Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Correction: Ligand design for Rh(III)-catalyzed C–H activation: an unsymmetrical cyclopentadienyl group enables a regioselective synthesis of dihydroisoquinolones
Todd K.
Hyster
a,
Derek M.
Dalton
a and
Tomislav
Rovis
*ab
aDepartment of Chemistry, Colorado State University, Fort Collins, Colorado 80523, USA. E-mail: rovis@lamar.colostate.edu
bDepartment of Chemistry, Columbia University, 3000 Broadway, New York, NY 12007, USA. E-mail: tr2504@columbia.edu
First published on 13th December 2016
Abstract
Correction for ‘Ligand design for Rh(III)-catalyzed C–H activation: an unsymmetrical cyclopentadienyl group enables a regioselective synthesis of dihydroisoquinolones’ by Todd K. Hyster et al., Chem. Sci., 2015, 6, 254–258.
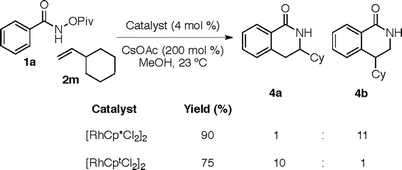
In the original article, Fig. 1 describes the coupling of O-pivaloyl benzhydroxamic acids with vinyl cyclohexane. Upon further analysis, the authors discovered that [RhCp*Cl2]2 provides lactam 4b preferentially (11
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 rr and 90% yield) while [RhCptCl2]2 provides lactam 4a (10
1 rr and 90% yield) while [RhCptCl2]2 provides lactam 4a (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 rr and 75% yield). The authors regret the error. A modified Fig. 1 is provided herein to clarify this error.
1 rr and 75% yield). The authors regret the error. A modified Fig. 1 is provided herein to clarify this error.
In addition, the authors wish to provide updated affiliation and contact details, and as such affiliation b has been added to the corresponding author of this article. Full details may be found herein.
The Royal Society of Chemistry apologises for these errors and any consequent inconvenience to authors and readers.
| This journal is © The Royal Society of Chemistry 2017 |