Open Access Article
Open Access ArticleMulti-electron reduction of sulfur and carbon disulfide using binuclear uranium(III) borohydride complexes†
Polly L.
Arnold
 *a,
Charlotte J.
Stevens
a,
Nicola L.
Bell
*a,
Charlotte J.
Stevens
a,
Nicola L.
Bell
 a,
Rianne M.
Lord
a,
Jonathan M.
Goldberg
b,
Gary S.
Nichol
a,
Rianne M.
Lord
a,
Jonathan M.
Goldberg
b,
Gary S.
Nichol
 a and
Jason B.
Love
a and
Jason B.
Love
 *a
*a
aEaStCHEM School of Chemistry, University of Edinburgh, The King's Buildings, Edinburgh EH9 3JF, UK. E-mail: Polly.Arnold@ed.ac.uk; Jason.Love@ed.ac.uk; Fax: +44 (0)131 6506453; Tel: +44 (0)131 6505429
bDepartment of Chemistry, University of Washington, Box 351700, Seattle, WA 98195-1700, USA
First published on 10th March 2017
Abstract
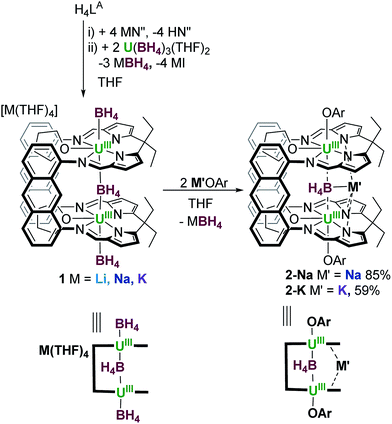
The first use of a dinuclear UIII/UIII complex in the activation of small molecules is reported. The octadentate Schiff-base pyrrole, anthracene-hinged ‘Pacman’ ligand LA combines two strongly reducing UIII centres and three borohydride ligands in [M(THF)4][{U(BH4)}2(μ-BH4)(LA)(THF)2] 1-M, (M = Li, Na, K). The two borohydride ligands bound to uranium outside the macrocyclic cleft are readily substituted by aryloxide ligands, resulting in a single, weakly-bound, encapsulated endo group 1 metal borohydride bridging the two UIII centres in [{U(OAr)}2(μ-MBH4)(LA)(THF)2] 2-M (OAr = OC6H2tBu3-2,4,6, M = Na, K). X-ray crystallographic analysis shows that, for 2-K, in addition to the endo-BH4 ligand the potassium counter-cation is also incorporated into the cleft through η5-interactions with the pyrrolides instead of extraneous donor solvent. As such, 2-K has a significantly higher solubility in non-polar solvents and a wider U–U separation compared to the ‘ate’ complex 1. The cooperative reducing capability of the two UIII centres now enforced by the large and relatively flexible macrocycle is compared for the two complexes, recognising that the borohydrides can provide additional reducing capability, and that the aryloxide-capped 2-K is constrained to reactions within the cleft. The reaction between 1-Na and S8 affords an insoluble, presumably polymeric paramagnetic complex with bridging uranium sulfides, while that with CS2 results in oxidation of each UIII to the notably high UV oxidation state, forming the unusual trithiocarbonate (CS3)2− as a ligand in [{U(CS3)}2(μ-κ2:κ2-CS3)(LA)] (4). The reaction between 2-K and S8 results in quantitative substitution of the endo-KBH4 by a bridging persulfido (S2)2− group and oxidation of each UIII to UIV, yielding [{U(OAr)}2(μ-κ2:κ2-S2)(LA)] (5). The reaction of 2-K with CS2 affords a thermally unstable adduct which is tentatively assigned as containing a carbon disulfido (CS2)2− ligand bridging the two U centres (6a), but only the mono-bridged sulfido (S)2− complex [{U(OAr)}2(μ-S)(LA)] (6) is isolated. The persulfido complex (5) can also be synthesised from the mono-bridged sulfido complex (6) by the addition of another equivalent of sulfur.
Introduction
The UIII oxidation state is strongly reducing and its molecular complexes are well known for their ability to activate small molecules1–3 such as arenes,4,5 N2,6–10 CO,11–19 and CO2.20–26 The coordination of actinides with chalcogenide ligands has begun to attract increasing interest.27–33 Understanding and controlling the activation and functionalisation of chalcogen elements and their compounds is important in the petroleum industry and in functional polymer technologies, and is increasingly of interest for new methods in organic and biomimetic syntheses,34 both with d-block35–43 and rare earth metal44,45 complexes. The kinetically facile nature of the soft atom transfer reactions with the harder metal cations suggests opportunities in catalytic chalcogen atom-transfer processes, yet the binding mode and stoichiometry of the incorporated chalcogen atoms/fragments is as yet unpredictable and so far appears to be primarily dependent on subtle differences in steric accessibility of the reducing metal centre(s). Furthermore, complexes that exhibit different binding modes with polarisable atoms such as these can provide new insight into the role of f- and other valence orbitals in actinide-ligand bonding which is fundamentally important to improving the safe handling of nuclear waste materials.46–49Almost all instances of the activation of sulfur or sulfur-containing small molecules by an actinide involve the assembly of two mononuclear UIII centres around one or more atoms of elemental sulfur, or an S atom from CS2, providing two reducing electrons to form [UIV]2 products, occasionally with further incorporation of CS2. Products are often formed as a mixture of the persulfido (E2)2−-bridged [UIV]2 complexes such as (μ-η2:η2-S2)[UX3]2 (where [UX3] = [U(C5H4Me)3],47 [U(N′′3)3] (N′′ = N(SiMe3)2),27 [U{(SiMe2NPh)3tacn}],50 and [U{(AdArO)3tacn}],51), and sulfido (E)2−-bridged [UIV]2 complexes such as (μ-S)[U(N′′3)3]2,27 and (μ-S)[U((SiMe2NPh)3tacn)]2.50 The first terminal uranium persulfido complex was U[(SiMe2NPh)3tacn](η2-S2).50 Incorporation of up to four S atoms has also been observed, e.g. in [K(18-crown-6)][(ηn-Sn)[U(N′′3)3] (n = 1–3),52 and (μ-S2)2[U{(AdArO)3tacn}]2.51 One monosulfido complex adds CS2 to form the [UIV]2CS3 adduct (μ-κ2:κ2-CS3)2[U{(AdArO)3tacn}]2, which can also be formed directly from the UIII precursor and CS2. Finally, the ‘ate’ UIII siloxide complex [K(18-c-6)U{OSi(OtBu)3}4] has been shown to react with CS2 to form a variety of potassium-bound reduction products including [K2(18-c-6)2U{OSi(OtBu)3}4(μ3-κ2:κ2:κ2-CS3)].53
We reasoned that the preorganisation of two UIII centres could enhance the rate and selectivity of small molecule activation reactions in the now two-body problem. In light of this we reported the first structurally characterised binuclear [UIII]2 complex of a single ligand using the small cavity macrocycle trans-calix[2]benzene[2]pyrrole.54 We further showed that the reaction between [U(BH4)3(THF)2] and the anions of the ‘Pacman’-shaped Schiff-base polypyrrolic macrocycles55–57 afforded another two classes of molecule that combine two UIII centres in a single ligand structure.58 The larger of the two ‘Pacman’ ligands, hinged by anthracenyl groups, forms the unusual ‘ate’ complex, [Na(THF)4][{UIII(BH4)}2(μ-BH4)(LA)(THF)2] 1-Na, Scheme 1.58
Herein, we report reactivity studies of 1 and a new derivative in which the exo-coordination sites of both UIII centres are protected by ‘capping’ aryloxide groups. We demonstrate the differences in reactivity between these compounds and their unique selectivity for the formation of (μ-S), (μ-S2) or (μ-CS3) in their reactions with S8 and CS2.
Results and discussion
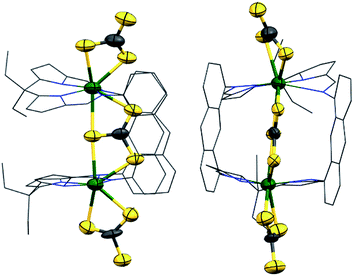
The reaction of H4LA with KN(SiMe3)2, followed by U(BH4)3(THF)2 affords [K(THF)4][{UIII(BH4)}2(μ-BH4)(LA)(THF)2] 1-K in good yield; 1-K is the potassium analogue of our recently reported sodium complex 1-Na.56,59 Reactions of 1-K to target exo-X ligand substitution with amide, alkoxide, aryloxide, cyclopentadienyl, alkyl and allyl anions were investigated (see ESI†).The most successful reactions, as evidenced by 1H NMR spectroscopy are those between 1-K and two equivalents of the aryloxide MOAr where M = K, Na and Ar = C6H2(tBu)3-2,4,6 (Scheme 1). The 1H NMR spectra of both reaction mixtures are very similar and each display a new set of very broad, paramagnetically shifted resonances of low intensity, which nevertheless are consistent with a single, symmetric macrocyclic ligand environment. A large quantity of dark green crystals formed over 4 h in the 1-Na/KOAr reaction mixture. Analysis of these by X-ray diffraction revealed their composition to be [{U(OAr)}2(endo-μ-KBH4)(LA)(THF)2] (2-K) in which the two exo BH4− ligands have been exchanged for aryloxides and the Na+ cation of 1-Na has been exchanged for a K+ cation which notably now binds within the macrocyclic cleft (Fig. 1). Single crystals also formed in the 1-Na/NaOAr reaction mixture, but only after standing for two weeks. These were characterised as the analogous Na+-containing product [{U(OAr)}2(endo-μ-NaBH4)(LA)(THF)2] (2-Na) in which again the Na+ cation is also located within the macrocyclic cleft (Fig. 1). The in situ NMR scale reaction between 1-K and NaOAr yielded resonances consistent with the formation of only 2-K. Interestingly, no reaction occurs between 1-K and two equivalents of LiOAr. On a preparative scale, the reaction of 1-Na with KOAr in THF allows crystalline 2-K to be isolated in 59% yield. Crystalline 2-K is insoluble in THF and pyridine but sparingly soluble in toluene and hot benzene. The 1H NMR spectrum of 2-K in C6D6 is sharper than that of the crude product formed from an in situ synthesis in d8-THF and contains paramagnetically shifted resonances corresponding to a symmetric macrocycle and two equivalent aryloxide ligands. One resonance that integrates to 18H is seen at 4.1 ppm for the two para-tBu groups and one of integral 36H at −0.1 ppm for the four ortho-tBu groups of the two aryloxides. The resonance corresponding to the four equivalent meta protons of the aryloxides cannot be distinguished from the macrocycle resonances of equal integral. A single broad resonance appears in the 11B NMR spectrum at 188 ppm, attributed to the endo-KBH4, in comparison to the two resonances seen at 212 ppm (1B, endo-BH4) and 207 (2B, exo-BH4) for 1-K. The solution state IR for complex 2-K in THF shows a single stretch at 2280 cm−1 corresponding to a symmetric U(μ2-η2-, μ2-η2-H2BH2)U ionic binding mode in solution, identical to that observed in the solid state for 1-Na.
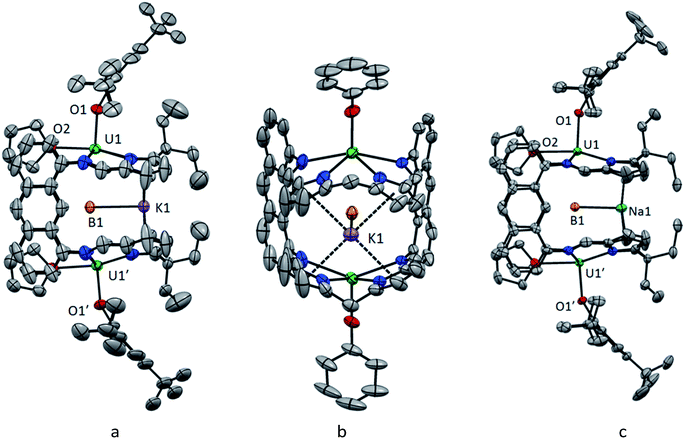 | ||
| Fig. 1 Solid-state structure of 2-K showing side view (a) and front view (b), and solid-state structure of 2-Na, side view (c). For clarity, the major orientation of the disordered tBu groups in 2-K is shown in (a) and the meso ethyl groups, aryloxide substituents, THF molecules, and tert-butyl groups are omitted from (b); all H atoms and lattice solvent are also omitted (displacement ellipsoids are drawn at 50% probability). Full details for 2-Na are in the ESI.† | ||
The geometry of each UIII centre in 2-K (Fig. 1) is best described as a distorted pentagonal bipyramid. The coordination environment of the UIII centre shows five equatorial donor atoms, comprising the four nitrogen atoms of the macrocycle and one oxygen atom of THF solvent, which sits between the macrocyclic hinges, and the borohydride. The aryloxide ligand occupies the exo axial coordination site and the BH4 ligand (hydrogens not located) sits within the macrocyclic cleft bridging the two UIII centres with long U–B distances of about 3.3 Å (Table 1).
| 2-K | 2-Na | |
|---|---|---|
| U1⋯U1′ | 6.5881(3) | 6.5265(7) |
| Mean U–Nim | 2.65 | 2.65 |
| Mean U–Npyr | 2.50 | 2.51 |
| U1–N4 plane | 0.70 | 0.69 |
| U1–O1 | 2.231(5) | 2.245(6) |
| U1⋯B1 | 3.312(1) | 3.269(1) |
| U1–O2 | 2.554(5) | 2.592(6) |
| B1–M1 | 3.036(11) | 2.747(2) |
| M1-[pyr]centroid | 3.154(2), 3.153(2) | 2.85(4), 3.04(2), 3.08(4), 3.61(2) |
| U1–B1–U1′ | 168.2(4) | 173.0(6) |
| O1–U1–B1 | 178.3(2) | 177.6(1) |
| U1–O1–Cipso | 154.0(5) | 153.3(6) |
The phenyl rings of the aryloxide ligands are perpendicular to the anthracenyl hinges of the macrocycle and the angle at the O atom (U1–O1–Cipso = 154.0(5)° (2-K), 153.3(6)° (2-Na)) orients the ortho-tBu groups away from the THF donor. The UIII cations are considerably displaced out of the macrocycle N4 donor planes, away from the intermetallic cleft, by 0.70 Å in 2-K and 0.69 Å in 2-Na, and the sum of the four N–U–N angles in the two structures is 337.9(8)° and 338.1(8)° respectively. The separation of the bulky aryloxide ligand from the N4 plane of the macrocycle is imposed by steric demand. Therefore, the displacement of the UIII centres out of the N4 plane is a compromise between optimised U–OAr and U–N bond lengths. The resulting mean U–N(imine) distances of 2.65 Å in both complexes and the mean U–N(pyrrolide) distances of 2.50 Å (2-K) and 2.51 Å (2-Na) are lengthened compared to those observed in 1-Na (2.62 Å and 2.49 Å). The U1–O1 bond lengths in 2-K and 2-Na are 2.231(5) Å and 2.245(6) Å respectively (Table 1). These are longer than the UIII-OAr distances in [U(OC6H3iPr2-2,6)3]54 and [U(OC6H3tBu2-2,6)3]59 which range from 2.149(4) to 2.214(7) Å but similar to the mean U-OAr distance of 2.22 Å observed in the constrained aryloxide TACN complexes U[(RArO)3(TACN)].60,61
The main difference between the structures of 2-K and 2-Na is the binding of the K+ and Na+ cations within the cleft. The larger K+ ion is sandwiched symmetrically between all four pyrrolide rings (Fig. 1a) with K1-[pyr]centroid separations of 3.154(2) Å and 3.153(2) Å. By contrast, the smaller Na+ ion is disordered over two sites about the crystallographic C2 axis, presumably because it cannot effectively bridge all four pyrrolides. This results in three shorter Na1-[pyr]centroid distances of 2.85(4), 3.04(2) and 3.08(4) Å and one long, non-bonding separation of 3.61(2) Å (Fig. 1c). The larger and more polarisable K+ is clearly a better match for the Pacman macrocyclic cleft than Na+. Based on the M1⋯B1 separations, the U ions form a standard bonding interaction with the BH4 anion.62,63 Reported terminal K⋯BH4 separations range from 2.947(3)64 to 3.091(4)65 Å with a mean value of 3.00 Å, while terminal Na⋯BH4 separations range from 2.600(6)66 to 2.841(2)67 Å with a mean value of 2.68 Å. The K1–B1 (3.036(11) Å) and Na1–B1 (2.747(2) Å) separations in 2-K and 2-Na lie within these ranges, close to the mean values. The elongated K1–B1 distance means that the BH4− ligand sits further back into the molecular cleft in 2-K and the U1–B1–U1′ angle in 2-K (168.2(4)°) is more acute than that in 2-Na (173.0(6)°).
The effect of the out-of-cleft distortion of the UIII centres is a marked lengthening of both the U⋯U and the U⋯(endo-BH4) separations. The U1⋯U1′ separation is 6.5881(3) Å in 2-K and 6.5265(7) Å in 2-Na compared to 5.9243(3) Å in 1-Na. U1–B1 is 3.312(1) Å in 2-K and 3.269(1) in 2-Na compared to 2.977(7) Å and 2.949(7) Å in 1-Na. The U–B distances in 2 are the longest observed for any uranium borohydride complex, with the next longest being complex 1-Na followed by 2.927(7) Å in [U(BH4)L′] (L′ = trans-calix[2]benzene[2]pyrrolide).59 This raises the question of whether there is a bond between the UIII ions and the endo BH4− group in 2-K and 2-Na or whether the BH4− group is held within the cleft by association with its M+ counter-ion. The observed 11B NMR shift of the endo BH4− group in 2-K (188 ppm) is significantly paramagnetically shifted from that of free KBH4 (−40 ppm) indicating that there is some electronic overlap between the UIII centres and the BH4− group in solution. Therefore, it is likely that in-cleft cation binding in 2-K and 2-Na contributes to the stabilisation of a very weak and long U(BH4)–U interaction.
Reactions of 1 and 2
Reactions to compare the small molecule activation chemistry of 1-Na and 2-K were carried out, noting both the high number of potential reducing equivalents in 1 and the weak binding of the central, and unsolvated MBH4 in 2.Complex 1-Na was dissolved in THF and 0.75 equivalents of S8 was added, immediately forming a red solution of a product we assign as [U2S3(LA)]n3 from elemental analysis, and analysis of the boron–sulfur containing by-products of the reaction, Scheme 2. The 1H NMR spectrum of a freshly made solution shows paramagnetically shifted resonances between +34 and −23 ppm that correspond to a symmetrical macrocycle environment; some H2 is also seen in solution. The 11B NMR spectrum contains two triplets in a 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio at −6.2 and −16.5 ppm, the latter of which can be assigned to Na2[(BH2)6S4], the caesium analogue of which has previously been made from the reaction between CsBH4, BH3 and H2S (eqn (1)).68 The initially-soluble reaction product precipitates from the reaction mixture over a 12 h period and remains insoluble in common polar aprotic solvents. This observation and the rarity with which S binds as a terminal multiply bonded ligand led us to assign a polymeric structure for 3 as drawn in Scheme 2.
1 ratio at −6.2 and −16.5 ppm, the latter of which can be assigned to Na2[(BH2)6S4], the caesium analogue of which has previously been made from the reaction between CsBH4, BH3 and H2S (eqn (1)).68 The initially-soluble reaction product precipitates from the reaction mixture over a 12 h period and remains insoluble in common polar aprotic solvents. This observation and the rarity with which S binds as a terminal multiply bonded ligand led us to assign a polymeric structure for 3 as drawn in Scheme 2.
| CsBH4 + 2THF:BH3 + 2H2S → ½Cs2[(BH2)6S4] + 4H2 | (1) |
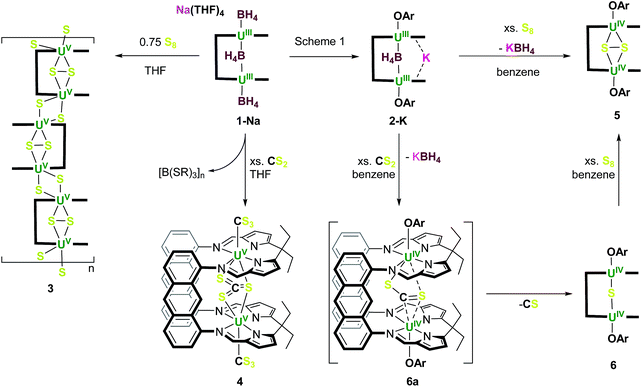 | ||
| Scheme 2 Contrasting reactions of [Na(THF)4][{U(BH4)}2(μ-BH4)(LA)(THF)2] (1-Na) and [{U(OAr)(THF)2}2(endo-μ-KBH4)(LA)] (2-K) and the synthesis of complexes 3–6 (OAr = OC6H2tBu3-2,4,6). | ||
A THF solution of 1-Na was treated with an excess (>9 equivalents) of CS2, upon which the reaction mixture immediately turned bright orange, and quantitative deposition of the product characterised as [{U(CS3)}2(μ-κ2:κ2-CS3)(LA)] 4 as an orange solid is observed after ca. 15 min. The 1H NMR spectrum of the reaction mixture before precipitation shows a single symmetrical paramagnetically shifted macrocycle environment with resonances between +25 and −44 ppm. The IR spectrum of solid 4 shows no absorptions in the region 2500–2000 cm−1 confirming that no borohydride ligands remain. The 11B NMR spectrum of the supernatant shows two sharp singlets at 0.29 and 0.5 ppm, attributed to boron-sulfide-containing by-products, and shows that the BH4 ligands have provided additional reducing capability to the UIII centres in 1. Related borohydride reduction reactions from simple group 1 salts are shown in eqn (2)–(5). Both resonances appear at a higher frequency than known reaction products of NaBH4 and BH3 with CS2, namely [CH2(BH2)5S4]− (−13.7/15.8 ppm)69 and [(BH2)4(SCH2S)2] (−17.0 ppm).70 The 11B NMR resonance at 0.5 ppm is attributed to the known anion [B(SCH2S)4]5− (eqn (4)) which is formed from the sub-stoichiometric reaction of NaBH4 with CS2. The corresponding CH2 group is observed as a quartet at 3.97 ppm in the 1H NMR spectrum.71 The second species in the 11B NMR appears closer to the polymeric species, formulated as {[B(SCH2S)2]−}n (0.0 ppm, eqn (5)) suggesting a similar formulation for the resonance at 0.29 ppm possibly with an intermediate charge (e.g. [B(SCH2S)3]3−).71
| NaBH4 + 2BH3 + 2CS2 → Na[(BH2)5S2(SCH2S)] + 3H2 | (2) |
| 2BH3 + CS2 → ½[(BH2)4(SCH2S)2] | (3) |
| 5NaBH4 + 4CS2 → Na5[B(SCH2S)4] + 2B2H6 | (4) |
| NaBH4 + 2CS2 → {Na[B(SCH2S)2]}n | (5) |
Small orange crystals of [{U(CS3)}2(μ-κ1:κ1:κ2-CS3)(LA)] (4) were obtained from the concentrated THF solution. X-ray crystallographic analysis of 4 shows the incorporation of the rare trithiocarbonate (CS3)2− motif in the endo and both of the exo uranium coordination sites from which charge balancing arguments assign the notably high formal oxidation state of UV/UV (Fig. 2). While the crystallographic data are poor and prevent a full discussion of structural parameters, the U⋯U separation is 5.85 Å (from an average of the three structures in the unit cell). This is the first case in which two uranium centres have been shown to provide a total of four reducing electrons (rather than just one each) in the rare formation of the (CS3)2− ligand, and the first time that more than one thiocarbonate ligand has been formed through reductive activation by a single molecule.
The reactivity of the more soluble complex 2-K provides an interesting comparison with that of 1-M. Reactions of 2-K were carried out with both S8 and CS2 in the anticipation of displacing the single, weakly bound endo-KBH4 molecule.
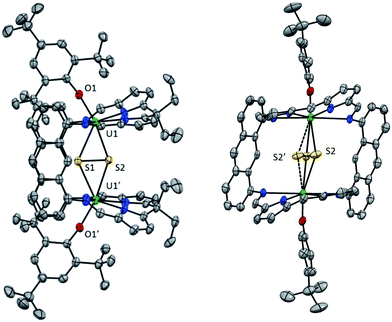
Addition of an excess of S8 to a slurry of 2-K in toluene resulted in the immediate formation of a pale orange solution and a pale yellow precipitate of KBH4. Addition of hexanes to the filtrate results in the deposition of orange crystals of the thermally stable product [{U(OAr)}2(μ-κ2:κ2-S2)(LA)] (5) in 41% yield (Scheme 2). In the solid-state structure (Fig. 3) the intermetallic cleft is occupied by a bridging persulfido ion, (S2)2− suggesting that both uranium centres have been oxidised to UIV. This is reinforced by the reduction of the U–L bond lengths (cf.2-K), in keeping with the values for known UIV complexes (see below). The 1H NMR spectrum of a solution of 5 displays paramagnetically shifted resonances corresponding to a single C2-symmetric macrocycle environment and two equivalent aryloxide ligands, as was observed in the 1H NMR spectrum of 2-K. However, in contrast to 2-K, the aryloxide rings appear to be rotating freely in solution as only three resonances in a 36![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 18
18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 ratio are seen. No resonances are seen in the 11B NMR spectrum confirming the loss of KBH4 from the cleft and its subsequent precipitation.
4 ratio are seen. No resonances are seen in the 11B NMR spectrum confirming the loss of KBH4 from the cleft and its subsequent precipitation.
Addition of an excess of CS2 to a suspension of 2-K in d8-toluene results in a slow colour change from dark green to orange-brown over the course of 10 min and formation of an orange precipitate (Scheme 2). The solution species were characterised on the basis of NMR spectroscopy as [{U(OAr)}2(μ-CS2)(LA)] (6a) and [{U(OAr)}2(μ-S)(LA)] (6). The resonances of the major species 6a indicate the presence of a single asymmetric macrocyclic compound in which the two compartments of the macrocycle are inequivalent. The two aryloxide ligands are also inequivalent; nine resonances are observed, five of intensity 9H corresponding to five of the six tBu groups (the resonance of the sixth group is assumed to be concealed by the solvent resonances) and four of intensity 1H corresponding to each meta proton. It is proposed from this that both aryloxide ligands are rigidly bound with the aryl rings coplanar with the anthracenyl groups of the macrocycle hinge. As no resonances are seen in the 11B NMR spectrum, it is probable that displacement of KBH4 by CS2 has occurred, and that a bent (CS2)2− unit binds asymmetrically between the two UIV centres, rendering the macrocyclic compartments and exo aryloxides inequivalent. Complex 6a is not stable in solution, and converts quantitatively to a new, C2-symmetric complex either on standing at room temperature for five days or heating in benzene for 2.5 h; the resulting complex was characterised as the orange sulfido-bridged compound [{U(OAr)}2(μ-S)(LA)] (6) (see below). No further reactivity of 6 with CS2 was observed, but boiling a benzene solution of 6 and excess S8 resulted in the formation of an orange solution which showed resonances in the 1H NMR spectrum corresponding to complex 5, Scheme 2.
By comparing the reactions of 1 and 2-K with excess CS2, it is seen that the exo-aryloxide groups direct the uranium centres to activate only one molecule of CS2 within the cleft, forming 6a initially and eventually the sulfido-bridged 6. However, without the aryloxide capping ligands, 1 is able to activate CS2 in both the exo and endo positions, with poor overall control, resulting in the formation of poorly soluble products.67
X-ray crystal structures of the endo-chalcogenido complexes 5 and 6
Orange single crystals of 5 suitable for X-ray structural analysis were obtained from a C6D6/hexane solution. In the solid-state, the UIV cations in 5 are seven coordinate, binding to the four N donors of the macrocycle, the exo-aryloxide ligand and both S atoms of the endo-bridging persulfido ion (Fig. 4). The solid-state structure of 5 confirms that, in contrast to 2, the aryloxide rings are indeed approximately coplanar with the anthracene hinges of the macrocycle with one ortho-tBu group on each ring sitting between the hinges. Also, the two THF molecules which were bound to the U centres in the equatorial sites in 2-K have dissociated during formation of 5. The U1–O1 bond length in 5 is 2.091(3) Å, which is reduced from 2.231(5) Å in 2-K and supports the oxidation of the UIII centres to UIV. The angle at the O atom of the aryloxides (U1–O1–Cipso = 169.0(3)°) is less acute than that observed in 2-K (154.0(5)°). The mean U1–N(pyrrolide) distance has contracted from 2.50 Å in 2-K to 2.41 Å in 5, though the difference in the mean U1–N(imine) distances is less marked (Table 2).| 5 | 6 | |
|---|---|---|
| U1⋯U1′ | 5.1571(5) | 5.1899(5) |
| Mean U–Nim | 2.63 | 2.59 |
| Mean U–Npyr | 2.41 | 2.42 |
| U–N4 plane | −0.07 | −0.10/−0.03 |
| U–O | 2.091(3) | 2.081(6)/2.099(6) |
| U1–S1 | 2.8229(8) | 2.608(2)/2.594(2) |
| U1–S2 | 2.707(3) | |
| S1–S2 | 2.118(3) | |
| O1–U1–S1 | 125.7(1) | 140.1(2)/143.2(2) |
| O1–U1–S2 | 166.6(1) | |
| U1–S1–U1′ | 131.98(7) | |
| U1–S2–U1′ | 135.0(1) | 172.0(1) |
| S1–S2–U1 | 70.40(8) | |
| S2–S1–U1 | 64.62(8) | |
| U1–(S2)cent–U1′ | 165.4 | |
| U1–O1–Cipso | 169.0(3) | 170.4(5)/167.8(6) |
The (S2)2− unit in 5 is symmetry defined to be equidistant from the two UIV centres but the U1–S1 bond length of 2.8229(8) Å is longer than the U1–S2 length of 2.707(3). S2 is disordered over two sites related by rotation about the C2 axis and the occupancy of each site was fixed at 0.5. U1, U1′, S1 and S2 are not coplanar but instead the {U2S2} unit forms a bent diamond with a dihedral angle of 165.4°. Bridging persulfido uranium complexes are rare, with the only two examples having been reported very recently, and both featuring a persulfido ion bridging symmetrically between two UIV centres in [{U(N{SiMe3}2)3}2(μ-κ2:κ2-S2)]27 and [{U((SiMe2NPh)3TACN)}2(μ-κ2:κ2-S2)].50
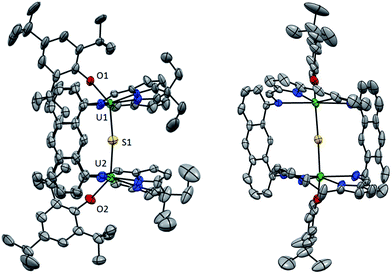
Orange block-shaped crystals of 6 suitable for X-ray crystallography were obtained by addition of hexanes to a toluene solution (Fig. 4). The coordination environment about the two UIV ions in 6 is distorted octahedral and the four N donors of the macrocycle occupy the equatorial plane with the exo aryloxide and endo bridging sulfido ligands axial. There is, however, a large deviation from idealised octahedral geometry; the angles between the trans axial ligands O1–U1–S1 and O2–U2–S2 are 143.2(2)° and 140.1(2)°, respectively. As with 5, the aryloxides are tilted back toward the hinges of the macrocycle to avoid unfavourable steric interactions between their ortho-tBu groups and the exo meso ethyl groups of the macrocycle. At 2.594(2) Å and 2.608(2) Å, the U–S bond lengths in 6 are reduced by ca. 0.16 Å compared to the mean U–S distance observed in the persulfido complex 5 (Table 2).
The geometry of the {U-(μ-S)-U} core in 6 is approaching linear (U1–S1–U2 is 172.0(1)°) and the U1⋯U2 separation is 5.1899(5) Å. Other mono-sulfido bridged complexes prepared to date include [{U(N{SiMe3}2)3}2(μ-S)]27 [{U(OAr)3}2(μ-S)] (Ar = 2,6-C6H3(tBu)2)65 and [{U((AdArO)3N)(DME)}2(μ-S)].66 In these compounds the U–S bond lengths range from 2.588(1) Å to 2.736(2) Å, the U⋯U separations vary from 5.176(3) Å to 5.4407(6) Å and the U–S–U angles range from 165.2(2)° to 180°. The structural parameters of the {U-(μ-S)-U} unit in 6 lie within these limits and so the rigid environment of the Pacman macrocycle does not appear to cause an excessive distortion.
We attribute the formation of complex 6 to the slow reductive cleavage of the bound CS2 molecule in 6a to form S2− and release CS. This is an unusual transformation since CS is not expected to be stable, and so not prone to eliminate, in contrast to reactions of CO2 with reducing metal complexes that often eliminate CO and form an oxo bridge.72,73 Despite this, CS formed from reductive disproportionation of CS2 has been trapped previously.43,74 To probe whether this transformation is accelerated by heating, a solution of 6a in C6D6 was boiled for 2.5 hours forming an orange solution and a brown precipitate. The subsequent 1H NMR spectrum displayed one major set of paramagnetically shifted resonances assignable to a single, symmetric Pacman product consistent with the transformation of 6a into 6. The 1H NMR spectrum of 6 exhibits just five aryloxide resonances in the ratio 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 18
18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 18
18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, as was seen for the similarly symmetric persulfido complex 5.
2, as was seen for the similarly symmetric persulfido complex 5.
Conclusions
The reactions of [Na(THF)4][{U(BH4)}2(μ-BH4)(LA)(THF)2] (1-Na) with two equivalents of MOAr (where M = K or Na and OAr = OC6H2tBu3-2,4,6), result in the exclusive substitution of the exo-BH4 for an aryloxide, yielding [{U(OAr)}2(endo-BH4M)(LA)(THF)2] (K = 2-K and Na = 2-Na). An unusual binding mode for MBH4 is seen in which the M+ counter-ion sits adjacent to the BH4 ligand in a cavity formed by the π-systems of four pyrrolide rings of the macrocycle. The U⋯U separation is increased by over 0.6 Å, presumably due to this additional endo-bound ion pair.The reaction of [Na(THF)4][{U(BH4)}2(μ-BH4)(LA)(THF)2] (1-Na) with excess S8 formed an insoluble paramagnetic species 3, with a molecular formula suggesting the formation of a bridging uranium(V) sulfido coordination polymer. In addition, treatment of 1-Na with CS2 results in the formation of [{U(CS3)}2(μ-η1:η1:η2-CS3)(LA)] (4) in which unusual trithiocarbonate (CS3)2− motifs are seen in both the endo and exo positions. To our knowledge, this is the first case in which two uranium(III) centres have been able to provide a total of four reducing electrons rather than just one each in the rare incorporation of the (CS3)2− ligand, and the first time that more than one thiocarbonate has been formed through reductive activation by a single molecule.
The larger cleft size and more loosely-bound endo-BH4 in 2 also provides a good site for the activation of S8 and CS2, affording the endo-(S2)2− [{U(OAr)}2(μ-η2:η2-S2)(LA)] (5) and endo-(S)2− [{U(OAr)}2(μ-S)(LA)] (6) complexes, respectively. It is clear that the addition of the aryloxide ligand in 2-K promotes the activation of the CS2 exclusively between the two UIII centres. In contrast, when the aryloxides are not present i.e. in 1, the BH4 groups are easily replaced and activation of CS2 occurs in both the exo and endo positions. Therefore, to control and localise the activation of CS2, the exo aryloxide ligands are essential.
The unusual reactivity of 2-K is attributed to the unique environment imposed by the Pacman macrocycle. It is concluded that the endo persulfido ion may be comfortably incorporated in 5 but further incorporation of sulfur is restricted. Similarly, the sulfido ion bridges the UIV centres effectively in 6 but in-cleft formation of the bulky thiocarbonate ion is disfavoured. Similarly to related UIV systems,49 sulfido 6 can be converted into persulfido 5 by the addition of elemental sulfur, suggesting the optimum cavity size between the two UIV centres that fits this polarisable anion has been found. These first small molecule activations within the di-uranium(III) Pacman cleft exemplify the flexibility of the anthracenyl-hinged macrocycle, with U⋯U separations ranging from 4.1927(3) Å to 6.5881(3) Å, and that the use of different endo ligands and bridging modes could lead to a wider application of these systems towards other less readily reducible molecules.
Acknowledgements
We thank EaStCHEM, the University of Edinburgh and the Engineering and Physical Sciences Research Council EPSRC, grants EP/H004823/1 and EP/M010554/1, and the European COST network CM1205. We thank Dr Markus Zegke for additional X-ray crystallographic analysis. We thank the NSF CCI Center for Enabling New Technologies through Catalysis (CENTC, CHE-1205189) and the EPSRC Centre for Doctoral Training in Critical Resource Catalysis (CRITICAT, Grant code EP/L016419/1) for funding the researcher exchange visit (JMG). PLA also thanks the Technische Universität München – Institute for Advanced Study, funded by the German Excellence Initiative.Notes and references
- H. S. La Pierre and K. Meyer, in Progress in Inorganic Chemistry, John Wiley & Sons, Inc., 2014, vol. 58, DOI:10.1002/9781118792797.ch05, pp. 303–416.
- B. M. Gardner and S. T. Liddle, Eur. J. Inorg. Chem., 2013, 3753–3770 CrossRef CAS.
- P. L. Arnold, Chem. Comm., 2011, 47, 9005–9010 RSC.
- S. M. Mansell, B. F. Perandones and P. L. Arnold, J. Organomet. Chem., 2010, 695, 2814–2821 CrossRef CAS.
- P. L. Arnold, A. Prescimone, J. H. Farnaby, S. M. Mansell, S. Parsons and N. Kaltsoyannis, Angew. Chem., Int. Ed., 2015, 54, 6735–6739 CrossRef CAS PubMed.
- P. Roussel and P. Scott, J. Am. Chem. Soc., 1998, 120, 1070–1071 CrossRef CAS.
- F. G. N. Cloke and P. B. Hitchcock, J. Am. Chem. Soc., 2002, 124, 9352–9353 CrossRef CAS PubMed.
- S. M. Mansell, N. Kaltsoyannis and P. L. Arnold, J. Am. Chem. Soc., 2011, 133, 9036–9051 CrossRef CAS PubMed.
- A. L. Odom, P. L. Arnold and C. C. Cummins, J. Am. Chem. Soc., 1998, 120, 5836–5837 CrossRef CAS.
- I. Korobkov, S. Gambarotta and G. P. A. Yap, Angew. Chem., Int. Ed., 2002, 41, 3433–3436 CrossRef CAS PubMed.
- C. Elschenbroich, Organometallics, Wiley-VCH, 2006 Search PubMed.
- J. Parry, E. Carmona, S. Coles and M. Hursthouse, J. Am. Chem. Soc., 1995, 117, 2649–2650 CrossRef CAS.
- W. J. Evans, S. A. Kozimor, G. W. Nyce and J. W. Ziller, J. Am. Chem. Soc., 2003, 125, 13831–13835 CrossRef CAS PubMed.
- I. Castro-Rodriguez and K. Meyer, J. Am. Chem. Soc., 2005, 127, 11242–11243 CrossRef CAS PubMed.
- O. T. Summerscales, F. G. N. Cloke, P. B. Hitchcock, J. C. Green and N. Hazari, Science, 2006, 311, 829–831 CrossRef CAS PubMed.
- A. S. P. Frey, F. G. N. Cloke, P. B. Hitchcock, I. J. Day, J. C. Green and G. Aitken, J. Am. Chem. Soc., 2008, 130, 13816–13817 CrossRef CAS PubMed.
- B. M. Gardner, J. C. Stewart, A. L. Davis, J. McMaster, W. Lewis, A. J. Blake and S. T. Liddle, Proc. Natl. Acad. Sci., 2012, 109, 9265–9270 CrossRef CAS PubMed.
- O. T. Summerscales, F. G. N. Cloke, P. B. Hitchcock, J. C. Green and N. Hazari, J. Am. Chem. Soc., 2006, 128, 9602–9603 CrossRef CAS PubMed.
- N. Tsoureas, O. T. Summerscales, F. G. N. Cloke and S. M. Roe, Organometallics, 2013, 32, 1353–1362 CrossRef CAS.
- J.-C. Berthet, J.-F. L. Maréchal, M. Nierlich, M. Lance, J. Vigner and M. Ephritikhine, J. Organomet. Chem., 1991, 408, 335–341 CrossRef CAS.
- I. Castro-Rodriguez, H. Nakai, L. N. Zakharov, A. L. Rheingold and K. Meyer, Science, 2004, 305, 1757–1759 CrossRef CAS PubMed.
- O. T. Summerscales, A. S. P. Frey, F. G. N. Cloke and P. B. Hitchcock, Chem. Commun., 2008, 198–200 RSC.
- O. P. Lam, S. C. Bart, H. Kameo, F. W. Heinemann and K. Meyer, Chem. Commun., 2010, 46, 3137–3139 RSC.
- A.-C. Schmidt, A. V. Nizovtsev, A. Scheurer, F. W. Heinemann and K. Meyer, Chem. Commun., 2012, 48, 8634–8636 RSC.
- V. Mougel, C. Camp, J. Pécaut, C. Copéret, L. Maron, C. E. Kefalidis and M. Mazzanti, Angew. Chem., Int. Ed., 2012, 51, 12280–12284 CrossRef CAS PubMed.
- A. R. Fox, S. C. Bart, K. Meyer and C. C. Cummins, Nature, 2008, 455, 341–349 CrossRef CAS PubMed.
- J. L. Brown, G. Wu and T. W. Hayton, Organometallics, 2013, 32, 1193–1198 CrossRef CAS.
- O. P. Lam, F. W. Heinemann and K. Meyer, Chem. Sci., 2011, 2, 1538–1547 RSC.
- J. L. Brown, S. Fortier, R. A. Lewis, G. Wu and T. W. Hayton, J. Am. Chem. Soc., 2012, 134, 15468–15475 CrossRef CAS PubMed.
- W. Ren, G. Zi, D.-C. Fang and M. D. Walter, J. Am. Chem. Soc., 2011, 133, 13183–13196 CrossRef CAS PubMed.
- L. P. Spencer, P. Yang, B. L. Scott, E. R. Batista and J. M. Boncella, Inorg. Chem., 2009, 48, 11615–11623 CrossRef CAS PubMed.
- O. P. Lam, S. M. Franke, F. W. Heinemann and K. Meyer, J. Am. Chem. Soc., 2012, 134, 16877–16881 CrossRef CAS PubMed.
- J. L. Brown, S. Fortier, G. Wu, N. Kaltsoyannis and T. W. Hayton, J. Am. Chem. Soc., 2013, 135, 5352–5355 CrossRef CAS PubMed.
- L. Wang, W. He and Z. Yu, Chem. Soc. Rev., 2013, 42, 599–621 RSC.
- M. Draganjac and T. B. Rauchfuss, Angew. Chem., Int. Ed. Engl., 1985, 24, 742–757 CrossRef.
- F. A. Cotton and G. Schmid, Inorg. Chem., 1997, 36, 2267–2278 CrossRef CAS PubMed.
- A. R. Johnson, W. M. Davis, C. C. Cummins, S. Serron, S. P. Nolan, D. G. Musaev and K. Morokuma, J. Am. Chem. Soc., 1998, 120, 2071–2085 CrossRef CAS.
- J. S. Figueroa and C. C. Cummins, J. Am. Chem. Soc., 2003, 125, 4020–4021 CrossRef CAS PubMed.
- A. Kayal, J. Kuncheria and S. C. Lee, Chem. Commun., 2001, 2482–2483 RSC.
- M. C. Kuchta and G. Parkin, J. Chem. Soc. Chem. Commun., 1994, 1351 RSC.
- Q. Zhang, G. Armatas and M. G. Kanatzidis, Inorg. Chem., 2009, 48, 8665–8667 CrossRef CAS PubMed.
- W. A. Howard, T. M. Trnka and G. Parkin, Organometallics, 1995, 14, 4037–4039 CrossRef CAS.
- B. Li, X. Tan, S. Xu, H. Song and B. Wang, J. Organomet. Chem., 2008, 693, 667–674 CrossRef CAS.
- D. J. Berg, C. J. Burns, R. A. Andersen and A. Zalkin, Organometallics, 1989, 8, 1865–1870 CrossRef CAS.
- A. Kornienko, J. H. Melman, G. Hall, T. J. Emge and J. G. Brennan, Inorg. Chem., 2002, 41, 121–126 CrossRef CAS PubMed.
- D. E. Smiles, G. Wu, P. Hrobárik and T. W. Hayton, J. Am. Chem. Soc., 2016, 138, 814–825 CrossRef CAS PubMed.
- M. Roger, L. Belkhiri, P. Thuéry, T. Arliguie, M. Fourmigué, A. Boucekkine and M. Ephritikhine, Organometallics, 2005, 24, 4940–4952 CrossRef CAS.
- M. J. Manos and M. G. Kanatzidis, J. Am. Chem. Soc., 2002, 134, 16441–16446 CrossRef PubMed.
- Q. Wu, B. V. Yakshinskiy, T. Gouder and T. E. Madey, Catal. Today, 2003, 85, 291–301 CrossRef CAS.
- C. Camp, M. A. Antunes, G. García, I. Ciofini, I. C. Santos, J. Pécaut, M. Almeida, J. Marçalo and M. Mazzanti, Chem. Sci., 2014, 5, 841–846 RSC.
- S. M. Franke, F. W. Heinemann and K. Meyer, Chem. Sci., 2014, 5, 942–950 RSC.
- D. E. Smiles, G. Wu and T. W. Hayton, Inorg. Chem., 2014, 53, 12683–12685 CrossRef CAS PubMed.
- C. Camp, O. Cooper, J. Andrez, J. Pecaut and M. Mazzanti, Dalton Trans., 2015, 44, 2650–2656 RSC.
- P. L. Arnold, J. H. Farnaby, R. C. White, N. Kaltsoyannis, M. G. Gardiner and J. B. Love, Chem. Sci., 2014, 5, 756–765 RSC.
- E. Askarizadeh, A. M. J. Devoille, D. M. Boghaei, A. M. Z. Slawin and J. B. Love, Inorg. Chem., 2009, 48, 7491–7500 CrossRef CAS PubMed.
- G. Givaja, A. J. Blake, C. Wilson, M. Schröder and J. B. Love, Chem. Commun., 2003, 2508–2509 RSC.
- T. Cantat, B. L. Scott and J. L. Kiplinger, Chem. Commun., 2010, 46, 919–921 RSC.
- P. L. Arnold, C. J. Stevens, J. H. Farnaby, M. G. Gardiner, G. S. Nichol and J. B. Love, J. Am. Chem. Soc., 2014, 136, 10218–10221 CrossRef CAS PubMed.
- C. D. Carmichael, N. A. Jones and P. L. Arnold, Inorg. Chem., 2008, 47, 8577–8579 CrossRef CAS PubMed.
- I. Castro-Rodriguez, H. Nakai, P. Gantzel, L. N. Zakharov, A. L. Rheingold and K. K. Meyer, J. Am. Chem. Soc., 2003, 125, 15734–15735 CrossRef CAS PubMed.
- S. M. Mansell, J. H. Farnaby, A. I. Germeroth and P. L. Arnold, Organometallics, 2013, 32, 4214–4222 CrossRef CAS.
- T. Arliguie, L. Belkhiri, S.-E. Bouaoud, P. Thuéry, C. Villiers, A. Boucekkine and M. Ephritikhine, Inorg. Chem., 2009, 48, 221–230 CrossRef CAS PubMed.
- M. Mancini, P. Bougeard, R. C. Burns, M. Mlekuz, B. G. Sayer, J. I. A. Thompson and M. J. McGlinchey, Inorg. Chem., 1984, 23, 1072–1078 CrossRef CAS.
- P. J. Fagan, J. M. Manriquez, E. A. aatta, A. M. Seyam and T. J. Marks, J. Am. Chem. Soc., 1981, 103, 6650–6667 CrossRef CAS.
- W. J. Evans, K. A. Miller, S. A. Kozimor, J. W. Ziller, A. G. DiPasquale and A. L. Rheingold, Organometallics, 2007, 26, 3568–3576 CrossRef CAS.
- J. G. Brennan, R. A. Andersen and A. Zalkin, Inorg. Chem., 1986, 25, 1761–1765 CrossRef CAS.
- O. P. Lam, L. Castro, B. Kosog, F. W. Heinemann, L. Maron and K. Meyer, Inorg. Chem., 2012, 51, 781–783 CrossRef CAS PubMed.
- H. Binder, H. Loos, K. Dermentzis, H. Borrmann and A. Simon, Chem. Ber., 1991, 124, 427–432 CrossRef CAS.
- K. Wolfer, H. D. Hausen and H. Binder, Z. Naturforsch., B: Anorg. Chem., Org. Chem., 1985, 40, 235–239 Search PubMed.
- H. Binder, K. Diamantikos, H. D. Dermentzis and Z. Hausen, Z. Naturforsch., B: Anorg. Chem., Org. Chem., 1982, 37, 1548–1552 Search PubMed.
- W. Diamantikos, H. Heinzelmann, E. Rath and H. Binder, Z. Anorg. Allg. Chem., 1984, 517, 111–117 CrossRef CAS.
- A. J. Boutland, I. Pernik, A. Stasch and C. Jones, Chem. Eur. J., 2015, 21, 15749–15758 CrossRef CAS PubMed.
- A. F. R. Kilpatrick, J. C. Green and F. G. N. Cloke, Organometallics, 2015, 34, 4816–4829 CrossRef CAS PubMed.
- J. Campora, E. Gutierrez, A. Monge, P. Palma, M. L. Poveda, C. Ruiz and E. Carmona, Organometallics, 1994, 13, 1728–1745 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Full synthetic and structural characterisation data. CCDC 1480072–1480076. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c7sc00382j |
| This journal is © The Royal Society of Chemistry 2017 |