Open Access Article
Open Access ArticleFluorinated EuII-based multimodal contrast agent for temperature- and redox-responsive magnetic resonance imaging†
Lina A.
Basal
 a,
Matthew D.
Bailey
a,
Matthew D.
Bailey
 a,
Jonathan
Romero
b,
Meser M.
Ali
c,
Lyazat
Kurenbekova
d,
Jason
Yustein
de,
Robia G.
Pautler
*b and
Matthew J.
Allen
a,
Jonathan
Romero
b,
Meser M.
Ali
c,
Lyazat
Kurenbekova
d,
Jason
Yustein
de,
Robia G.
Pautler
*b and
Matthew J.
Allen
 *a
*a
aDepartment of Chemistry, Wayne State University, 5101 Cass Avenue, Detroit, Michigan 48202, USA. E-mail: mallen@chem.wayne.edu
bDepartment of Molecular Physiology and Biophysics, Baylor College of Medicine, One Baylor Plaza, Houston, Texas 77030, USA. E-mail: rpautler@bcm.edu
cDepartment of Neurosurgery, Henry Ford Hospital, 1 Ford Place, Detroit, Michigan 48202, USA
dIntegrative Molecular and Biomedical Sciences, Baylor College of Medicine, Houston, TX 77030, USA
eDepartment of Pediatrics, Texas Children's Cancer and Hematology Centers, Baylor College of Medicine, Houston, TX 77030, USA
First published on 26th October 2017
Abstract
Magnetic resonance imaging (MRI) using redox-active, EuII-containing complexes is one of the most promising techniques for noninvasively imaging hypoxia in vivo. In this technique, positive (T1-weighted) contrast enhancement persists in areas of relatively low oxidizing ability, such as hypoxic tissue. Herein, we describe a fluorinated, EuII-containing complex in which the redox-active metal is caged by intramolecular interactions. The position of the fluorine atoms enables temperature-responsive contrast enhancement in the reduced form of the contrast agent and detection of the oxidized contrast agent via MRI in vivo. Positive contrast is observed in 1H-MRI with Eu in the +2 oxidation state, and chemical exchange saturation transfer and 19F-MRI signal are observed with Eu in the +3 oxidation state. Contrast enhancement is controlled by the redox state of Eu, and modulated by the fluorous interactions that cage a bound water molecule reduce relaxivity in a temperature-dependent fashion. Together, these advancements constitute the first report of in vivo, redox-responsive imaging using 19F-MRI.
Introduction
Oxygen is critical to all forms of aerobic life, and imbalances of oxygen supply and consumption, such as hypoxia, are associated with disease.1–7 For example, hypoxic and nonhypoxic tumors have different responses to therapies, and regions of hypoxic tissue can be extremely heterogenous,8 complicating the differentiation of different types of tissues. Consequently, many visualization techniques, including optical imaging, positron emission tomography, photoacoustic imaging, and magnetic resonance imaging (MRI), are being investigated for the imaging of oxygen levels to study the progress and treatment of diseases.9 MRI is capable of producing images of the inside of living organisms with excellent spatial resolution, and 19F has no detectable background signal in vivo. As a result of these properties, 19F-MRI has been used in vivo with perfluorocarbon emulsions,10 perfluorocarbon nanoparticles,11,12 perfluorocarbon-labelled cells,13,14 and discrete lanthanide complexes.15 These examples of in vivo imaging with 19F-MRI make the technique one of the most promising for noninvasive imaging, and metal-based multimodal oxygen-responsive contrast agents for 19F-MRI would expand that promise to the imaging of hypoxia-related diseases. The design of contrast agents for 19F-MRI is complicated by the conflict between the need for a large number of chemically equivalent fluorine atoms and the hydrophobicity of fluorine. Too few 19F nuclei lead to undetectable signal, but too many 19F nuclei are associated with low solubility.12 Ideally, oxygen-responsive contrast agents for 19F-MRI would be soluble in water to enable distribution in vivo and be redox-responsive to yield molecular information regarding oxidation. Furthermore, because the 19F atoms in ligands are not intrinsically redox-active, redox-responsive probes for 19F-MRI need to include a redox-active element to impart a responsive nature to probes. EuII/III-based contrast agents react with oxygen to change MRI-relevant properties: EuII enhances contrast in T1-weighted MRI and EuIII does not.16–22 We hypothesized that combining the favorable imaging properties of 19F-MRI with 1H-MRI-active EuII-based complexes would produce oxidation-state-dependent imaging and enable detection of both oxidation states of Eu. We overcame the solubility challenges associated with 19F via strategic placement of 19F atoms in ligands for europium that sequestered the fluorine atoms via intramolecular interactions. An important consequence of these intramolecular interactions was the formation of a cage that trapped a molecule of water bound to europium, enabling control over contrast enhancement in a temperature-responsive manner. Here, we report a new redox-active contrast system, 2EuII/III, that has twelve chemically equivalent 19F nuclei, is water-soluble, and is detectable in vivo before and after oxidation using different imaging protocols with a single MRI scanner.Our strategy was based upon the ability of EuII, but not EuIII that has a diamagnetic ground state, to dramatically influence the relaxation rates of nearby nuclei.17 Although EuIII has low-lying excited paramagnetic electronic states that are thermally accessible, no measurable effect on relaxation rates have been observed at concentrations up to 6 mM.16,17,19 EuII is isoelectronic with GdIII, which is commonly used in contrast agents for MRI. Fluorinated GdIII-containing complexes influence the signal of 19F in a distance-dependent manner;22–28 consequently, we hypothesized that introducing 19F nuclei near EuII would cause severe line-broadening (via shortening of the transverse relaxation time of 19F) to the point of no observable signal. However, unlike GdIII that is not redox-active in vivo, oxidation of EuII to EuIII would be expected to remove quenching of 19F signal. Therefore, Eu-containing complexes would act as T1-shortening contrast agents for 1H-MRI in the +2 oxidation state and “turn-on” 19F imaging probes in response to oxidation of EuII to EuIII. Based on recent reports of EuII-based contrast agents,16,17,29 we thought that a cyclen scaffold would provide a synthetically viable opportunity to coordinate both oxidation states of Eu near multiple chemically equivalent 19F nuclei while maintaining solubility in water. Our design incorporates twelve equivalent 19F nuclei that enable detection in vivo but also provides solubility for clearance via the excretory system. Because a single MRI scanner can be used for 19F- and 1H-MRI, the 2EuII/III system would be detectable before and after oxidation using the same instrument.
Results and discussion
Fluorinated ligand 2 was synthesized in two steps from commercially available starting materials (see the ESI† for detailed procedures, yields, and characterization). Ligand 2 was metalated with EuCl3 to produce 2EuIII (59% yield), and 2EuII was synthesized in quantitative yield by reducing 2EuIII with Zn0. Complex 2EuIII can be recovered by oxidizing solutions of 2EuII with air as evidenced by UV-visible, 19F-NMR, and luminescence spectroscopies (ESI Fig. S2†). Both 2EuIII and 2EuII are soluble in neutral, buffered water suitable for in vivo injections. Furthermore, both complexes share similar conformations in the solid state (Fig. 1). All four amide arms are pseudo-axial, resulting in caged structures around the Eu ions. This conformation is unusual for tetraamide complexes with lanthanides. In all reported examples, amide arms favor pseudo-equatorial positions unless forced to be pseudo-axial by intramolecular steric interactions.30–32 The structures of 2EuIII and 2EuII suggest that the fluorophilic character of the CF3 groups and π-stacking of the benzene rings contribute to the formation of a cage around the metal. Additionally, in both 2EuIII and 2EuII, one coordinated water molecule is held within the pocket formed by the p-trifluoromethylbenzyl groups, effectively caging the coordinated water molecule from the surroundings. Although trapping of water has implications for T1-weighted MRI because impeded water exchange influences relaxivity,33 the pseudo-axial amides could be useful for temperature control of relaxivity. However, crystal structures describe solid-state conformations and do not account for dynamics in solution; therefore, we turned to solution-phase characterization.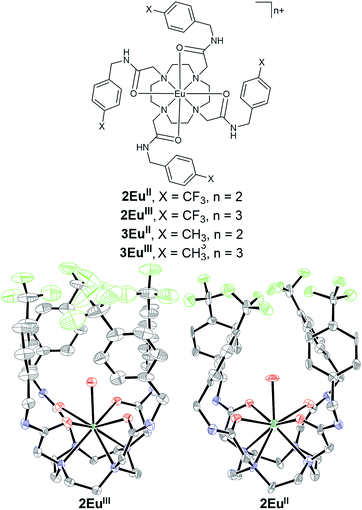 | ||
| Fig. 1 (Left) Chemical structures of 2EuII, 2EuIII, 3EuII, and 3EuIII. Crystal structures of (center) 2EuII and (right) 2EuIII grown from solutions in methanol and ethanol, respectively. Chloride counterions, outer-sphere water molecules, and hydrogen atoms are not shown for clarity. Thermal ellipsoids are drawn at 50% probability. Grey = C; blue = N; red = O; yellow-green = F; sea green = Eu. Crystallographic data for these structures have been deposited at the Cambridge Crystallographic Data Centre under deposition numbers CCDC 1562330 (2EuII) and CCDC 1562331 (2EuIII).† | ||
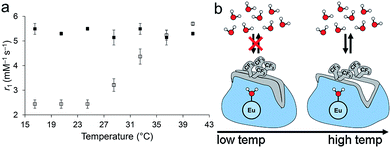
To probe the stability of the pseudo-axial conformation in solution, relaxivity was measured as a function of temperature at 11.7 T between 16.5 and 40.5 °C (Fig. 2). This temperature range was selected because it spans from slightly below room temperature to slightly above body temperature. The plot of relaxivity versus temperature of 2EuII shows two regimes: there is no change in relaxivity between 16.5 and 24.5 °C, and there is a linear increase in relaxation rate between 24.5 and 40.5 °C. The relaxivity at 36.5 °C is 2.0× higher than the relaxivity at 16.5 °C. At the colder temperature, the relaxivity of 2EuII is similar to EuII-containing complexes with purely outer-sphere contrast enhancement (a coordinated water molecule does not measurably influence relaxivity if it is not exchanging with bulk water),34 and the relaxivity at 36.5 °C is within the range of reported EuII-containing complexes that have exchanging water.17,18,34–39 The observed dependence on temperature is the opposite of what would be expected for a system that does not change the number of coordinated water molecules: increased temperatures lead to more rapid molecular rotations that are associated with lower relaxivity values.40 A similar, but smaller, effect in relaxivity caused by hydrophobic groups around a caged water molecule has been previously observed with a structurally similar GdIII complex that has four N-(1-phenylethyl)propionamide arms and exhibits a similar increase in relaxivity over the same temperature range.41,42 Additionally, the change in relaxivity is consistent with GdIII-based responsive contrast agents that modulate water coordination number.43 A possible explanation for this temperature-dependent shift in relaxivity is that thermal energy overcomes the fluorous and π-stacking interactions above 24.5 °C, resulting in accessibility of bulk water to participate in exchange with the coordinated water inside of the cage formed by the p-trifluoromethylbenzyl arms. Conformational changes of similar EuIII- and GdIII-containing, cyclen-based complexes have been reported to influence water-exchange rates.42,44–47 Finally, to check for the possibility of aggregation, we performed dynamic light scattering measurements of 2EuII (3.1 mM in aqueous 3-morpholinopropane-1-sulfonic acid buffer) at 5 and 37 °C: No aggregates were detected at either temperature, supporting a mechanism of relaxivity modulation based on water exchange instead of molecular reorientation. The observed changes in relaxivity are consistent with what would be expected if pseudo-axial amides in the solid-state structure were similar to solution-phase at lower temperatures, and thermal energy at higher temperatures overcame intramolecular fluorous interactions to enable water exchange.
To explore the influence of the fluorous interactions between the trifluoromethyl moieties on the temperature-dependent behavior of 2EuII, we synthesized the nonfluorinated methyl analog, 3EuII, and measured relaxivity over the same range of temperatures. Ligand 3 was synthesized in two steps from commercially available starting materials, and the corresponding Eu-containing complexes, 3EuIII and 3EuII, were synthesized similarly to 2EuIII and 2EuII (ESI Fig. S1†). The methylated complex, 3EuII, did not exhibit a temperature-dependent change in relaxivity like the fluorinated complex 2EuII. Between 16.5 and 36.5 °C, 3EuII displayed relaxivities from 5.14 to 5.49 mM−1 s−1, similar to the relaxivity of the trifluoromethyl complex, 2EuII, at temperatures ≥36.5 °C. The lack of temperature dependence of the methyl analog 3EuII suggests that intramolecular fluorous interactions are the cause of the temperature-dependent relaxivity of 2EuII. Therefore, the fluorous interactions impart an element of kinetic control that has implications for long-term storage and could be used to measure temperature using relaxivity.
To test if the p-trifluoromethylbenzylamide arms would enable detection after oxidation of EuII to EuIII, we studied the spectroscopic properties of 2EuII/III. To verify that the 19F-NMR signal changes with oxidation state, we acquired 19F-NMR spectra of aqueous, buffered solutions of 2EuIII and 2EuII (5.4 mM in aqueous 3-morpholinopropane-1-sulfonic acid buffer, pH 7.0). A singlet at −62.1 ppm relative to sodium triflate was observed for 2EuIII, and no detectable signal was observed for 2EuII. Upon oxidation of the solution of 2EuII, 19F-signal appeared consistent with the presence of 2EuIII (ESI Fig. S2†). The 19F-NMR signal was too broad to be observed for 2EuII because the EuII ion shortened the transverse relaxation times of 19F to the point of no observable signal. Upon oxidation to EuIII, 19F signal appeared. Furthermore, because not all MRI scanners are capable of imaging 19F, we also acquired the chemical exchange saturation transfer (CEST) spectrum of 2EuIII. Amides near EuIII and EuII provide a means of imaging oxygen content via CEST MRI.17 Complex 2EuIII has a saturation offset of 49 ppm (ESI Fig. S3†), which is similar to other EuIII-containing tetraamide complexes.17,30,31,48,49 Therefore, based on spectroscopic studies, 2EuIII has detectable 19F- and CEST MRI after oxidation.
To verify that the oxidation-state-dependent signal was observable with MRI at imaging-relevant concentrations, we imaged solutions of complexes 2EuIII, 2EuII, and 2EuIII post-oxidation (Fig. 3). 19F- and CEST-MRI signals were observed for EuIII-containing complex 2EuIII, but no T1-weighted enhancement was observed relative to buffer. No detectable 19F- or CEST-MRI signals were observed for EuII-containing complex 2EuII, but a 2.0× increase in brightness in the T1-weighted scan was observed relative to buffer (see ESI Fig. S8 and Table S4† for signal-to-noise ratios). This observation is consistent with another EuII-containing tetraamide complex without benzyl groups that does not influence CEST-MRI but does shorten T1.17 Therefore, the pair of complexes, 2EuII and 2EuIII, provide oxidation-dependent signal: the EuII-containing complex acts as a T1-enhancing contrast agent, and the EuIII-containing complex acts as an imaging probe for 19F- and CEST-MRI. Due to the well-behaved redox- and temperature-responsive contrast system 2EuII/IIIin vitro, we investigated the behavior of the system in mice.
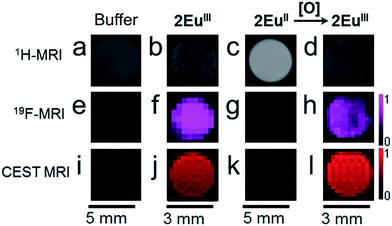 | ||
| Fig. 3 MR images of solutions of samples in aqueous 3-morpholinopropane-1-sulfonic acid buffer (20 mM, pH 7.0) in glass (3 mm) or plastic tubes (5 mm). Scale bars correspond to the three images in the column above each bar. T1-weighted MRI images of solutions of (a) 3-morpholinopropane-1-sulfonic acid (20 mM, pH 7.0) (b) 2EuIII (3.5 mM); (c) 2EuII (3.5 mM); and (d) 2EuIII after oxidation of 2EuII (3.5 mM). 19F-MRI images of (e) 3-morpholinopropane-1-sulfonic acid (20 mM, pH 7.0); (f) 2EuIII (3.5 mM); (g) 2EuII (3.5 mM); and (h) 2EuIII after oxidation of 2EuII (3.5 mM). The scale bar on the right of (h) represents signal intensity for all 19F- MRI images in Fig. 3. CEST MRI images of (i) 3-morpholinopropane-1-sulfonic acid (20 mM, pH 7.0); (j) 2EuIII (3.5 mM); (k) 2EuII (3.5 mM); and (l) 2EuIII after oxidation of 2EuII (3.5 mM). The scale bar on the right of (l) represents signal intensity for all CEST MRI images in Fig. 3. | ||
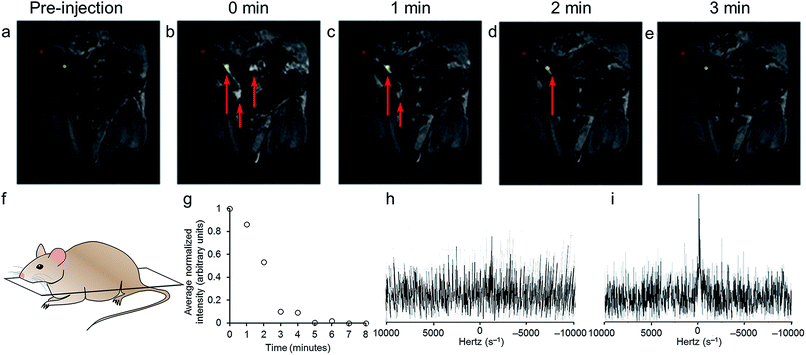
To verify that oxidation-state-dependent signals were observable with MRI in vivo, mice were injected in the peritoneal cavities with solutions of 2EuII (200 μL, 5.4 mM in aqueous 3-morpholinopropane-1-sulfonic acid buffer, pH 7.0) and imaged with T1-weighted and 19F-MRI scans (Fig. 4a–e). All animal studies were done in accordance with protocols preapproved by the Institutional Animal Care and Use Committee of Baylor College of Medicine. Intraperitoneal injections were selected to enable comparison with previous EuII studies.20 Because intraperitoneal injections in a normal mouse are in non-hypoxic conditions, it is expected that the contrast agent would lose T1 contrast and develop 19F signal intensity upon oxidation. T1-weighted MRI images of the coronal planes of mice showed contrast that appeared immediately upon injection and faded over the course of three minutes. The duration of T1-enhancement is consistent with intraperitoneal injection of a EuII-containing cryptate.20 To detect 2EuIII post-oxidation in vivo, we collected 19F spectra of mice between T1-weighted scans. 19F signal intensity appeared nine minutes post injection (Fig. 4i). The in vivo19F spectra demonstrated that 2EuIII could be detected post-oxidation in vivo using a 19F coil of an MRI scanner. Other 19F-MRI, redox-responsive contrast agents have been demonstrated to display oxidation-dependent signal in vitro or ex vivo,50–53 but to the best of our knowledge, this is the first demonstration of detection of 19F-MR signal in vivo in a redox-responsive manner.
Encouraged by the T1 and 19F oxidation-response in vivo, we investigated the in vivo solubility and clearance by collecting a sample of urine after imaging and within one hour of injection. The urine was analyzed using coupled high-performance liquid chromatography mass spectrometry. The [M + Cl]2+ adduct of 2EuIII was observed with the correct mass-to-charge ratio and expected isotope pattern (ESI Fig. S4†). Although this technique is not quantitative, evidence of intact complex post-injection is promising for future in vivo studies with respect to minimization of toxicity from decomplexation or transmetallation.54 Furthermore, evidence of intact complex indicates that 2EuII/III remained soluble, diffused through the mouse, and was cleared intact.
Conclusions
In conclusion, we have synthesized a new redox-active contrast agent, 2EuII/III, that also displays temperature-dependent relaxivity, which was used as a tool for probing mechanism. The intramolecular fluorous interactions among p-trifluoromethylbenzylamides lead to the formation of a cage-like structure that modulates the interactions of water with Eu as a function of temperature, evidenced by the change in relaxivity. Because of the presence of fluorine atoms and amide protons, the system can be tracked in vivo after oxidation with a variety of instruments that are equipped with either CEST or 19F-MR imaging capabilities. We also observed intact complex in mouse urine post-injection, which is promising for future toxicity studies and in vivo imaging. Together, our results allude to a new molecular strategy for rationally designing ligand architectures by modulating interactions between EuII and water with intramolecular fluorous attractions. These results could pave the way to using existing imaging techniques with new chemical agents to access previously unattainable oxidative information noninvasively in vivo.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors acknowledge the National Institutes of Health for support (R01EB013663). L. A. B. is thankful for a Chemical Biology Interface research experience award (Wayne State University). We thank Kyle Blakeney, Levi Ekanger, and Michael Pirrone for technical assistance and helpful discussions. We thank the Small Animal Imaging Facility, Texas Children's Hospital, Houston, TX, and Baylor College of Medicine Small Animal MRI ATC, Houston, TX, for use of their imaging facility.References
- A. Facciabene, X. Peng, I. S. Hagemann, K. Balint, A. Barchetti, L.-P. Wang, P. A. Gimotty, C. B. Gilks, P. Lal, L. Zhang and G. Coukos, Nature, 2011, 475, 226 CrossRef CAS PubMed
.
- K. Ishikawa, K. Takenaga, M. Akimoto, N. Koshikawa, A. Yamaguchi, H. Imanishi, K. Nakada, Y. Honma and J.-I. Hayashi, Science, 2008, 320, 661 CrossRef CAS PubMed
.
- D. Shweiki, A. Itin, D. Soffer and E. Keshet, Nature, 1992, 359, 843 CrossRef CAS PubMed
.
- L. Park, P. Zhou, R. Pitstick, C. Capone, J. Anrather, E. H. Norris, L. Younkin, S. Younkin, G. Carlson, B. S. McEwen and C. Iadecola, Proc. Natl. Acad. Sci. U. S. A., 2008, 105, 1347 CrossRef CAS PubMed
.
- M. P. Mattson, Nature, 2004, 430, 631 CrossRef CAS PubMed
.
- M. T. Lin and M. F. Beal, Nature, 2006, 443, 787 CrossRef CAS PubMed
.
- K. J. Barnham, C. L. Masters and A. I. Bush, Nature Reviews, 2004, 3, 205 CAS
.
- M. Jamal-Hanjani, S. A. Quezada, J. Larkin and C. Swanton, Clin. Cancer Res., 2015, 21, 1258 CrossRef CAS PubMed
.
- J. Liu, W. Bu and J. Shi, Chem. Rev., 2017, 117, 6160 CrossRef CAS PubMed
.
- R. P. Mason, W. Rodbumrung and P. Antich, NMR Biomed., 1996, 9, 125 CrossRef CAS PubMed
.
- H. Matsushita, S. Mizukami, F. Sugihara, Y. Nakanishi, Y. Yoshioka and K. Kikuchi, Angew. Chem., Int. Ed., 2014, 53, 1008 CrossRef CAS PubMed
.
- R. Díaz-López, N. Tsapis and E. Fattal, Pharm. Res., 2009, 27, 1 Search PubMed
.
- M. Srinivas, P. A. Morel, L. A. Ernst, D. H. Laidlaw and E. T. Ahrens, Magn. Reson. Med., 2007, 58, 725 CrossRef CAS PubMed
.
- E. T. Ahrens, R. Flores, H. Xu and P. A. Morel, Nat. Biotechnol., 2005, 23, 983 CrossRef CAS PubMed
.
- E. De Luca, P. Harvey, K. H. Chalmers, A. Mishra, P. K. Senanayake, J. I. Wilson, M. Botta, M. Fekete, A. M. Blamire and D. Parker, J. Biol. Inorg. Chem., 2014, 19, 215 CrossRef PubMed
.
- L. A. Basal, Y. Yan, Y. Shen, E. M. Haacke, M. Mehrmohammadi and M. J. Allen, ACS Omega, 2017, 2, 800 CrossRef CAS PubMed
.
- L. A. Ekanger, D. R. Mills, M. M. Ali, L. A. Polin, Y. Shen, E. M. Haacke and M. J. Allen, Inorg. Chem., 2016, 55, 9981 CrossRef CAS PubMed
.
- P. Caravan, É. Tóth, A. Rockenbauer and A. E. Merbach, J. Am. Chem. Soc., 1999, 121, 10403 CrossRef CAS
.
- L. A. Ekanger, M. M. Ali and M. J. Allen, Chem. Commun., 2014, 50, 14835 RSC
.
- L. A. Ekanger, L. A. Polin, Y. Shen, E. M. Haacke and M. J. Allen, Contrast Media Mol. Imaging, 2016, 11, 299 CrossRef CAS PubMed
.
- L. A. Ekanger, L. A. Polin, Y. Shen, E. M. Haacke, P. D. Martin and M. J. Allen, Angew. Chem., Int. Ed., 2015, 54, 14398 CrossRef CAS PubMed
.
- C. U. Lenora, F. Carniato, Y. Shen, Z. Latif, E. M. Haacke, P. D. Martin, M. Botta and M. J. Allen, Chem.–Eur. J., 2017 DOI:10.1002/chem.201702158
.
- S. Mizukami, R. Takikawa, F. Sugihara, Y. Hori, H. Tochio, M. Wälchli, M. Shirakawa and K. Kikuchi, J. Am. Chem. Soc., 2008, 130, 794 CrossRef CAS PubMed
.
- T. Nakamura, H. Matsushita, F. Sugihara, Y. Yoshioka, S. Mizukami and K. Kikuchi, Angew. Chem., Int. Ed., 2015, 54, 1007 CrossRef CAS PubMed
.
- A. Keliris, I. Mamedov, G. E. Hagberg, N. K. Logothetis, K. Scheffler and J. Engelmann, Contrast Media Mol. Imaging, 2012, 7, 478 CrossRef CAS PubMed
.
- T. Sakamoto, Y.-k. Shimizu, J. Sasaki, H. Hayakawa and K. Fujimoto, Bioorg. Med. Chem. Lett., 2011, 21, 303 CrossRef CAS PubMed
.
- X. Yue, Z. Wang, L. Zhu, Y. Wang, C. Qian, Y. Ma, D. O. Kiesewetter, G. Niu and X. Chen, Mol. Pharmaceutics, 2014, 11, 4208 CrossRef CAS PubMed
.
- K. Srivastava, E. A. Weitz, K. L. Peterson, M. Marjańska and V. C. Pierre, Inorg. Chem., 2017, 56, 1546 CrossRef CAS PubMed
.
- L. A. Ekanger, L. A. Basal and M. J. Allen, Chem.–Eur. J., 2017, 23, 1145 CrossRef CAS PubMed
.
- J. R. Slack and M. Woods, J. Biol. Inorg Chem., 2014, 19, 173 CrossRef CAS PubMed
.
- T. Mani, A. C. L. Opina, P. Zhao, O. M. Evbuomwan, N. Milburn, G. Tircsó, C. Kumas and A. D. Sherry, J. Biol. Inorg Chem., 2014, 19, 161 CrossRef CAS PubMed
.
- T. Mani, G. Tircso, P. Zhao, A. D. Sherry and M. Woods, Inorg. Chem., 2009, 48, 10338 CrossRef CAS PubMed
.
- B. N. Siriwardena-Mahanama and M. J. Allen, Molecules, 2013, 18, 9352 CrossRef CAS PubMed
.
- L. Burai, É. Tóth, S. Seibig, R. Scopelliti and A. E. Merbach, Chem.–Eur. J., 2000, 6, 3761 CrossRef CAS
.
- J. Garcia and M. J. Allen, Inorg. Chim. Acta, 2012, 393, 324 CrossRef CAS PubMed
.
- J. Garcia, A. N. W. Kuda-Wedagedara and M. J. Allen, Eur. J. Inorg. Chem., 2012, 2135 CrossRef CAS PubMed
.
- J. Garcia, J. Neelavalli, E. M. Haacke and M. J. Allen, Chem. Commun., 2011, 47, 12858 RSC
.
- L. Burai, R. Scopelliti and É. Tóth, Chem. Commun., 2002, 2366 RSC
.
- L. Burai, É. Tóth, G. Moreau, A. Sour, R. Scopelliti and A. E. Merbach, Chem.–Eur. J., 2003, 9, 1394 CrossRef CAS PubMed
.
- P. Caravan, J. J. Ellison, T. J. McMurry and R. B. Lauffer, Chem. Rev., 1999, 99, 2293 CrossRef CAS PubMed
.
- K. H. Chalmers, E. De Luca, N. H. M. Hogg, A. M. Kenwright, I. Kuprov, D. Parker, M. Botta, J. I. Wilson and A. M. Blamire, Chem.–Eur. J., 2010, 16, 134 CrossRef CAS PubMed
.
- A. L. Thompson, D. Parker, D. A. Fulton, J. A. K. Howard, S. U. Pandya, H. Puschmann, K. Senanayake, P. A. Stenson, A. Badari, M. Botta, S. Avedano and S. Aime, Dalton Trans., 2006, 5605 RSC
.
- L. M. Matosziuk, J. H. Leibowitz, M. C. Heffern, K. W. MacRenaris, M. A. Ratner and T. J. Meade, Inorg. Chem., 2013, 52, 12250 CrossRef CAS PubMed
.
- S. Aime, A. Barge, M. Botta, A. S. De Sousa and D. Parker, Angew. Chem., Int. Ed., 1998, 37, 2673 CrossRef CAS
.
- S. Aime, A. Barge, J. I. Bruce, M. Botta, J. A. K. Howard, J. M. Moloney, D. Parker, A. S. de Sousa, D. Parker and M. Woods, J. Am. Chem. Soc., 1999, 121, 5762 CrossRef CAS
.
- R. S. Dickins, J. A. K. Howard, C. W. Lehmann, J. Moloney, D. Parker and R. Peacock, Angew. Chem., Int. Ed., 1997, 36, 521 CrossRef CAS
.
- R. S. Dickins, J. A. K. Howard, C. L. Maupin, J. M. Moloney, D. Parker, J. P. Riehl, G. Siligardi and J. A. G. Williams, Chem.–Eur. J., 1999, 5, 1095 CrossRef CAS
.
- O. M. Evbuomwan, J. Lee, M. Woods and A. D. Sherry, Inorg. Chem., 2014, 53, 10012 CrossRef CAS PubMed
.
- S. Viswanathan, S. J. Ratnakar, K. N. Green, Z. Kovacs, L. M. De León-Rodríguez and A. D. Sherry, Angew. Chem., Int. Ed., 2009, 121, 9494 CrossRef
.
- D. Xie, T. L. King, A. Banerjee, V. Kohli and E. L. Que, J. Am. Chem. Soc., 2016, 138, 2937 CrossRef CAS PubMed
.
- D. Xie, S. Kim, V. Kohli, A. Banerjee, M. Yu, J. S. Enriquez, J. J. Luci and E. L. Que, Inorg. Chem., 2017, 56, 6429 CrossRef CAS PubMed
.
- K. Tanabe, H. Harada, M. Narazaki, K. Tanaka, K. Inafuku, H. Komatsu, T. Ito, H. Yamada, Y. Chujo, T. Matsuda, M. Hiraoka and S.-i. Nishimoto, J. Am. Chem. Soc., 2009, 131, 15982 CrossRef CAS PubMed
.
- M. Yu, D. Xie, K. P. Phan, J. S. Enriquez, J. J. Luci and E. L. Que, Chem. Commun., 2016, 52, 13885 RSC
.
- J.-M. Idée, M. Port, I. Raynal, M. Schaefer, S. Le Greneur and C. Corot, Fundam. Clin. Pharmacol., 2006, 20, 563 CrossRef PubMed
.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 1562330 and 1562331. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c7sc03142d |
| This journal is © The Royal Society of Chemistry 2017 |