 Open Access Article
Open Access ArticleHighly selective one-step dehydration, decarboxylation and hydrogenation of citric acid to methylsuccinic acid†
Jasper
Verduyckt
 and
Dirk E.
De Vos
and
Dirk E.
De Vos
 *
*
Centre for Surface Chemistry and Catalysis, Department of Microbial and Molecular Systems, KU Leuven – University of Leuven, Leuven Chem&Tech, Celestijnenlaan 200F, Post Box 2461, 3001 Heverlee, Belgium. E-mail: dirk.devos@kuleuven.be
First published on 16th January 2017
Abstract
The one-step dehydration, decarboxylation and hydrogenation of the bio-based and widely available citric acid is presented. This reaction sequence yields methylsuccinic acid with yields of up to 89%. Optimal balances between the reaction rates of the different steps were found by varying the hydrogenation catalyst and the reaction parameters (H2 pressure, pH, temperature, time and catalyst-to-substrate ratio).
Introduction
Methylsuccinic acid constitutes a very interesting bio-based building block for the chemical industry. This bifunctional carboxylic acid is used for the production of biodegradable polyesters,1–4 binders,5 and cosmetics.6 Currently, it is only available via the hydrogenation of itaconic acid.7–9 Research is still being conducted to optimise this transformation.10 Itaconic acid itself is synthesised via fermentation with an annual production volume of 41.4 thousand tons in 2011.11Citric acid is a much more widely available renewable resource. It is produced via a high yield fermentation with an annual production volume of 2 million tons in 2016.12 Sanders and co-workers recently reported the use of citric acid as a platform chemical for the synthesis of methacrylic acid.13 The dehydration of citric acid was followed by a double decarboxylation, catalyzed by Pd/Al2O3; this reaction sequence resulted however in a selectivity of only 41% at full conversion with 44% selectivity to volatile degradation products.
In this edge article we present the one-step dehydration, decarboxylation and hydrogenation of citric acid. This surprising reaction sequence was discovered by performing the decarboxylation reaction under a low H2 pressure in the presence of Pd and allows to obtain methylsuccinic acid in very high yield.
Results and discussion
In the initial proof of concept, Pd/C, a well-known hydrogenation catalyst, was compared to Pt/C under an inert atmosphere as well as under a H2-rich atmosphere; citric acid was reacted at 225 °C for 6 h in water (Table 1, entries 1–4). These initial reaction conditions were inspired by the decarboxylation of L-proline to pyrrolidine, which was recently reported by our group.14 In addition, NaOH was added in order to reduce unwanted side reactions, such as hydration of the generated double bond.13 In general, citric acid is very reactive; full conversions are readily obtained in all reactions. Scheme 1 gives an overview of the different products observed via1H-NMR. Citric acid (1) is first dehydrated to aconitic acid, which can spontaneously decarboxylate under the reaction conditions (Table 1, entry 25).15 Although this reaction proceeds spontaneously, it is also accelerated by metal catalysts based on e.g. Pd or Pt.13 The catalyst support (at least in case of BaSO4) appears to have no influence on the reaction (Table 1, entry 26). Via this first decarboxylation, itaconic acid (2) is formed (Scheme 2).15,16 This unsaturated carboxylic acid can quickly isomerise to mesaconic (3) and citraconic acid (4).15 In the presence of H2 these isomers can either be hydrogenated to the desired methylsuccinic acid (5) or further decarboxylated to methacrylic (6) and crotonic acid. Hydrogenation of the latter then leads to the formation of isobutyric (7) and butyric acid (8), respectively. The monofunctional unsaturated carboxylic acids can also react through a third consecutive decarboxylation, yielding propene, which is hydrogenated to propane (9) in a H2-rich environment. The presence of lower alkanes like propane (9) leads to mass loss from solution and was confirmed by performing gas phase Fourier transform infrared spectroscopy (FTIR) on the headspace of the reaction in entry 9. When hydrogenation of the double bond occurs before the first decarboxylation, propane-1,2,3-tricarboxylic acid (10) can be obtained. Finally, acetone (11), pyruvic (12) and acetic acid (13) were observed as fragmentation products (cfr. infra). The detailed identification of the observed (numbered) compounds is given in the ESI.† The identity of the desired methylsuccinic acid (5) was additionally confirmed by GC-MS after derivatisation with methanol to dimethyl methylsuccinic acid.| Catalyst | C/Sb [mol%] | P H2 [bar] | T [°C] | t [h] | Additive (eq.) | Conversion [%] | Selectivity [%] | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MSAc | PTAd | 2nd decae | Fragmentationf | Itaconic isomersg | ||||||||
| a Reaction conditions: citric acid (0.2 mmol), water (2 mL), 2 bar N2. b Catalyst-to-substrate ratio. c Methylsuccinic acid. d Propane-1,2,3-tricarboxylic acid. e ‘2nd deca’ represents isobutyric acid, butyric acid and methacrylic acid. f ‘Fragmentation’ represents acetone, acetic acid, pyruvic acid and lactic acid. g ‘Itaconic isomers’ represents itaconic acid, mesaconic acid and citraconic acid. h 6 bar N2. i Includes 12% of 2-hydroxyisobutyric acid. j Ce(SO4)2. k p-Methoxyphenol. l Includes 10% of β-carboxy-γ-butyrolactone, as a hydration and ring-closure product of itaconic acid. m 17 mg BaSO4. | ||||||||||||
| 1 | Pt/C | 4 | 0h | 225 | 6 | NaOH (0.8) | >99 | 23 | <1 | 28 | 16 | <1 |
| 2 | Pt/C | 4 | 4 | 225 | 6 | NaOH (0.8) | >99 | 31 | <1 | 23 | 9 | <1 |
| 3 | Pd/C | 4 | 0h | 225 | 6 | NaOH (0.8) | >99 | 13 | <1 | 46i | 27 | <1 |
| 4 | Pd/C | 4 | 4 | 225 | 6 | NaOH (0.8) | >99 | 67 | 2 | 12 | 7 | <1 |
| 5 | Pd/ZrO2 | 4 | 4 | 225 | 6 | NaOH (0.8) | >99 | 81 | <1 | 7 | 6 | <1 |
| 6 | Pd/MgAl2O4 | 4 | 4 | 225 | 6 | NaOH (0.8) | >99 | 71 | 7 | 5 | 12 | <1 |
| 7 | Pd/BaSO4 | 4 | 4 | 225 | 6 | NaOH (0.8) | >99 | 81 | <1 | 3 | 10 | <1 |
| 8 | Pd/Al2O3 | 4 | 4 | 225 | 6 | NaOH (0.8) | >99 | 71 | <1 | 14 | 9 | <1 |
| 9 | Pd/BaSO4 | 4 | 4 | 225 | 6 | NaOH (1.0) | >99 | 75 | <1 | 5 | 8 | <1 |
| 10 | Pd/BaSO4 | 4 | 8 | 225 | 6 | NaOH (1.0) | >99 | 75 | <1 | 6 | 9 | <1 |
| 11 | Pd/BaSO4 | 4 | 12 | 225 | 6 | NaOH (1.0) | >99 | 74 | 4 | 3 | 15 | <1 |
| 12 | Pd/BaSO4 | 4 | 4 | 225 | 6 | — | >99 | 84 | <1 | 3 | 7 | <1 |
| 13 | Pd/BaSO4 | 4 | 4 | 225 | 6 | NaOH (1.5) | >99 | 64 | <1 | 4 | 18 | <1 |
| 14 | Pd/BaSO4 | 4 | 4 | 225 | 6 | NaOH (2.0) | >99 | 54 | <1 | 2 | 26 | <1 |
| 15 | Pd/BaSO4 | 4 | 4 | 175 | 6 | — | >99 | 82 | 7 | 1 | 5 | <1 |
| 16 | Pd/BaSO4 | 4 | 4 | 200 | 6 | — | >99 | 83 | 7 | 1 | 5 | <1 |
| 17 | Pd/BaSO4 | 4 | 4 | 250 | 6 | — | >99 | 58 | <1 | 10 | 9 | <1 |
| 18 | Pd/BaSO4 | 4 | 4 | 175 | 0.67 | — | 12 | 67 | 33 | <1 | <1 | <1 |
| 19 | Pd/BaSO 4 | 4 | 4 | 225 | 0.67 | — | >99 | 86 | <1 | 3 | 6 | <1 |
| 20 | Pd/C | 0.5 | 8 | 225 | 0.67 | — | >99 | 84 | 5 | 2 | 7 | <1 |
| 21 | Pd/C | 0.5 | 20 | 225 | 0.67 | — | >99 | 77 | 5 | 2 | 9 | <1 |
| 22 | Pd/BaSO4 | 0.5 | 20 | 225 | 0.67 | — | >99 | 36 | 0 | 11 | 29 | 17 |
| 23 | Pd/BaSO4 | 4 | 4 | 225 | 0.67 | CeIV (0.03)j | >99 | 68 | <1 | 6 | 13 | <1 |
| 24 | Pd/BaSO 4 | 4 | 4 | 225 | 0.67 | p-MP (0.05) | >99 | 89 | <1 | 3 | 6 | <1 |
| 25 | — | — | 4 | 225 | 6 | — | >99 | <1 | <1 | 24 | 18 | 33l |
| 26 | BaSO4 | —m | 4 | 225 | 6 | — | >99 | <1 | <1 | 26 | 22 | 32l |
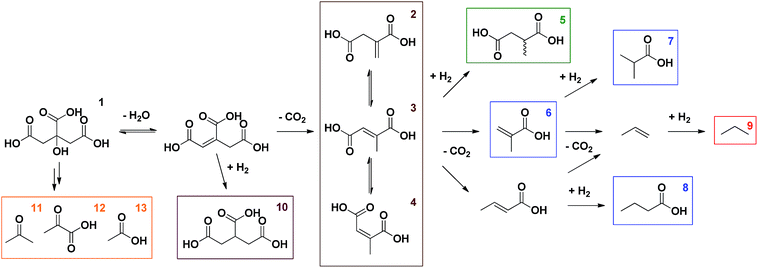 | ||
| Scheme 1 Reaction network for the decarboxylation of citric acid in the presence of H2. The detailed identification of the observed (numbered) compounds is given in the ESI.† | ||
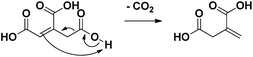 | ||
| Scheme 2 Heterolytic reaction mechanism of the spontaneous decarboxylation of aconitic acid to itaconic acid.15,16 | ||
In the first reactions, a substantial amount of methylsuccinic acid (5, 67%) was only formed in the presence of Pd/C and under a H2-rich atmosphere (Table 1, entry 4). Using Pt/C the hydrogenation was rather slow, resulting in only 31% selectivity for methylsuccinic acid (5; Table 1, entry 2). Although hydrogenated products were observed in the reactions without added H2, hydrogenation is not fast enough to suppress the formation of further decarboxylation products; non-reduced Pd or Pt species at the surface of the metal particles might facilitate the radical fragmentation (cfr. infra; Table 1, entries 1, 3). Especially acetic (13), methacrylic (6) and isobutyric acid (7) are formed. In these reactions H2 most likely originates from CO via the water–gas shift reaction, which occurs readily under the reaction conditions.14 CO itself is formed during the fragmentation of citric acid (1, cfr. infra).13,15 In addition, in the absence of H2 2-hydroxyisobutyric acid is formed with a selectivity of 12% (Table 1, entry 3). This side product most probably stems from the hydration of mesaconic or citraconic acid (3, 4), followed by decarboxylation. The hydration of methacrylic acid (6) is not likely, since one would expect the formation of 3-hydroxyisobutyric acid.17,18
Next, various supported Pd catalysts were screened for the conversion of citric acid (1) to methylsuccinic acid (5) using the conditions of the reaction in entry 4 (Table 1, entries 4–8). Data on metal dispersion and texture of the catalysts are given in the ESI.† The highest yield so far of methylsuccinic acid (5, 81%) was obtained with Pd supported on ZrO2 and BaSO4 (entries 5, 7). With catalysts containing highly dispersed Pd (e.g. Pd/C, Pd/Al2O3 or Pd/MgAl2O4; see ESI†),19,20 side or consecutive reactions are occurring somewhat more prominently. Examples are the further decarboxylation of itaconic acid and its isomers (2–4) on Pd/C or Pd/Al2O3 (entries 4, 8),13 or the hydrogenation to propane-1,2,3-tricarboxylic acid (10), even before the first decarboxylation can occur, as in the reaction with Pd/MgAl2O4 (entry 6).
The commercial Pd/BaSO4 was further used to optimise the reaction conditions. First, the effect of an increasing H2 pressure was investigated (Table 1, entries 9–11). An increase to 8 bar has no impact, which indicates that the formation of fragmentation products is not in direct competition with the hydrogenation and thus that these products most probably originate directly from citric acid (1, cfr. infra). Further increase of the H2 pressure to 12 bar however leads to the production of propane-1,2,3-tricarboxylic acid (10) and to an increased production of acetic acid (13), indicating that a higher H2 pressure is not favourable in this case.
Subsequently, the pH of the aqueous reaction mixture was varied (Table 1, entries 9, 12–14). By adding various amounts of NaOH (0–2 eq.), the pH of the starting solution was varied gradually between 2 and 5. A yield of 84% was obtained for methylsuccinic acid (5) when no NaOH was added. This rapidly decreased with increasing pH, especially due to the production of acetic acid (13). Besides, from the addition of 1.5 eq. NaOH onwards, also lactic acid was produced. This compound presumably arises from the hydrogenation of pyruvic acid (12). The beneficial effect of an acidic environment on the production of methylsuccinic acid (5) can be threefold: (i) the formation of fragmentation products may be promoted by a more alkaline medium; (ii) the initial dehydration of citric acid to aconitic acid is acid-catalyzed; (iii) the (partly spontaneous) decarboxylation of aconitic acid requires the uptake of a proton by the double bond (Scheme 2).
Next, the influence of the temperature was studied (Table 1, entries 15–17). A higher temperature of 250 °C results in more fragmentation products, more further decarboxylation and therefore in a methylsuccinic acid yield of only 58%. When the temperature was decreased to 200 °C and further to 175 °C, less fragmentation products and less further decarboxylation were observed. However, at these temperatures the decarboxylation is slower and hydrogenation competes with the first decarboxylation, resulting in the formation of propane-1,2,3-tricarboxylic acid (10). Therefore 225 °C is considered as the optimal temperature. Moreover, from the time course it is clear that a reaction time of only 40 min suffices at this temperature (Fig. S1†). The yield of methylsuccinic acid (5) even reaches an optimum of 86% at this point (Table 1, entry 19). At 175 °C the reaction is markedly slower; only 12% conversion of citric acid (1) is reached after 40 min (Table 1, entry 18).
Summarising, to reach a high yield of methylsuccinic acid (5) the initial dehydration rate must surpass the fragmentation rate (cfr. infra) and the first decarboxylation has to occur faster than the hydrogenation, which in turn must be quicker than the subsequent decarboxylation. The first decarboxylation readily occurs at 225 °C and the hydrogenation is fast enough using 4 mol% Pd/BaSO4 and 4 bar H2. The question arises whether this hydrogenation can also be rapid enough using only 0.5 mol% Pd, which is an industrially relevant catalyst-to-substrate ratio. For a more rapid hydrogenation either a larger amount of Pd, a higher dispersion of Pd or a higher H2 pressure is needed. Since the former is now lowered to 0.5 mol% Pd, compensation by the other two parameters is required. Considering the much higher dispersion of Pd/C compared to Pd/BaSO4 (32% vs. 6%) and given that the promoting effect of Pd/C on further decarboxylation would be suppressed at higher H2 pressures, the focus turned to Pd/C.20–23 A range of H2 pressures was screened using 0.5 mol% Pd/C (Table S3†). From 8 bar H2 onwards, the formation of ‘2nd decarboxylation’ products was minimal, resulting in an optimal yield of 84% methylsuccinic acid (5; Table 1, entry 20). For the lower dispersed Pd/BaSO4 even a H2 pressure of 20 bar was not enough for an adequate hydrogenation: only 36% methylsuccinic acid (5) was produced and there were still unsaturated products (methacrylic acid, itaconic acid and its isomers) left in solution (Table 1, entry 22 vs. 21).
Finally, to sort out where acetone (11), pyruvic (12) and acetic acid (13) originate from, first a reactivity study was performed on several intermediate compounds using the optimized conditions of entry 19 (Table 2, entries 1–4). The unsaturated tricarboxylic acid aconitic acid is quickly decarboxylated and hydrogenated to methylsuccinic acid (5, entry 1). The relatively large amount of propane-1,2,3-tricarboxylic acid (10) formed, can be explained by the lower onset temperature for hydrogenation in comparison to decarboxylation. In contrast, unsaturated dicarboxylic and monocarboxylic acids are preferentially hydrogenated to the corresponding saturated carboxylic acids (entries 2–4). No fragmentation products were observed starting from these compounds. Moreover, further examination shows that the saturated carboxylic acids are rather stable, as shown by the limited conversion after 6 h (Table 2, entries 5–7). Apparently, carboxylic acids need to be activated for a smooth decarboxylation, like in α,β-unsaturated carboxylic acids. All this means that the fragmentation products originate directly from citric acid. To check whether this process has radical-type intermediates, an attempt was made to both accelerate and slow down the fragmentation by the addition of a one-electron acceptor (CeIV) and a radical trap (p-methoxyphenol), respectively (Table 1, entries 23–24). The addition of CeIV increases the amount of fragmentation products from 6% to 15%; this undoubtedly shows that the fragmentation process is radical in nature. Although the amount of fragmentation products was not reduced by adding p-methoxyphenol, the yield of methylsuccinic acid (5) did increase from 86% to 89%. This indicates that the mass loss from solution partly occurs through radical intermediates. Based on these observations a mechanism for the formation of acetone (11), pyruvic (12) and acetic acid (13) is proposed in Scheme 3. Since this process is both radical in nature and promoted by a more alkaline environment, a carboxylate anion is proposed to lose an electron in the first step. The generated radical then eliminates CO2, forming another radical that further reacts via a β-cleavage. The β-cleavage in reaction A results in the formation of pyruvic (12) and acetic acid (13). Pyruvic acid is not stable and further decarboxylates to acetaldehyde (Fig. S1†).24 This aldehyde is then decarbonylated on Pd to form CO and methane, which is lost from solution. The presence of methane and CO (traces), was also confirmed by gas phase Fourier transform infrared spectroscopy. In reaction B the β-cleavage forms acetoacetic acid, which further decomposes to acetone (11).24 Lastly, the β-cleavage in reaction C produces itaconic acid (2), which further reacts according to Scheme 1. To corroborate this degradation mechanism an isotopic labelling experiment with citric acid-2,4-13C was performed under the ‘blank’ reaction conditions, without a catalyst (ESI†). The distribution of 13C throughout the different side products is entirely consistent with the proposed reaction mechanisms.
| Substrate | t [h] | Conversion [%] | Selectivity [%] | |||||
|---|---|---|---|---|---|---|---|---|
| PTAb | MSAc | BAd | IBAe | Fragmentationf | ||||
| a Reaction conditions: substrate (0.2 mmol), 4 mol% Pd/BaSO4, water (2 mL), 2 bar N2 + 4 bar H2, 225 °C. b Propane-1,2,3-tricarboxylic acid. c Methylsuccinic acid. d Butyric acid. e Isobutyric acid. f ‘Fragmentation’ represents acetone, acetic acid, pyruvic acid and lactic acid. | ||||||||
| 1 | Aconitic acid | 0.33 | >99 | 18 | 81 | <1 | 1 | <1 |
| 2 | Mesaconic acid | 0.33 | >99 | — | 88 | 1 | 2 | <1 |
| 3 | Itaconic acid | 0.33 | >99 | — | 86 | 1 | 2 | <1 |
| 4 | Methacrylic acid | 0.33 | >99 | — | — | — | 93 | <1 |
| 5 | Propane-1,2,3-tricarboxylic acid | 6 | 8 | — | >99 | <1 | <1 | <1 |
| 6 | Methylsuccinic acid | 6 | 12 | — | — | <1 | 25 | <1 |
| 7 | Isobutyric acid | 6 | 19 | — | — | — | — | <1 |
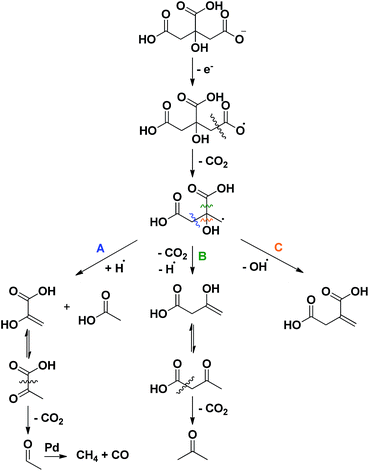 | ||
| Scheme 3 Radical fragmentation routes for citric acid, explaining the formation of acetone, acetic acid, pyruvic acid, methane and CO. | ||
Conclusions
In conclusion, the direct formation of methylsuccinic acid from citric acid was successfully achieved in water via the new reaction sequence of dehydration, decarboxylation and hydrogenation. An optimal balance between the different reaction rates was reached at 225 °C for a reaction time of merely 40 min using 4 mol% Pd/BaSO4 with 4 bar H2 or using only 0.5 mol% Pd/C with 8 bar H2, leading to a methylsuccinic acid yield of 86% and 84%, respectively. In addition, the radical fragmentation of citric acid to acetone, acetic and pyruvic acid could drastically be reduced by working in a more acidic environment, i.e. at the natural pH of citric acid.Acknowledgements
J. V. thanks FWO for a doctoral fellowship. D. E. D. V. acknowledges IWT and FWO for research project funding, the Flemish government for long-term structural funding through Methusalem and Belspo (IAP-PAI 7/05) for financial support. The assistance of Karel Duerinckx and Werner Wouters with 1H-NMR and pressure reactors is very much appreciated. Finally, Free De Schouwer is thanked for our fruitful discussions.Notes and references
- A. Takasu, Y. Iio, Y. Oishi, Y. Narukawa and T. Hirabayashi, Macromolecules, 2005, 38, 1048 CrossRef CAS.
- H. G. Chae, S. H. Park, B. C. Kim and D. K. Kim, J. Polym. Sci., Part B: Polym. Phys., 2004, 42, 1759 CrossRef CAS.
- T. Xie, C. Gao, C. Wang, S. Shen and Y. Wu, Polym.–Plast. Technol. Eng., 2014, 53, 465 CrossRef CAS.
- R. Loos, D. Mijolovic, J. Heimann and Z. J. Szarka, U.S. Pat. Appl., US 20120245256, 2012.
- D. Mijolovic, Z. J. Szarka, J. Heimann and S. Garnier, Ger. Pat., DE 102011080722, 2011.
- H. Richard and B. Muller, WO Pat., WO/2012/119861, 2012.
- A. Bavley and C. J. Knuth, U.S. Pat., 2773897, 1956.
- H. Shi, W. Shen and H. Xu, SIPO Pat., CN 1609089, 2005.
- Y. Wu, C. Gao, Y. Wang, C. Wang and J. Xu, SIPO Pat., CN 102617326, 2012.
- Q. Huang, W. Yu, R. Lu, F. Lu, J. Gao, H. Miao and J. Xu, RSC Adv., 2015, 5, 97256 RSC.
- Weastra, http://www.bioconsept.eu/wp-content/uploads/BioConSepT_Market-potential-for-selected-platform-chemicals_report1.pdf, accessed November 21, 2016.
- IHS Chemical, http://blog.ihs.com/video-bon-appetit-a-taste-of-the-citric-acid-and-monosodium-glutamate-msg-markets, accessed November 21, 2016.
- J. Le Nôtre, S. C. M. Witte-van Dijk, J. van Haveren, E. L. Scott and J. P. M. Sanders, ChemSusChem, 2014, 7, 2712 CrossRef PubMed.
- J. Verduyckt, M. Van Hoof, F. De Schouwer, M. Wolberg, M. Kurttepeli, P. Eloy, E. M. Gaigneaux, S. Bals, C. E. A. Kirschhock and D. E. De Vos, ACS Catal., 2016, 6, 7303 CrossRef CAS.
- M. Carlsson, C. Habenicht, L. C. Kam, M. J. Antal, N. Bian, R. J. Cunningham and M. Jones, Ind. Eng. Chem. Res., 1994, 33, 1989 CrossRef CAS.
- R. T. Arnold, O. C. Elmer and R. M. Dodson, J. Am. Chem. Soc., 1950, 72, 4359 CrossRef CAS.
- X. Meng, T. Abraham and P. Tsobanakis, WO Pat., WO/2004/076398, 2004.
- S. Wang, Z. Wang, L. Yang, J. Dong, C. Chi, D. Sui, Y. Wang, J. Ren, M. Hung and Y. Jiang, J. Mol. Catal. A: Chem., 2007, 264, 60 CrossRef CAS.
- F. De Schouwer, L. Claes, N. Claes, S. Bals, J. Degrève and D. E. De Vos, Green Chem., 2015, 17, 2263 RSC.
- M. Casagrande, S. Franceschini, M. Lenarda, O. Piccolo and A. Vaccari, J. Mol. Catal. A: Chem., 2006, 246, 263 CrossRef CAS.
- J. Su, M. Lu and H. Lin, Green Chem., 2015, 17, 2769 RSC.
- J. G. Immer and H. H. Lamb, Energy Fuels, 2010, 24, 5291 CrossRef CAS.
- J. P. Ford, J. G. Immer and H. H. Lamb, Top. Catal., 2012, 55, 175 CrossRef CAS.
- C. Ferri, Reaktionen der organischen Synthese, Georg Thieme Verlag, Stuttgart, 1978 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details, catalyst characterisation, time course, PH2 optimisation for 0.5 mol% Pd, isotopic labelling experiment and product identification. See DOI: 10.1039/c6sc04541c |
| This journal is © The Royal Society of Chemistry 2017 |
