5g had the same configuration as that obtained by the direct addition of p-bromobenzoyl chloride, thereby demonstrating that the sulfide 5c (and hence also likely the sulfides 5a and 5b) has the stereochemistry as shown, and was formed by reaction with retention of configuration. We have not been able to determine the absolute configurations of the alcohols 5e and 5f, but these are likely to be as shown with reaction by retention of configuration, and this would be in line with other known electrophilic quenches of metallated N-Boc-piperidines.17 A similar reaction was carried out, in which sulfur–magnesium exchange was followed, after 10 s, by addition of the electrophile PhSSO2Ph to give the product 5a (Scheme 3). This was formed in moderate yield without significant loss of enantiopurity, together with what appeared to be an alkene by-product from elimination.
 | ||
| Scheme 3 Sulfur–magnesium exchange and electrophilic quench to determine the absolute configuration of nitrile 5c. | ||
The impressive enantioselectivities that can be obtained with the simple N-Boc-2-cyanopiperidine 4 and base TMPMgCl demonstrate that this method has potential for asymmetric synthesis. The magnesium metal likely has a preference for attachment to the carbon atom at least initially. A possible intermediate, supported by analogy to that proposed by Carlier and co-workers for magnesiated cyclopropylnitriles,15 would have two magnesium atoms, one on the carbon atom and one on the nitrile nitrogen atom, connected by a bridging chloride. Dimeric magnesium amides with bridging chloride ligands are well known.18,19
To investigate this further, Density Functional Theory (DFT) calculations were performed [6-311G(d,p) basis set with B3LYP functional: see Computational methods section below]. Our calculations show, as expected, that nitrile 4 should be present as two rotamers corresponding to different orientations of the Boc group with approximate equal probability. The rate of rotation of the Boc group is slow at the temperatures used for the reaction. Upon deprotonation this will lead to either the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O group or the C–OtBu group pointing towards the deprotonated carbon. Complexes of these rotamers, with a bridging chloride ligand between two magnesium ions, were found and are shown in the ESI.† These complexes have very different conformations for the two rotamers. In the case where the OtBu group points towards the magnesium, a ring-flip occurs to provide a lower energy structure in which the Boc group sits on the opposite side of the ring from the magnesium.
O group or the C–OtBu group pointing towards the deprotonated carbon. Complexes of these rotamers, with a bridging chloride ligand between two magnesium ions, were found and are shown in the ESI.† These complexes have very different conformations for the two rotamers. In the case where the OtBu group points towards the magnesium, a ring-flip occurs to provide a lower energy structure in which the Boc group sits on the opposite side of the ring from the magnesium.
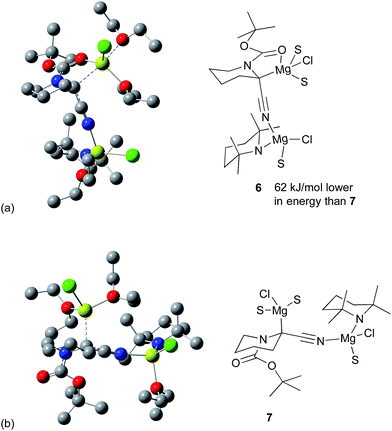
The case where an additional Et2O coordinates at the proximal magnesium was also considered. In contrast to that of Carlier and co-workers,15 this leads to an additional stabilization energy and opens up the possibility of structures without the bridging Cl atom, which could be expected to be less strained. The two rotamers with the lowest energy are shown in Fig. 6. Other orientations were attempted as well, but all lead to higher energy structures. Fig. 6a (structure 6) has chelation of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O group to the magnesium. Fig. 6b (structure 7) derives from the other rotamer without this chelation and this structure is 62 kJ mol−1 higher in Gibbs energy than 6. As the Boc group is not rotating at low temperature, both species should be present in solution and able to react with the electrophiles as shown earlier.
O group to the magnesium. Fig. 6b (structure 7) derives from the other rotamer without this chelation and this structure is 62 kJ mol−1 higher in Gibbs energy than 6. As the Boc group is not rotating at low temperature, both species should be present in solution and able to react with the electrophiles as shown earlier.
The organomagnesium species 6 and 7 could racemise by breakage of the C–Mg bond followed by carbanion inversion and reattachment of the magnesium to the opposite face. Alternatively racemisation could take place by formation of the N-magnesiated ketene imine type structure. However, by whatever mechanism racemisation occurs, the experimental data show that the C-magnesiated intermediates have sufficient lifetime at low temperature for addition of an electrophile and reaction to give highly enantiomerically enriched products.
Experimental
A representative method for the deprotonation and quench of nitrile 4 is given below. For further details and all data, see ESI.†TMPMgCl (1.6 mL, 0.75 mmol) was added to the nitrile 4 (54 mg, 0.25 mmol) in Et2O (1 mL) at −104 °C. After 10 s, cyclobutanone (0.056 mL, 0.75 mmol) was added. After 30 min, saturated aqueous NH4Cl (0.3 mL) was added. The mixture was allowed to warm to room temperature and was extracted with Et2O (3 × 1 mL), dried (MgSO4) and the solvent was evaporated. Purification by column chromatography on silica gel, eluting with petrol–EtOAc (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), gave the alcohol 5f (47 mg, 68%); [α]21D −25.7 (c 0.4, CHCl3); er 95
1), gave the alcohol 5f (47 mg, 68%); [α]21D −25.7 (c 0.4, CHCl3); er 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 by CSP-GC.
5 by CSP-GC.
Computational methods
All calculations were performed using the D.01 version of Gaussian 09.20 Density functional theory was used throughout using the B3LYP21 functional including dispersion interactions via the GD3-BJ22 correction. All calculations used the 6-311G(d,p)23 basis set. Solvent was included via the PCM method24 as implemented in Gaussian with the default parameters for Et2O. Frequency calculations were performed on all optimized structures to confirm that these were all true minima as evidenced by the absence of imaginary frequencies. No complete conformational search for any added Et2O molecule was performed. Instead, the calculations were all started with Et2O in the conformation of its free molecule. All Gibbs energies were evaluated at 298.15 K.Conclusions
In conclusion, the base TMPMgCl can be used at low temperatures to deprotonate a chiral nitrile without significant loss of enantiopurity even in the absence of an in situ electrophile. The intermediate magnesiated nitrile can be trapped with a variety of electrophiles to give enantioenriched substituted nitrile products with overall retention of configuration. The organomagnesium intermediate racemises fairly rapidly and the half-life is slightly slower in the presence of the less polar solvent Et2O than in THF. In addition we have shown that sulfur–magnesium exchange can occur with retention of configuration. Calculations support the experimental that two magnesium ions are present in the intermediate complexes. These results suggest that, despite their general lack of use for asymmetric synthesis, chiral nitrile anions can be valuable intermediates that do not always lose their configuration but can be converted to highly enantiomerically enriched products.Acknowledgements
We acknowledge support from the European Union under a Marie Curie International Incoming Fellowship award PIIF-GA-2013-625471, the EPSRC, and the University of Sheffield. We thank Harry Adams for the single crystal X-ray analysis.Notes and references
- D. Enders, J. Kirchhoff, P. Gerdes, D. Mannes, G. Raabe, J. Runsink, G. Boche, M. Marsch, H. Ahlbrecht and H. Sommer, Eur. J. Org. Chem., 1998, 63 CrossRef CAS; F. Fleming and B. Shook, Tetrahedron, 2002, 58, 1 CrossRef; T. Opatz, Synthesis, 2009, 1941 CrossRef; N. Otto and T. Opatz, Chem.–Eur. J., 2014, 20, 13064 CrossRef PubMed; R. López and C. Palomo, Angew. Chem., Int. Ed., 2015, 54, 13170 CrossRef PubMed.
- M. Purzycki, W. Liu, G. Hilmersson and F. F. Fleming, Chem. Commun., 2013, 49, 4700 RSC.
- P. R. Carlier and C. W. S. Lo, J. Am. Chem. Soc., 2000, 122, 12819 CrossRef CAS.
- G. Boche, M. Marsch and K. Harms, Angew. Chem., Int. Ed., 1986, 25, 373 CrossRef.
- R. Sott, J. Granander and G. Hilmersson, J. Am. Chem. Soc., 2004, 126, 6798 CrossRef CAS PubMed.
- M. Sasaki, E. Kawanishi, Y. Shirakawa, M. Kawahata, H. Masu, K. Yamaguchi and K. Takeda, Eur. J. Org. Chem., 2008, 3061 CrossRef CAS.
- For examples of forming enantiomerically enriched nitriles from silyl ketene imines, see S. E. Denmark, T. W. Wilson and M. T. Burk, Chem.–Eur. J., 2014, 20, 9268 CrossRef CAS PubMed; J. Guin, G. Varseev and B. List, J. Am. Chem. Soc., 2013, 135, 2100 CrossRef PubMed; J. Zhao, B. Fang, W. Luo, X. Hao, X. Liu, L. Lin and X. Feng, Angew. Chem., Int. Ed., 2015, 54, 241 CrossRef PubMed.
- For examples of forming enantiomerically enriched products from racemic nitriles in the presence of an electrophile and a chiral catalyst, see S. Shirakawa, K. Liu, H. Ito, T. N. Le and K. Maruoka, Adv. Synth. Catal., 2011, 353, 2614 CrossRef CAS; B. M. Trost, J. R. Miller and C. M. Hoffman, J. Am. Chem. Soc., 2011, 133, 8165 CrossRef PubMed; P. Breistein, J. Johansson, I. Ibrahem, S. Lin, L. Deiana, J. Sun and A. Cordova, Adv. Synth. Catal., 2012, 354, 1156 CrossRef; L. Yin, M. Kanai and M. Shibasaki, Tetrahedron, 2012, 68, 3497 CrossRef; K. Ohmatsu, A. Goto and T. Ooi, Chem. Commun., 2012, 48, 7913 RSC; T.-Y. Qin, W.-W. Liao, Y.-J. Zhang and S. X.-A. Zhang, Org. Biomol. Chem., 2013, 11, 984 Search PubMed; S. H. Eitel, S. Jautze, W. Frey and R. Peters, Chem. Sci., 2013, 4, 2218 RSC; D. Sureshkumar, V. Ganesh, N. Kumagai and M. Shibasaki, Chem.–Eur. J., 2014, 20, 15723 CrossRef PubMed; E. Badiola, B. Fiser, E. Gómez-Bengoa, A. Mielgo, I. Olaizola, I. Urruzuno, J. M. García, J. M. Odriozola, J. Razkin, M. Oiarbide and C. Palomo, J. Am. Chem. Soc., 2014, 136, 17869 CrossRef PubMed; B. W. H. Turnbull and P. A. Evans, J. Am. Chem. Soc., 2015, 137, 6156 CrossRef PubMed; Z.-P. Hu, Z. Zhuang and W.-W. Liao, J. Org. Chem., 2015, 80, 4627 CrossRef PubMed; M. V. Vita, P. Caramenti and J. Waser, Org. Lett., 2015, 17, 5832 CrossRef PubMed; J. Izquierdo, A. Landa, I. Bastida, R. López, M. Oiarbide and C. Palomo, J. Am. Chem. Soc., 2016, 138, 3282 CrossRef PubMed; G.-F. Zou, S.-Q. Zhuang, J.-X. Wang and W.-W. Liao, J. Org. Chem., 2016, 81, 5717 CrossRef PubMed.
- M. Sasaki, T. Takegawa, H. Ikemoto, M. Kawahata, K. Yamaguchi and K. Takeda, Chem. Commun., 2012, 48, 2897 RSC.
- M. Sasaki, T. Takegawa, K. Sakamoto, Y. Kotomori, Y. Otani, T. Ohwada, M. Kawahata, K. Yamaguchi and K. Takeda, Angew. Chem., Int. Ed., 2013, 52, 12956 CrossRef CAS PubMed . See also, Y. Kotomori, M. Sasaki, M. Kawahata, K. Yamaguchi and K. Takeda, J. Org. Chem., 2015, 80, 11013 CrossRef PubMed.
- G. Barker, M. R. Alshawish, M. C. Skilbeck and I. Coldham, Angew. Chem., Int. Ed., 2013, 52, 7700 CrossRef CAS PubMed.
- P. R. Carlier and Y. Zhang, Org. Lett., 2007, 9, 1319 CrossRef CAS PubMed.
- F. F. Fleming, Y. Wei, W. Liu and Z. Zhang, Tetrahedron, 2008, 64, 7477 CrossRef CAS PubMed.
- X. Yang, D. Nath and F. F. Fleming, Org. Lett., 2015, 17, 4906 CrossRef CAS PubMed.
- M. Gao, N. N. Patwardhan and P. R. Carlier, J. Am. Chem. Soc., 2013, 135, 14390 CrossRef CAS PubMed; N. N. Patwardhan, M. Gao and P. R. Carlier, Chem.–Eur. J., 2011, 17, 12250 CrossRef PubMed.
- D. Nath, M. C. Skilbeck, I. Coldham and F. F. Fleming, Org. Lett., 2014, 16, 62 CrossRef CAS PubMed . See also, P. J. Rayner, P. O'Brien and R. A. J. Horan, J. Am. Chem. Soc., 2013, 135, 8071 CrossRef PubMed; D. Nath and F. F. Fleming, Chem.–Eur. J., 2012, 19, 2023 CrossRef PubMed.
- N. S. Sheikh, D. Leonori, G. Barker, J. D. Firth, K. R. Campos, A. J. H. M. Meijer, P. O'Brien and I. Coldham, J. Am. Chem. Soc., 2012, 134, 5300 CrossRef CAS PubMed.
- D. R. Armstrong, P. García-Álvarez, A. R. Kennedy, R. E. Mulvey and J. A. Parkinson, Angew. Chem., Int. Ed., 2010, 49, 3185 CrossRef CAS PubMed.
- R. Neufeld, T. L. Teuteberg, R. Herbst-Irmer, R. A. Mata and D. Stalke, J. Am. Chem. Soc., 2016, 138, 4796 CrossRef CAS PubMed.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G. A. Petersson, H. Nakatsuji, M. Caricato, X. Li, H. P. Hratchian, A. F. Izmaylov, J. Bloino, G. Zheng, J. L. Sonnenberg, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, J. A. Montgomery Jr, J. E. Peralta, F. Ogliaro, M. Bearpark, J. J. Heyd, E. Brothers, K. N. Kudin, V. N. Staroverov, R. Kobayashi, J. Normand, K. Raghavachari, A. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, N. Rega, J. M. Millam, M. Klene, J. E. Knox, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, R. L. Martin, K. Morokuma, V. G. Zakrzewski, G. A. Voth, P. Salvador, J. J. Dannenberg, S. Dapprich, A. D. Daniels, Ö. Farkas, J. B. Foresman, J. V. Ortiz, J. Cioslowski and D. J. Fox, Gaussian 09, Revision D.01, Gaussian, Inc., Wallingford CT, 2009 Search PubMed.
- A. D. Becke, J. Chem. Phys., 1993, 98, 5648 CrossRef CAS.
- S. Grimme, S. Ehrlich and L. Goerigk, J. Comput. Chem., 2011, 32, 1456 CrossRef CAS PubMed.
- A. D. McLean and G. S. Chandler, J. Chem. Phys., 1980, 72, 5639 CrossRef CAS; K. Raghavachari, J. S. Binkley, R. Seeger and J. A. Pople, J. Chem. Phys., 1980, 72, 650 CrossRef.
- G. Scalmani and M. J. Frisch, J. Chem. Phys., 2010, 132, 114110 CrossRef CAS PubMed; M. Cossi, N. Rega, G. Scalmani and V. Barone, J. Comput. Chem., 2003, 24, 669 CrossRef PubMed and references therein.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details, spectroscopic data, ReactIR, kinetics details, X-ray and DFT data, and NMR spectra. CCDC 1477823. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c6sc03712g |
| This journal is © The Royal Society of Chemistry 2017 |