Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Single crystal structures and theoretical calculations of uranium endohedral metallofullerenes (U@C2n, 2n = 74, 82) show cage isomer dependent oxidation states for U†
Wenting
Cai
a,
Roser
Morales-Martínez
b,
Xingxing
Zhang
c,
Daniel
Najera
a,
Elkin L.
Romero
a,
Alejandro
Metta-Magaña
a,
Antonio
Rodríguez-Fortea
 b,
Skye
Fortier
b,
Skye
Fortier
 a,
Ning
Chen
a,
Ning
Chen
 *c,
Josep M.
Poblet
*c,
Josep M.
Poblet
 *b and
Luis
Echegoyen
*b and
Luis
Echegoyen
 *a
*a
aDepartment of Chemistry, University of Texas at El Paso, 500 W University Avenue, El Paso, Texas 79968, USA. E-mail: echegoyen@utep.edu
bDepartament de Química Física i Inorgànica, Universitat Rovira i Virgili, C/Marcel⋅lí Domingo 1, 43007 Tarragona, Spain
cLaboratory of Advanced Optoelectronic Materials, College of Chemistry, Chemical Engineering and Materials Science, Soochow University, Suzhou, Jiangsu 215123, P. R. China
First published on 22nd May 2017
Abstract
Charge transfer is a general phenomenon observed for all endohedral mono-metallofullerenes. Since the detection of the first endohedral metallofullerene (EMF), La@C82, in 1991, it has always been observed that the oxidation state of a given encapsulated metal is always the same, regardless of the cage size. No crystallographic data exist for any early actinide endohedrals and little is known about the oxidation states for the few compounds that have been reported. Here we report the X-ray structures of three uranium metallofullerenes, U@D3h-C74, U@C2(5)-C82 and U@C2v(9)-C82, and provide theoretical evidence for cage isomer dependent charge transfer states for U. Results from DFT calculations show that U@D3h-C74 and U@C2(5)-C82 have tetravalent electronic configurations corresponding to U4+@D3h-C744− and U4+@C2(5)-C824−. Surprisingly, the isomeric U@C2v(9)-C82 has a trivalent electronic configuration corresponding to U3+@C2v(9)-C823−. These are the first X-ray crystallographic structures of uranium EMFs and this is first observation of metal oxidation state dependence on carbon cage isomerism for mono-EMFs.
Introduction
Endohedral metallofullerenes (EMFs) have attracted considerable attention due to their unique electronic properties, which arise mainly as a consequence of the intramolecular metal-to-cage charge transfer.1–4 Of these systems, mono-EMFs are particularly interesting because they represent the simplest model to probe the specific interactions between the inner metal and the outer cage. To date, many rare-earth metals and neighboring elements (groups II, III, IV) have been reported to form air-stable mono-EMFs that exhibit +2 or +3 oxidation states inside the fullerene cages.5–10 Notably, the charge of the metal ion determines the isomeric forms of the host mono-EMF cages. For instance, for C82-EMFs, isomers C2(5)-C82, Cs(6)-C82, C3v(7)-C82 and C2v(9)-C82 containing divalent rare-earth metals have been isolated and structurally characterized,11–14 while C2v(9)-C82 and Cs(6)-C82 are known to contain encapsulated trivalent lanthanides.15–17 The number of electrons that are transferred from the metal to the cage has a significant effect on the properties of mono-EMFs. For example, the divalent metallofullerenes M@C74 (M = Ba,18 Ca,19 Sr,20 Sm,21 Eu22 and Yb23,24) exhibit improved solubility relative to La@C74, which is almost insoluble in most common organic solvents.Actinide EMFs represent an exciting and underexplored area of EMF chemistry. In comparison to the rare-earth elements, the early actinides (Th–Pu) are highly redox active metals possessing several accessible oxidation states. For instance, uranium oxidation states typically range from U(III) to U(VI) while U(II) has been recently reported.25–27 Thus, in principle, uranium offers five accessible oxidation states, in contrast with the rather limited Ln(II)/Ln(III) redox states of the rare-earth series. Additionally, the 5f-orbitals of the actinides are chemically accessible,25,28 contrasting the core-like 4f-orbitals of the lanthanides, suggesting potentially new EMF electronic structures and cage isomer preferences for the actinides.
Understanding the structures and electronic properties of U-EMFs is interesting and potentially significant. Perhaps due to the radioactivity of the actinide elements, the investigation of U-EMFs has generally been confined to theoretical model calculations and a few spectroscopic investigations. In 1992, Smalley and co-workers29 observed the formation of U@Td-C28. X-ray photoelectron spectroscopy (XPS) and calculations suggested a four electron uranium-to-cage charge transfer, U4+@C284−. In a separate study, the electronic structure of U@C82 was assigned as U3+@C823− according to similarities in the UV-Vis-NIR absorptions between U@C82 and other M@C82 containing trivalent lanthanide ions.30 Furthermore, the X-ray absorption near edge structure (XANES) spectrum of U@C82 shows some similarity to that for UCl3, supporting a U(III) charge assignment.31,32 The corresponding theoretical results for the molecular structure and associated electronic properties of U@C82 indicated that the most thermodynamically stable isomer, U@C2v(9)-C82, is a trivalent EMF with an electronic configuration of U3+@C823−, while C2(5)-C82 and C3v(8)-C82 would favor the encapsulation of U+ and U4+, respectively.33
Herein we report the isolation and first single crystal X-ray crystallographic characterization of three mono-EMFs containing a single encapsulated uranium atom, namely, U@D3h-C74, U@C2(5)-C82 and U@C2v(9)-C82. Surprisingly, we find that the oxidation state of uranium in U@C74 and the two U@C82 isomers depends on the cage that encapsulates the U atom: U4+@D3h-C744−, U4+@C2(5)-C824− and U3+@C2v(9)-C823−.
Experimental
General instruments
HPLC separations were accomplished using a Varian Prostar instrument. Laser desorption/ionization time-of-flight (LDI-TOF) mass spectrometry was conducted on a Bruker Microflex LRF mass spectrometer. Vis-NIR spectra were obtained with a Cary 5000 UV-Vis-NIR spectrophotometer in toluene. Cyclic voltammograms (CV) and square wave voltammograms (SWV) were measured in o-dichlorobenzene with 0.05 M n-Bu4NPF6 as the supporting electrolyte using a CH Instrument Potentiostat. A 1 mm diameter glassy carbon disk was used as the working electrode, with a platinum wire and silver wire as the counter reference electrodes, respectively. All potentials were reported relative to the Fc/Fc+ couple.Synthesis and isolation of U@C74 and U@C82 (I, II)
Soot containing uranium metallofullerenes was synthesized using a direct-current arc discharge method. The raw soot was refluxed in CS2 for 12 h. After removal of CS2, the residue was dissolved in toluene and the solution was subjected to a multi-stage HPLC separation process. Further details are described in the ESI.†Single-crystal X-ray diffraction
Crystalline blocks of U@C2n (2n = 74, 82) were obtained by layering a benzene solution of NiII(OEP) over a nearly saturated solution of the endohedral in CS2 in a glass tube. Over a 20 day period, the two solutions diffused into each other and black crystals formed. XRD measurements were performed at 150 K on a Bruker P4 machine equipped with a graphite monochromator. The multi-scan method was used for absorption corrections. The structures were solved by a direct method and were refined with SHELXL-2013.34Crystal data for U@D3h-C74·NiII(OEP)·2(toluene): C122H56N4NiU, Mw = 1874.28, monoclinic, space group C2/c, a = 25.102(3) Å, b = 14.9208(19) Å, c = 38.636(5) Å, β = 94.044(2)°, V = 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 435(3) Å3, Z = 8, T = 150 K, ρcalcd = 1.725 Mg m−3, μ(MoKα) = 2.569 mm−1, 72
435(3) Å3, Z = 8, T = 150 K, ρcalcd = 1.725 Mg m−3, μ(MoKα) = 2.569 mm−1, 72![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 450 reflections measured, 18
450 reflections measured, 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 022 unique (Rint = 0.0981) used in all calculations. The final wR2 was 0.3355 (all data) and R1 (11
022 unique (Rint = 0.0981) used in all calculations. The final wR2 was 0.3355 (all data) and R1 (11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 937 with I > 2\s(I)) = 0.1247. The relatively high R1 and wR2 values are due to the severe disorder in the cage, the metal atom and the intercalated solvent molecules. CCDC 1508508 contains the crystallographic data.†
937 with I > 2\s(I)) = 0.1247. The relatively high R1 and wR2 values are due to the severe disorder in the cage, the metal atom and the intercalated solvent molecules. CCDC 1508508 contains the crystallographic data.†
Crystal data for U@C2(5)-C82·NiII(OEP)·2(toluene): C130H56N4NiU, Mw = 1970.37, monoclinic, space group C2/m, a = 25.0308(10) Å, b = 15.3288(6) Å, c = 19.9410(8) Å, V = 7632.7(5) Å3, Z = 4, T = 150 K, ρcalcd = 1.715 Mg m−3, μ(MoKα) = 2.434 mm−1, 42![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 398 reflections measured, 9067 unique (Rint = 0.0328) used in all calculations. The final wR2 was 0.2290 (all data) and R1 (7016 with I > 2\s(I)) = 0.0779. CCDC 1508509 contains the crystallographic data.†
398 reflections measured, 9067 unique (Rint = 0.0328) used in all calculations. The final wR2 was 0.2290 (all data) and R1 (7016 with I > 2\s(I)) = 0.0779. CCDC 1508509 contains the crystallographic data.†
Crystal data for U@C2v(9)-C82·NiII(OEP)·1.5(toluene)·CS2: C128H53N4NiS2U, Mw = 2007.56, monoclinic, space group P21/c, a = 17.7846(5) Å, b = 17.2807(5) Å, c = 26.6533(7) Å, V = 7817.6(4) Å3, Z = 4, T = 150 K, ρcalcd = 1.706 Mg m−3, μ(MoKα) = 2.431 mm−1, 96![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 891 reflections measured, 14
891 reflections measured, 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 336 unique (Rint = 0.0840) used in all calculations. The final wR2 was 0.2849 (all data) and R1 (9344 with I > 2\s(I)) = 0.0987. CCDC 1522558 contains the crystallographic data.†
336 unique (Rint = 0.0840) used in all calculations. The final wR2 was 0.2849 (all data) and R1 (9344 with I > 2\s(I)) = 0.0987. CCDC 1522558 contains the crystallographic data.†
Computational details
All calculations were carried out using density functional theory (DFT) with the ADF 2013 package.35 The exchange-correlation functionals of Becke and Perdew (BP86) were used.36,37 Slater triple-zeta polarization (TZP) basis sets were used to describe the valence electrons of Ba, La, Hf, U and C. Frozen cores were described by means of single Slater functions, consisting of the 1s shell for C, the 1s to 5d shells for U, 1s to 4d shells for La and Hf, and 1s to 4p shells for Ba. Scalar and spin–orbit relativistic corrections were included by means of the ZORA formalism. Open-shell calculations were performed at an unrestricted level. For the spin–orbit calculations, we carried out single-point energy calculations at the geometry optimized at BP86/TZP + scalar relativistic level, with a basis set of TZ2P quality with no core for U. Electrochemistry calculations were performed at the same level of theory BP86/TZP, with dichlorobenzene as solvent. A data set collection of computational results is available in the ioChem-BD repository and can be accessed viahttp://doi.org/10.19061/iochem-bd-2-14.38Results and discussion
Characterization of U@C2n (2n = 74, 82)
Soot containing uranium endohedral fullerenes was obtained from graphite rods filled with uranium oxide and graphite powder (weight ratio of U/C = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
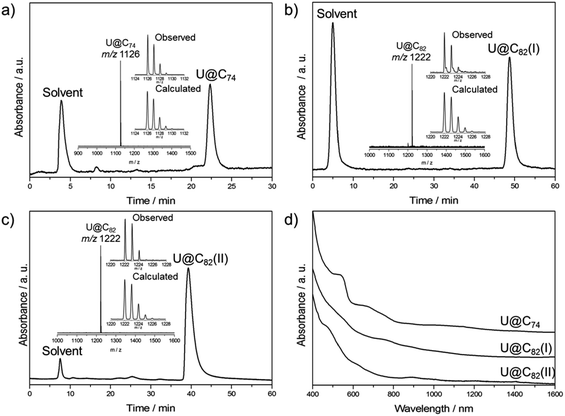
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) in a conventional Krätschmer-Huffman arc-discharge reactor under a 200 Torr helium atmosphere.39 The as-produced fullerene soot was Soxhlet-extracted with carbon disulfide (CS2) for 24 h. Multi-stage HPLC separations gave pure isomers of U@C74 and U@C82 (I, II) (see Fig. S1–S4†). Fig. 1 shows the chromatograms and mass spectra of the purified samples. The Vis-NIR spectra of the three compounds are shown in Fig. 1d. Their spectral onsets are located at around 1500 nm and 1300 nm, respectively, thus reflecting rather small HOMO–LUMO gaps. U@C74 displays absorption bands at 1399, 1018, 878, 743, 672 and 538 nm, U@C82 (I) exhibits distinct absorptions at 885, 760 and 535 nm, and U@C82 (II) exhibits distinct absorptions at 1405, 1204, 1003, 893, 620 and 469 nm.
4) in a conventional Krätschmer-Huffman arc-discharge reactor under a 200 Torr helium atmosphere.39 The as-produced fullerene soot was Soxhlet-extracted with carbon disulfide (CS2) for 24 h. Multi-stage HPLC separations gave pure isomers of U@C74 and U@C82 (I, II) (see Fig. S1–S4†). Fig. 1 shows the chromatograms and mass spectra of the purified samples. The Vis-NIR spectra of the three compounds are shown in Fig. 1d. Their spectral onsets are located at around 1500 nm and 1300 nm, respectively, thus reflecting rather small HOMO–LUMO gaps. U@C74 displays absorption bands at 1399, 1018, 878, 743, 672 and 538 nm, U@C82 (I) exhibits distinct absorptions at 885, 760 and 535 nm, and U@C82 (II) exhibits distinct absorptions at 1405, 1204, 1003, 893, 620 and 469 nm.
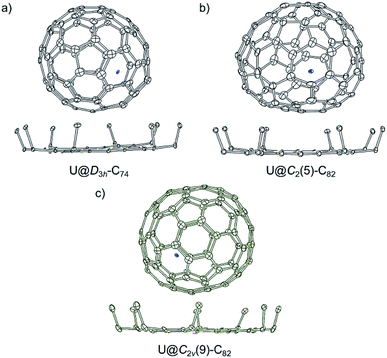
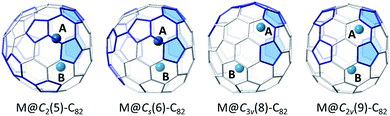
Single-crystal X-ray structure of U@D3h-C74, U@C2(5)-C82 and U@C2v(9)-C82
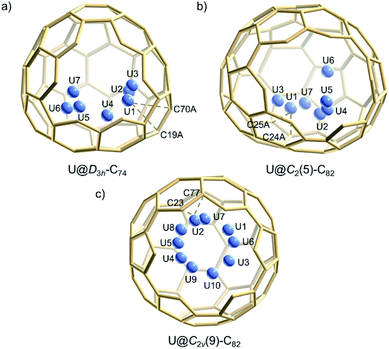
Single crystals of U@C74 and U@C82 (I, II) were obtained by layering a benzene solution of NiII(OEP) (OEP = 2,3,7,8,12,13,17,18-octaethylporphyrin dianion) over a nearly saturated solution of the EMFs in CS2 in a glass tube. The molecular structures of U@C74 and U@C82 (I, II) were unambiguously determined using single crystal X-ray diffraction. Analysis of cage connectivity reveals the cage isomers D3h-C74, C2(5)-C82 and C2v(9)-C82 (nomenclature in accordance with the spiro algorithm).40 Notably, the above-mentioned Vis-NIR spectra of the first two compounds are substantially different from those of crystallographically characterized endohedral fullerenes possessing the same cage, such as Sm@D3h-C74,21 Ba@D3h-C74,18 and Sm@C2(5)-C82,11 whereas the absorption features of U@C2v(9)-C82 are similar to those of La@C2v(9)-C82. These results suggest that U@D3h-C74 and U@C2(5)-C82 have formal charge transfers different (likely larger) from +2 while U@C2v(9)-C82 likely involves a +3 charge transfer, as observed for La3+.16Fig. 2 shows the single crystal X-ray structure of U@D3h-C74, U@C2(5)-C82 and U@C2v(9)-C82 relative to NiII(OEP). The porphyrin moiety faces a flat region for the three systems D3h-C74, C2(5)-C82 and C2v(9)-C82, with the shortest nickel-to-cage carbon distance ranging from 2.787 to 2.929 Å, indicative of strong π–π stacking interactions. The U ions in all three compounds show some degree of disorder, reflecting restricted motion of the U atom within the cage. Fig. 3 shows the positions of the disordered U atoms. For all three compounds, the U ions are highly localized near one part of the molecule. For U@D3h-C74, seven U ion positions have been assigned, with fractional occupancies ranging from 0.01 to 0.46. Similarly, there are also seven U ion positions with fractional occupancies that range from 0.02 to 0.26 for U@C2(5)-C82. Ten different positions, which are arranged in a circle, are found for the U atom in U@C2v(9)-C82. These disorder features are quite similar to those observed for mono-metallofullerenes encapsulating Group II, Group III and lanthanide metals. The predominant U position with fractional occupancies of 0.46 in U@D3h-C74 is situated over the central [6,6]-bond of one of the three symmetry-equivalent pyracylene units (Fig. S5a†). Somewhat surprisingly, similar arrangements have been reported previously for two other divalent mono metallofullerenes: Sm2+@D3h-C742− and Ba2+@D3h-C742−.18,21 The major U position for U@C2(5)-C82 (with 0.26 fractional occupancy) is situated over a [5,6]-bond and is identical to the mirror-related major Sm site for Sm2+@C2(5)-C822− (Fig. S5b†).11 The U ion in U@C2v(9)-C82 (with 0.22 fractional occupancy) is situated over the center of a hexagon that radiates from the C2 axis, which differs from that of the Sm2+ atom in Sm@C2v(9)-C82 but is very close to the La3+ site in La3+@C2v(9)-C822− (Fig. S5c†). Considering the spectroscopic similarities between U@C2v(9)-C82 and La@C2v(9)-C82,16 it is reasonable to speculate that the oxidation state of the U atom in C2v(9)-C82 is +3. However, the most populated sites of the U ion in D3h-C74 and C2(5)-C82 are similar to those observed for divalent metallofullerenes to some extent, but their corresponding Vis-NIR spectra are totally different. Such inconsistencies require reconsideration of the electronic structures of U@D3h-C74 and U@C2(5)-C82, which seem to be somewhat unique.
In-depth analysis of the electronic structures of U-EMFs
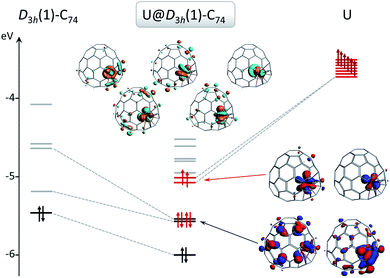
Without exception, the oxidation state of any given encapsulated metal atom (Group-2, Group-3 or lanthanides) in mono-EMFs is always the same regardless of the size or geometry of the corresponding fullerene cages. Based on the electronic absorption spectra and solid-state structures of the three uranium mono-EMFs, it seems that the oxidation state of uranium can depend on the cage isomer. In order to gain insight about the uranium charge oxidation states for the three uranium mono-EMFs we performed DFT calculations at the BP86/TZP level, taking into account relativistic effects (scalar relativistic as well as spin–orbit coupling, see ESI for more details†), which are of major importance for actinides. Below, we show for the first time that the cage isomer plays a critical role in determining the corresponding ground-state electronic configuration of U-EMFs.On the basis of the ionic model,41–43 different possible situations could exist for the mono-EMFs, the most likely ones being: U2+@C2n2−, U3+@C2n3− or U4+@C2n4− (Fig. 4). We have not considered explicitly oxidation states for U larger than four because the amount of charge transferred in medium-size mono-EMF has never been observed to exceed three. We have computed U@D3h-C74 in triplet and quintet states, and the triplet is 8.3 kcal mol−1 lower in energy than the quintet. The optimized structures for both multiplicities are essentially the same, with the uranium located in one of the crystallographically determined sites exhibiting a high occupancy value. To probe the electron transfer properties we analyzed the molecular orbital diagram of U@D3h-C74 in the triplet state (Fig. 5). Although calculations were carried out at an unrestricted level in Fig. 5, we preferred not to differentiate between alpha and beta orbitals, and a schematic representation is presented for simplicity. The four highest-energy electrons of the uranium are transferred to those unoccupied orbitals of the fullerene cage with lowest energies. Consequently, only two electrons remain in the uranium f-orbitals, a fact that is confirmed by the Mulliken spin density of the uranium atom, which is close to 2. Therefore, the electronic structure can be represented within the ionic model as U4+@D3h-C744−. For the quintet state, however, we found a three-electron transfer with one unpaired electron on the cage ferromagnetically coupled with the three 5f uranium electrons.
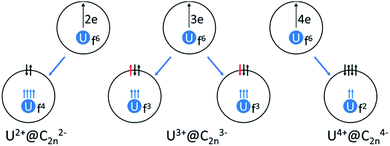 | ||
| Fig. 4 Schematic representation of the electronic structure of U2+@C2n2− (quintet state), U3+@C2n3− (two different spin multiplicities: quintet or triplet) and U4+@C2n4− (triplet state). | ||
For the C82 cage, out of 39![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 718 isomers, there are only nine that satisfy the isolated pentagon rule (IPR). In general, it is rather useful to analyze the neutral and anionic species to estimate the ability of the cage to encapsulate metals in different oxidation states. Indeed, Liu et al.33 performed an exhaustive study of the ionic forms of C82 cages concluding that (1) IPR isomers are, in general, much more stable than the non-IPR forms with a pentalene (i.e. one adjacent pentagon pair, APP1) and (2) C2(5)-C82 and C3v(8)-C82 are the best cages for the encapsulation of monovalent and tetravalent cations, respectively, whereas C2v(9)-C82 is the optimal cage to encapsulate M2+, M3+, M5+and M6+ species. It is worth mentioning that C2(5)-C82 was not found as an optimal cage to encapsulate a tetravalent ion but rather a monovalent species.
718 isomers, there are only nine that satisfy the isolated pentagon rule (IPR). In general, it is rather useful to analyze the neutral and anionic species to estimate the ability of the cage to encapsulate metals in different oxidation states. Indeed, Liu et al.33 performed an exhaustive study of the ionic forms of C82 cages concluding that (1) IPR isomers are, in general, much more stable than the non-IPR forms with a pentalene (i.e. one adjacent pentagon pair, APP1) and (2) C2(5)-C82 and C3v(8)-C82 are the best cages for the encapsulation of monovalent and tetravalent cations, respectively, whereas C2v(9)-C82 is the optimal cage to encapsulate M2+, M3+, M5+and M6+ species. It is worth mentioning that C2(5)-C82 was not found as an optimal cage to encapsulate a tetravalent ion but rather a monovalent species.
Based on the known oxidation states of uranium, U+ encapsulated inside a fullerene cage is highly unlikely. To determine the likely oxidation states for the U@C82 endohedrals we performed a series of calculations for cages #5, #6, #8 and #9 using U as the encapsulated atom, and also with Ba, La and Hf, which are useful models for two, three and four electron transfers between the captured atom and the carbon cage. These four C82 isomers are related by Stone–Wales (SW) transformations (see Fig. S6†). One structural pattern that is conserved in these four isomers is the chain of three pyracylene units (four pentagons) depicted at the right of cage #9 in Fig. 6. Taking this pattern as the reference, we oriented the cages so that the pyracylene units are fixed in the same position. We have then considered metal ions encapsulated in these four cages at two different positions A (at the top) and B (at the bottom), following the work of Liu et al.33 We have verified that other starting positions collapse to one of these two sites A or B.
As shown in Table 1 the relative stabilities of dianionic, trianionic, and tetraanionic cages match rather well with those of endohedral models containing Ba, La and Hf when the cation is located in site B. Remarkably, cage #5 seems to have the dual ability of stabilizing divalent cations in the bottom hemisphere (site B), or tetravalent cations in the top hemisphere (site A). For La3+, there is no preferential region inside this cage (see Table 1).
| Isomer | C822− | C823− | C824− | Siteb | Ba@C82 (singlet) | La@C82 (singlet) | Hf@C82 (singlet) | U@C82 (triplet) | U@C82 (quintet) |
|---|---|---|---|---|---|---|---|---|---|
| a Energies are in kcal mol−1. b Computed positions for the metal ion (see Fig. 7). c Charge transfer for U@C82 endohedral fullerenes is 3+ except for U@C2(5)-C82 site A and for U@C3v(8)-C82 site B (triplet) for which it is 4+ (see text for more details). | |||||||||
| C 2(5)-C82 | 3.9 | 11.1 | 22.3 | B | 5.0 | 13.1 | 17.6 | 15.3 | 14.7 |
| A | — | 14.7 | 5.0 | 1.9c | 11.0 | ||||
| C s(6)-C82 | 2.7 | 3.5 | 8.4 | B | 2.4 | 4.9 | 6.0 | 4.7 | 4.8 |
| A | — | 17.9 | 12.8 | 8.0 | 12.5 | ||||
| C 3v(8)-C82 | 8.8 | 2.9 | 0.0 | B | 10.8 | 4.8 | 0.0 | 2.2c | 5.0 |
| A | 31.8 | 31.5 | 30.2 | 25.3 | 20.9 | ||||
| C 2v(9)-C82 | 0.0 | 0.0 | 3.0 | B | 0.0 | 0.0 | 0.0 | 0.0 | 0.6 |
| A | 21.4 | 29.9 | — | 28.3 | 25.1 | ||||
We proceeded to study in detail the electronic structure of U@C2(5)-C82, U@Cs(6)-C82, U@C3v(8)-C82 and U@C2v(9)-C82 for triplet and quintet states (Fig. 4). The energy difference between these two states is in general small, with the triplet state favored over the quintet (see Table 1). U@C2v(9)-C82 B and U@C2(5)-C82 A are the lowest-energy isomers in good agreement with experiments, and the uranium sites predicted by the calculations correspond to the positions with major occupancy factors derived from the crystallographic structures (see Fig. S7†). We have checked that the relative energies computed so far are essentially kept (within 1.5 kcal mol−1) once the spin–orbit corrections are introduced in the calculations (see Table S1†). Relative energies for U@C82 in the triplet state with the guest ion located in site B correlate rather well with energies computed for the trianionic cages. For U@C2(5)-C82 we can observe a similar behavior to that found for Hf@C2(5)-C82, resulting in position A being more favored than B. These results clearly suggest that the oxidation state of uranium is U4+ for U@C2(5)-C82 and likely U3+ or U4+ for the other three cages.
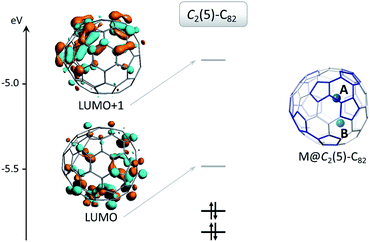
To understand the electronic structure of U@C2(5)-C82 and U@C2v(9)-C82, we analyzed the molecular orbital diagram in the same way as for U@D3h-C74. For U@C2(5)-C82 in the triplet state (see Fig. S8†), there is a formal transfer of four electrons from uranium to the fullerene cage with a spin density close to 2 for the uranium atom as for U@D3h-C74. For the quintet state we also found a four-electron transfer. For U@C2v(9)-C82, we have seen that triplet and quintet states are almost degenerate. In Fig. S8† we present the molecular orbital diagram of the quintet state, since it is easier to interpret than that for the triplet. Interestingly, only three electrons are transferred to the cage, instead of four as in U@D3h-C74 and U@C2(5)-C82. The spin density of uranium is close to 3 in this case. For the triplet state, an intermediate situation between a three and a four-electron transfer is observed. Therefore, for U@C2v(9)-C82, the oxidation state of uranium is closer to three than four. To conclude, the degree of charge transfer for U@C82 isomers depends on the specific cage that encapsulates the U atom: U4+@C2(5)-C824− and U3+@C2v(9)-C823−. For the other two computed isomers, not yet experimentally obtained, we have U3+@Cs(6)-C823− and U4+@C3v(8)-C824− for the triplet state, which changes to U3+@C3v(8)-C823− for the energetically accessible quintet state (only 2.5 kcal mol−1 higher in energy, see Table 1). These results are consistent with previous experimental observations where cage C2v(9)-C82 was found to encapsulate M3+ ions whereas cages Cs(6)-C82 and C3v(8)-C82 preferentially encapsulate tetravalent clusters (M2O, M2S and M2C2).44–48 It is interesting to point out that even though the C74 and C82 cages that encapsulate Sm and Yb are the same as the two found for U, M2+@D3h(1)-C742− and M2+@C2(5)-C822−, we have not observed a transfer of two electrons for any of the U-EMFs analyzed here. The reason for this apparent molecular electronic promiscuity is that D3h(1)-C74 and C2(5)-C82 cages are able to stabilize both 4 electron (U) as well 2 electron transfers (Sm and Yb). For example, for cage C2(5)-C82, the bottom hemisphere location is optimal for hosting M2+ (site B) whereas M4+ preferentially locate at site A. When a divalent metal ion is encapsulated, two electrons are transferred from the metal to the LUMO of the fullerene cage, which is mainly located at the bottom, as shown in Fig. 7. Consequently, the metal-cage interaction is optimal when the metal atom is located in this hemisphere. Alternatively, when the C2(5)-C82 cage encapsulates a tetravalent cation the LUMO and LUMO+1 are occupied and in this case the optimal metal-carbon cage interactions occur when the metal ion is near site A at the top hemisphere region where the LUMO+1 is mainly localized.
Finally, to incorporate the effect of the high temperatures at which the fullerenes are formed, which can be critical to determine their relative stabilities and abundances,48 we have also computed the molar fractions of these four isomers as a function of temperature (0–4000 K) using the rigid rotor and harmonic approximation (RRHO) and the related free-encapsulating model (FEM) as proposed by Slanina.49,50Fig. 8 shows molar fractions using the FEM approximation, where U@C2v(9)-C82 is the most abundant up to 3000 K followed by U@C2(5)-C82 in good agreement with experimental results. Isomers U@Cs(6)-C82 and U@C3v(8)-C82, which also show appreciable molar fractions at high temperatures, might also be detected in future experiments.
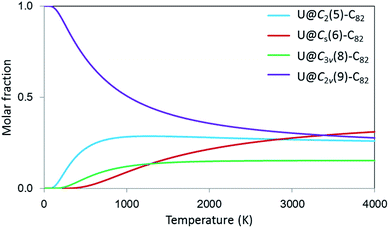 | ||
| Fig. 8 Computed molar fraction as a function of the temperature (K) using the free-encapsulating model (FEM) for U@C2(5)-C82, U@Cs(6)-C82, U@C3v(8)-C82 and U@C2v(9)-C82. | ||
Electrochemical properties of U4+@D3h-C744−, U4+@C2(5)-C824− and U3+@C2v(9)-C823−
The redox properties of U4+@D3h-C744−, U4+@C2(5)-C824− and U3+@C2v(9)-C823− were measured by cyclic voltammetry (CV) (Fig. 9) and square wave voltammetry (SWV) (see Fig. S9†) in o-dichlorobenzene (o-DCB) solution containing 0.05 M n-Bu4NPF6 as the supporting electrolyte. Four reversible one-electron reductive steps with nearly equal distance and two reversible one-electron oxidative steps were observed for U4+@D3h-C744−. Interestingly, despite the different metal-to-cage charge transfer, the redox behavior of U4+@D3h-C744−shows remarkable resemblance to those reported for Sm2+@D3h-C742− and Eu2+@D3h-C742−, which also exhibit four reversible reductive steps and two reversible oxidative steps.21,22 In addition, the electrochemical band gap of U4+@D3h-C744− (1.06 eV) is also very close to those of the Sm2+@D3h-C742− (0.97 eV) and Eu2+@D3h-C742− (1.00 eV), a clear indication of their common closed-shell electronic structures. However, despite the similarity in the number and regularity of the redox steps, it is worth mentioning that all potential values for U4+@D3h-C744− are markedly shifted cathodically (by 200 to 400 mV), indicating that it is much easier to oxidize and more difficult to reduce than the corresponding Sm2+@D3h-C742−. This likely due to the much higher negative charge localized on the cage for U4+@D3h-C744−.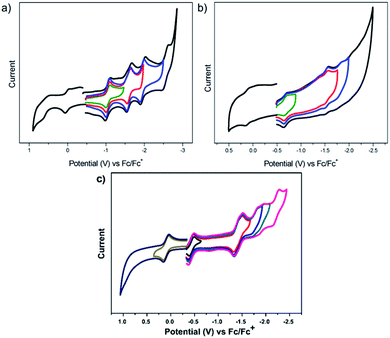 | ||
| Fig. 9 Cyclic voltammograms of U@D3h-C74 (a), U@C2(5)-C82 (b) and U@C2v(9)-C82 (c) in 0.05 M n-Bu4NPF6/o-DCB solution, (scan rate: 20 mV s−1). | ||
For the larger C82 cages, specifically for U4+@C2(5)-C824−, the electrochemical behavior is very different when compared with those of other mono-metallofullerenes with the same C2(5)-C82 cage. U4+@C2(5)-C824− exhibits two reversible and two irreversible one-electron reductive processes, whereas all of the one-electron reductive processes of Yb2+@C2(5)-C822− and Sm2+@C2(5)-C822− are perfectly reversible.12,51 As expected, the overall redox behavior of U3+@C2v(9)-C823− shows remarkable resemblance to those of other M3+@C2v(9)-C823− (M = La, Y, Ce),52,53 while it is very different compared with those of divalent mono-EMFs (e.g. Yb2+@C2v(9)-C822−, Sm2+@C2v(9)-C822−).12,14 Not surprisingly, the degree of metal-to-cage charge transfer strongly influences the electrochemical properties of mono-EMFs as well as the isomeric structure. Remarkably, despite the size and structural diversity of the cages, all U-EMFs share one common electrochemical feature: they are all reasonably easy to oxidize 0.01 V (U@D3h-C74), 0.11 V (U@C2(5)-C82) and 0.10 V (U3+@C2v(9)-C823−), making them reasonably good electron-donors (Table 2).
We also computed the first reduction and oxidation potentials for U4+@D3h-C744−, U4+@C2(5)-C824− and U3+@C2v(9)-C823−, in order to compare with experimental results (see Table S2†). The computed potentials are comparable to the experimental values within the error of the methodology,54 although the deviation of the electrochemical gap for U4+@D3h-C744− was larger than for the other uranium fullerenes. After a detailed analysis of the molecular orbitals involved in the redox processes we concluded that for U4+@D3h-C744− and U4+@C2(5)-C824− reduction takes place on uranium, whereas for U3+@C2v(9)-C823− reduction occurs on the carbon cage. Oxidation processes are rather specific for each case. Hence, we have found that U(III) is oxidized to U(IV) when it is encapsulated by C2v(9)-C82, and U(IV) oxidizes up to U(V) in the smaller cage C74. However, the first oxidation of U4+@C2(5)-C824− occurs at the carbon cage. It is essential to remark that in some cases after oxidation or reduction several states remain close in energy. For example, the first reduction of U@C2v(9)-C82 at the uranium ion or on the carbon cage have similar reduction potentials. This may explain why the experimental first reduction potentials for U@C2v(9)-C82 and La@C2v(9)-C82 are similar. Estimated oxidation states for neutral and ionic species are compiled in Table S3.† Finally, we would like to point out that the monoconfigurational DFT description of these quasidegenerated states is an approximation to the real multiconfigurational wavefunction and this might be the origin of the larger discrepancies found in the estimation of the potentials and electrochemical gap for U@D3h-C74 compared to other urano- or clusterfullerenes.
| Species | ox E 2 [V] | ox E 1 [V] | red E 1 [V] | red E 2 [V] | red E 3 [V] | red E 4 [V] | red E 5 [V] | ΔEgap [V] |
|---|---|---|---|---|---|---|---|---|
| a Half-cell potentials are given unless otherwise addressed. b Irreversible. Square wave voltammetry peak values. | ||||||||
| Sm@D3h-C74 (ref. 21) | 0.76 | 0.20 | −0.77 | −1.21 | −1.72 | −2.14 | — | 0.97 |
| U@D3h-C74 | 0.61 | 0.01 | −1.05 | −1.60 | −1.96 | −2.55 | — | 1.06 |
| Sm@C2(5)-C82 (ref. 51) | — | 0.42 | −0.84 | −1.01 | −1.51 | −1.90 | — | 1.26 |
| Yb@C2(5)-C82 (ref. 12) | 0.90 | 0.38 | −0.86 | −0.98 | −1.50 | −1.87 | — | 1.24 |
| U@C2(5)-C82 | — | 0.11 | −0.67 | −1.54 | −1.83b | −2.05b | — | 0.78 |
| Sm@C2v(9)-C82 (ref. 14) | — | 0.52 | −0.42 | −0.77 | −1.60 | −1.94 | — | 0.94 |
| Yb@C2v(9)-C82 (ref. 12) | — | 0.61 | −0.46 | −0.78 | −1.60 | −1.90 | — | 1.07 |
| La@C2v(9)-C82 (ref. 53) | 1.07 | 0.07 | −0.42 | −1.37 | −1.53 | −2.26 | — | 0.49 |
| U@C2v(9)-C82 | 0.92 | 0.10 | −0.43 | −1.42 | −1.76b | −1.77b | −2.21b | 0.53 |
Conclusions
In this work, single crystal X-ray structures and theoretical calculations of three U-EMFs, U@D3h-C74, U@C2(5)-C82 and U@C2v(9)-C82, reveal that a variable valence state of the encapsulated metal atom can result from isomeric fullerene cages. Notably, we find that the oxidation state of uranium for U@C74 and U@C82 isomers depend on the cage structures that encapsulates the U atom: U4+@D3h-C744−, U4+@C2(5)-C824− and U3+@C2v(9)-C823−. Formal transfers of two or six electrons, U2+@C2n2− or U6+@C2n6−, are not observed for any of the systems analyzed here. The first two compounds are the first examples of tetravalent (M4+) containing mono-EMFs and the two U@C82 isomers represent the first pair where the oxidation state of the encapsulated ion depends on the isomeric cage structure (4+ or 3+). Detailed analyses further reveal that reduction takes place at the endohedral metal ion when the oxidation state of U is IV and on the fullerene cage when it is III. We also present the first single-crystal X-ray crystallographic structures for U endohedrals.Acknowledgements
L. E. thanks the US National Science Foundation (NSF) for generous support of this work under the NSF-PREM program (DMR 1205302) and CHE-1408865. The Robert A. Welch Foundation is also gratefully acknowledged for an endowed chair to L. E. (grant AH-0033). J. M. P. thanks the Spanish Ministry of Science (CTQ2014-52774-P) and the Generalitat de Catalunya (2013SGR199 and XRQTC) for support. J. M. P. also thanks ICREA foundation for an ICREA ACADEMIA award. R. M.-M. thanks Spanish Ministry of Science for a PhD fellowship. C. N thanks the National Science Foundation China (NSFC 51302178) and Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).Notes and references
- T. Akasaka and S. Nagase, Endofullerenes: A New Family of Carbon Clusters, Kluwer Academic Publishers, Dordrecht, The Netherlands, 2002 Search PubMed.
- X. Lu, L. Feng, T. Akasaka and S. Nagase, Chem. Soc. Rev., 2012, 41, 7723–7760 RSC.
- A. Popov, S. Yang and L. Dunsch, Chem. Rev., 2013, 113, 5989–6113 CrossRef CAS PubMed.
- M. N. Chaur, F. Melin, A. L. Ortiz and L. Echegoyen, Angew. Chem., Int. Ed., 2009, 48, 7514–7538 CrossRef CAS PubMed.
- S. Nagase and K. Kobayashi, Chem. Phys. Lett., 1993, 214, 57–63 CrossRef CAS.
- K. Suenaga, S. Iijima, H. Kato and H. Shinohara, Phys. Rev. B: Condens. Matter Mater. Phys., 2000, 62, 1627–1630 CrossRef CAS.
- H. Yamaoka, H. Sugiyama, Y. Kubozono, A. Kotani, R. Nouchi, A. M. Vlaicu, H. Oohashi, T. Tochio, Y. Ito and H. Yoshikawa, Phys. Rev. B: Condens. Matter Mater. Phys., 2009, 80, 205403 CrossRef.
- T. Pichler, M. Knupfer, M. S. Golden, T. Boske, J. Fink, U. Kirbach, P. Kuran, L. Dunsch and C. Jung, Appl. Phys. A: Mater. Sci. Process., 1998, 66, 281–285 CrossRef CAS.
- Y. F. Lian, Z. J. Shi, X. H. Zhou and Z. N. Gu, Chem. Mater., 2004, 16, 1704–1714 CrossRef CAS.
- Z. D. Xu, T. Nakane and H. Shinohara, J. Am. Chem. Soc., 1996, 118, 11309–11310 CrossRef CAS.
- H. Yang, H. X. Jin, X. Q. Wang, Z. Y. Liu, M. L. Yu, F. K. Zhao, B. Q. Mercado, M. M. Olmstead and A. L. Balch, J. Am. Chem. Soc., 2012, 134, 14127–14136 CrossRef CAS PubMed.
- X. Lu, Z. Slanina, T. Akasaka, T. Tsuchiya, N. Mizorogi and S. Nagase, J. Am. Chem. Soc., 2010, 132, 5896–5905 CrossRef CAS PubMed.
- M. Suzuki, Z. Slanina, N. Mizorogi, X. Lu, S. Nagase, M. M. Olmstead, A. L. Balch and T. Akasaka, J. Am. Chem. Soc., 2012, 134, 18772–18778 CrossRef CAS PubMed.
- Z. Q. Hu, Y. J. Hao, Z. Slanina, Z. G. Gu, Z. J. Shi, F. Uhlik, Y. F. Zhao and L. Feng, Inorg. Chem., 2015, 54, 2103–2108 CrossRef CAS PubMed.
- M. Suzuki, X. Lu, S. Sato, H. Nikawa, N. Mizorogi, Z. Slanina, T. Tsuchiya, S. Nagase and T. Akasaka, Inorg. Chem., 2012, 51, 5270–5273 CrossRef CAS PubMed.
- S. Sato, H. Nikawa, S. Seki, L. Wang, G. F. Luo, J. Lu, M. Haranaka, T. Tsuchiya, S. Nagase and T. Akasaka, Angew. Chem., Int. Ed., 2012, 51, 1589–1591 CrossRef CAS PubMed.
- T. Akasaka, T. Wakahara, S. Nagase, K. Kobayashi, M. Waelchli, K. Yamamoto, M. Kondo, S. Shirakura, Y. Maeda, T. Kato, M. Kako, Y. Nakadaira, X. Gao, E. Van Caemelbecke and K. M. Kadish, J. Phys. Chem. B, 2001, 105, 2971–2974 CrossRef CAS.
- A. Reich, M. Panthofer, H. Modrow, U. Wedig and M. Jansen, J. Am. Chem. Soc., 2004, 126, 14428–14429 CrossRef CAS PubMed.
- T. Kodama, R. Fujii, Y. Miyake, S. Suzuki, H. Nishikawa, I. Ikemoto, K. Kikuchi and Y. Achiba, Chem. Phys. Lett., 2004, 399, 94–97 CrossRef CAS.
- O. Haufe, M. Hecht, A. Grupp, M. Mehring and M. Jansen, Z. Anorg. Allg. Chem., 2005, 631, 126–130 CrossRef CAS.
- W. Xu, Y. J. Hao, F. Uhlik, Z. J. Shi, Z. Slanina and L. Feng, Nanoscale, 2013, 5, 10409–10413 RSC.
- P. Kuran, M. Krause, A. Bartl and L. Dunsch, Chem. Phys. Lett., 1998, 292, 580–586 CrossRef CAS.
- J. X. Xu, T. Tsuchiya, C. Hao, Z. J. Shi, T. Wakahara, W. H. Mi, Z. N. Gu and T. Akasaka, Chem. Phys. Lett., 2006, 419, 44–47 CrossRef CAS.
- J. X. Xu, X. Lu, X. H. Zhou, X. R. He, Z. J. Shi and Z. N. Gu, Chem. Mater., 2004, 16, 2959–2964 CrossRef CAS.
- S. T. Liddle, Angew. Chem., Int. Ed., 2015, 54, 8604–8641 CrossRef CAS PubMed.
- M. R. MacDonald, M. E. Fieser, J. E. Bates, J. W. Ziller, F. Furche and W. J. Evans, J. Am. Chem. Soc., 2013, 135, 13310–13313 CrossRef CAS PubMed.
- H. S. La Pierre, A. Scheurer, F. W. Heinemann, W. Hieringer and K. Meyer, Angew. Chem., Int. Ed., 2014, 53, 7158–7162 CrossRef CAS PubMed.
- T. W. Hayton, Chem. Commun., 2013, 49, 2956–2973 RSC.
- T. Guo, M. Diener, Y. Chai, M. Alford, R. Haufler, S. McClure, T. Ohno, J. Weaver, G. Scuseria and R. Smalley, Science, 1992, 257, 1661–1664 CAS.
- K. Akiyama, Y. L. Zhao, K. Sueki, K. Tsukada, H. Haba, Y. Nagame, T. Kodama, S. Suzuki, T. Ohtsuki, M. Sakaguchi, K. Kikuchi, M. Katada and H. Nakahara, J. Am. Chem. Soc., 2001, 123, 181–182 CrossRef CAS PubMed.
- K. Akiyama, K. Sueki, K. Tsukada, T. Yaita, Y. Miyake, H. Haba, M. Asai, T. Kodama, K. Kikuchi, T. Ohtsuki, Y. Nagame, M. Katada and H. Nakahara, J. Nucl. Radiochem. Sci., 2002, 3, 151–154 CrossRef.
- K. Akiyama, K. Sueki, H. Haba, K. Tsukada, M. Asai, T. Yaita, Y. Nagame, K. Kikuchi, M. Katada and H. Nakahara, J. Radioanal. Nucl. Chem., 2003, 255, 155–158 CrossRef CAS.
- X. Liu, L. Li, B. Liu, D. Wang, Y. Zhao and X. Gao, J. Phys. Chem. A, 2012, 116, 11651–11655 CrossRef CAS PubMed.
- G. M. Sheldrick, Acta Crystallogr., Sect. A: Found. Crystallogr., 2008, 64, 112–122 CrossRef CAS PubMed.
- G. te Velde, F. M. Bickelhaupt, E. J. Baerends, C. Fonseca Guerra, S. J. A. van Gisbergen, J. G. Snijders and T. Ziegler, J. Comput. Chem., 2001, 22, 931–967 CrossRef CAS.
- A. D. Becke, J. Chem. Phys., 1986, 84, 4524–4529 CrossRef CAS.
- J. P. Perdew, Phys. Rev. B: Condens. Matter Mater. Phys., 1986, 33, 8822–8824 CrossRef.
- M. Álvarez-Moreno, C. de Graaf, N. López, F. Maseras, J. M. Poblet and C. Bo, J. Chem. Inf. Model., 2015, 55, 95–103 CrossRef PubMed.
- W. Krätschmer, L. D. Lamb, K. Fostiropoulos and D. R. Huffman, Nature, 1990, 347, 354–358 CrossRef.
- P. W. Fowler and D. E. Manolopoulos, An Atlas of Fullerenes, Dover Publications, Inc, Mineola, New York, 2006 Search PubMed.
- A. Rodriguez-Fortea, N. Alegret, A. L. Balch and J. M. Poblet, Nat. Chem., 2010, 2, 955–961 CrossRef CAS PubMed.
- M. N. Chaur, R. Valencia, A. Rodriguez-Fortea, J. M. Poblet and L. Echegoyen, Angew. Chem., Int. Ed., 2009, 48, 1425–1428 CrossRef CAS PubMed.
- A. Rodriguez-Fortea, A. L. Balch and J. M. Poblet, Chem. Soc. Rev., 2011, 40, 3551–3563 RSC.
- Q. Tang, L. Abella, Y. Hao, X. Li, Y. Wan, A. Rodríguez-Fortea, J. M. Poblet, L. Feng and N. Chen, Inorg. Chem., 2016, 55, 1926–1933 CrossRef CAS PubMed.
- B. Q. Mercado, N. Chen, A. Rodriguez-Fortea, M. A. Mackey, S. Stevenson, L. Echegoyen, J. M. Poblet, M. H. Olmstead and A. L. Balch, J. Am. Chem. Soc., 2011, 133, 6752–6760 CrossRef CAS PubMed.
- R. Valencia, A. Rodriguez-Fortea and J. M. Poblet, J. Phys. Chem. A, 2008, 112, 4550–4555 CrossRef CAS PubMed.
- Y. Iiduka, T. Wakahara, K. Nakajima, T. Nakahodo, T. Tsuchiya, Y. Maeda, T. Akasaka, K. Yoza, M. T. H. Liu, N. Mizorogi and S. Nagase, Angew. Chem., Int. Ed., 2007, 46, 5562–5564 CrossRef CAS PubMed.
- B. Q. Mercado, M. A. Stuart, M. A. Mackey, J. E. Pickens, B. S. Confait, S. Stevenson, M. L. Easterling, R. Valencia, A. Rodriguez-Fortea, J. M. Poblet, M. M. Olmstead and A. L. Balch, J. Am. Chem. Soc., 2010, 132, 12098–12105 CrossRef CAS PubMed.
- Z. Slanina, S. L. Lee, F. Uhlik, L. Adamowicz and S. Nagase, Theor. Chem. Acc., 2007, 117, 315–322 CrossRef CAS.
- Z. Slanina and S. Nagase, ChemPhysChem, 2005, 6, 2060–2063 CrossRef CAS PubMed.
- W. Xu, B. Niu, L. Feng, Z. Shi and Y. Lian, Chem.–Eur. J., 2012, 18, 14246–14249 CrossRef CAS PubMed.
- T. Suzuki, K. Kikuchi, F. Oguri, Y. Nakao, S. Suzuki, Y. Achiba, K. Yamamoto, H. Funasaka and T. Takahashi, Tetrahedron, 1996, 52, 4973–4982 CrossRef CAS.
- T. Akasaka, T. Wakahara, S. Nagase, K. Kobayashi, M. Waelchli, K. Yamamoto, M. Kondo, S. Shirakura, S. Okubo, Y. Maeda, T. Kato, M. Kako, Y. Nakadaira, R. Nagahata, X. Gao, E. Van Caemelbecke and K. M. Kadish, J. Am. Chem. Soc., 2000, 122, 9316–9317 CrossRef CAS.
- R. Valencia, A. Rodriguez-Fortea, A. Clotet, C. de Graaf, M. N. Chaur, L. Echegoyen and J. M. Poblet, Chem.–Eur. J., 2009, 15, 10997–11009 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 150850815085091522558. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c7sc01711a |
| This journal is © The Royal Society of Chemistry 2017 |