Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Highly selective acylation of polyamines and aminoglycosides by 5-acyl-5-phenyl-1,5-dihydro-4H-pyrazol-4-ones†
Kostiantyn O.
Marichev
 ,
Estevan C.
Garcia
,
Kartick C.
Bhowmick
,
Daniel J.
Wherritt
,
Estevan C.
Garcia
,
Kartick C.
Bhowmick
,
Daniel J.
Wherritt
 ,
Hadi
Arman
and
Michael P.
Doyle
,
Hadi
Arman
and
Michael P.
Doyle
 *
*
Department of Chemistry, The University of Texas at San Antonio, San Antonio, Texas 78249, USA. E-mail: michael.doyle@utsa.edu
First published on 30th August 2017
Abstract
5-Acyl-5-phenyl-1,5-dihydro-4H-pyrazol-4-ones, accessible from arylpropargyl phenyldiazoacetates, are highly selective acyl transfer reagents for di- and polyamines, as well as aminoalcohols and aminothiols. As reagents with a carbon-based leaving group, they have been applied for benzoyl transfer with a broad selection of substrates containing aliphatic amino in combination with other competing nucleophilic functional groups. The substrate scope and levels of selectivity for direct benzoyl transfer exceed those of known benzoylating reagents. With exceptional selectivity for acylation between primary amines bound to primary and secondary carbons, these new reagents have been used in direct site-selective monobenzoylation of aminoglycoside antibiotics.
Introduction
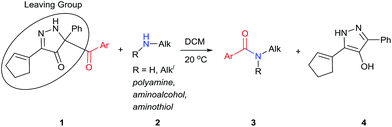
The formation of an amide bond by acyl transfer is a classic chemical reaction1 that has been extensively studied2 and widely applied.3 Over the years numerous acyl transfer agents have been investigated; their activities have been dependent on the leaving group, which has ranged from those of the highly reactive acyl chlorides and anhydrides to those that are conjugate bases of relatively weak acids. The linkage to the acyl group has included N-imidazolyl4a–g and N-benzotriazolyl,4h–m succinimidyloxy,5 cyanide,6 sulfonamide,7 trichloromethyl,8 enolates,9 and, most recently, boron trifluoride.10 Selectivity in acyl transfer has become an important goal with achievements evolving beyond chemoselection (OH vs. SH vs. NH acylation)11 to selective acylation of primary amines over secondary amines.10,11 Although the use of enzymes12 or RNA-based aptameric protective groups13 for highly regio- and site-selective acylation of di- and polyamines has been reported, small molecule acyl transfer reagents that provide high to exclusive regiocontrol remain under-investigated.Moderate to high,10,14 and even exclusive,10a,14d,e selectivities in the acylation of the primary over the secondary amine in aliphatic diamines have been reported. Secondary amines are more basic, but they are also more sterically encumbered and, thereby, subject to steric restrictions. Although high selectivity has been reported for acylation of the amino group at the 1-position of 1,2-diaminopropane,14d selective acylation of primary amines whose carbon attachment is primary, secondary or tertiary, has received scant attention.15
We have recently prepared a novel heterocyclic compound that appeared to have the potential of being a selective benzoyl transfer reagent. This compound, 5-benzoyl-3-(cyclopent-1-en-1-yl)-5-phenyl-1,5-dihydro-4H-pyrazol-4-one (BCPP, 1a), which is formed in one step from a propargyl phenyldiazoacetate by gold(I) catalysis,16 has its benzoyl group attached to carbon-5 of a pyrazolone ring so that the conjugate base of hydroxypyrazole is the leaving group (Scheme 1). Acyl transfer reagents that, like BCPP, have carbon-linked leaving groups, principally cyano,6,14a trichloromethyl,8 2-imidazolyl,11f,17 among a few others,14d,18 generally exhibit higher chemoselectivities than their heteroatom-linked counterparts. However, to our knowledge, acylating agents whose leaving group undergoes aromatization upon acyl transfer (transformation of 1 to 4, Scheme 1) have not been reported.
Results and discussion
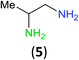
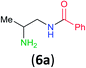
Having established that BCPP undergoes quantitative stoichiometric benzoylation of simple aliphatic amines (benzyl- and cyclohexyl-amines)16 at room temperature in dichloromethane, we investigated the benzoylation of 1,2-diaminopropane (5) by BCPP to determine its selectivity for benzoylation of the primary amino group attached to either a primary or secondary carbon atom (Scheme 2). The addition of BCPP to 5 resulted in the formation of a single regioisomer 6a (1H NMR analysis) in 98% isolated yield along with 4-hydroxypyrazole 4. Neither the 2-benzoylamido-1-aminopropane (6b) nor the dibenzoylated 1,2-propanediamine were detected. For a more precise analysis, however, the reaction mixture was subjected to HPLC analysis using as standards a mixture of mono- and dibenzoylated compounds formed from unselective benzoylation of 5 by benzoyl chloride. HPLC analysis of the reaction mixture from the BCPP reaction detected a minor amount of 6b and showed the ratio 6a/6b to be 98.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 (average of two experiments, ±0.3%); dibenzoylated 1,2-diaminopropane was not detected. Previously, Lewis acid activated N-acyl-2-oxazolidinone gave benzoylated products in 83% yield with a 6a/6b/dibenzoylation ratio determined by 1H NMR of 85
1.5 (average of two experiments, ±0.3%); dibenzoylated 1,2-diaminopropane was not detected. Previously, Lewis acid activated N-acyl-2-oxazolidinone gave benzoylated products in 83% yield with a 6a/6b/dibenzoylation ratio determined by 1H NMR of 85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7
7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8,15 and with an α-aryl-β-ketoester benzoyl transfer agent 100% selectivity for the formation of 6a was reported by 1H NMR analysis but was not confirmed by HPLC analysis.14d
8,15 and with an α-aryl-β-ketoester benzoyl transfer agent 100% selectivity for the formation of 6a was reported by 1H NMR analysis but was not confirmed by HPLC analysis.14d
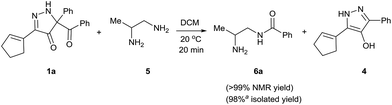 | ||
| Scheme 2 Site-selective benzoylation of 1,2-propanediamine (5) by BCPP. aAverage yield obtained from two parallel runs (±1%). | ||
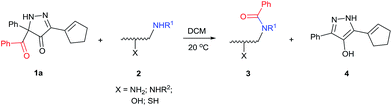
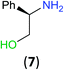
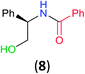
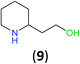
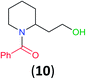
With the high regioselectivity established in the benzoylation of 1,2-diaminopropane, other examples that would illustrate the selectivity of BCPP were chosen. The results provided in Table 1 illustrate the substrate scope of selective benzoylation for aminoalcohols, aminothiols, and di- and polyamines by reactions with stoichiometric amounts of BCPP at room temperature in dichloromethane. (R)-2-Amino-2-phenylethan-1-ol (7) containing both primary amino and hydroxyl groups reacts with BCPP exclusively at the amino functionality and affords amide 8 in quantitative yield. In contrast, direct acylation of 7 by benzoyl chloride provides 8 in 68–85% yields along with the diacylated product.19 2-(Piperidin-2-yl)ethan-1-ol (9), as an example with the combination of secondary amino and primary hydroxyl groups, affords only N-benzoylated product 10 from the reaction with BCPP in 95% isolated yield, whereas benzoyl chloride forms 10 in only 82% yield;20 however, the reaction of 9 with BCPP is sluggish, and complete transfer occurs only after 12 h.
| Entrya | Reactant | Product | NMR yieldb (%) | Isolated yieldc (%) |
|---|---|---|---|---|
a Reactions were carried out on a 1.0 mmol scale: to a stirred solution of amine in 8 mL DCM a solution of acylating reagent 1a in 10 mL DCM was added dropwise over 20 min at 20 °C. The resulting reaction mixture was allowed to stir at 20 °C for 30 min.
b Yields were determined by 1H NMR analysis of reaction mixtures with 1,3,5-trimethoxybenzene as the internal standard.
c Yields calculated from the mass of chromatographed products as average of two runs with a deviation of ±1%.
d Reaction time was 12 h.
e Reaction time was 4 h.
f A commercial mixture of cis- and trans-isomers was used (trans/cis = 85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15).
g C18-reversed phase silica gel was used to purify the product.
h Two equivalents of 1a were used to avoid the formation of a mixture of two monoacylation products; reaction time 24 h.
i Ratio 32a/32b = 86 15).
g C18-reversed phase silica gel was used to purify the product.
h Two equivalents of 1a were used to avoid the formation of a mixture of two monoacylation products; reaction time 24 h.
i Ratio 32a/32b = 86![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 14 determined by 1H NMR as an average of two runs (±0.6%). 14 determined by 1H NMR as an average of two runs (±0.6%).
|
||||
| 1 |
|
|
>99 | 98 |
| 2 |
|
|
>99 | >99 |
| 3d |
|
|
97 | 95 |
| 4 |

|
|
>99 | 97 |
| 5e |
|
|
99 | 93 |
| 6 |
|
|
>99 | 98 |
| 7 |

|
|
>99 | 98 |
| 8 |
|
|
>99 | 94 |
| 9 |
|
|
>99 | >99 |
| 10f |
|
|
>99 | 97 |
| 11 |
|
|
>99 | >99 |
| 12g |
|
|
98 | 93 |
| 13h |
|
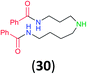
|
99 | 98 |
| 14i |
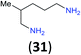
|
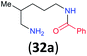
|
99 | 95 |
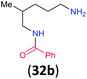
|
32a/32b 86![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 14 14 |
|||
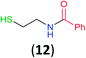
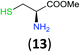
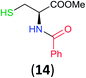
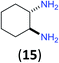
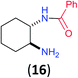
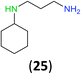
Like aminoalcohols, aminothiols 11 and 13 react with BCPP selectively, giving only the products from benzoylation of the amino group (97% yield for 12, and 92% isolated yield for 14 without evidence of any benzoylation of the sulfhydryl group). A previous report described the formation of 12 in 93% yield using benzoyl chloride, but only 67% of 14 was obtained when benzoic anhydride was used.11d Only monobenzoylation of trans-1,2-diaminocyclohexane (15) or 1,3-diaminopropane (17) occurred with BCPP (both obtained in 98% isolated yield); monoamide 16 was obtained previously in 90% yield (95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 monobenzoylation
5 monobenzoylation![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) dibenzoylation), and 18 was obtained in 80% yield with a ratio 18 to dibenzoylated product of 83
dibenzoylation), and 18 was obtained in 80% yield with a ratio 18 to dibenzoylated product of 83![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
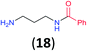
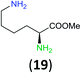
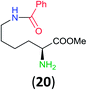
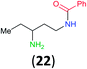
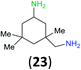
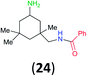
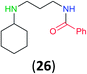
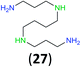
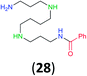
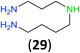
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 17 using complexation with 9-BBN to supress dibenzoylation.21 Exclusive benzoylation of the primary amine bonded to a primary carbon occurred in compounds 19, 21, and 23, where there was a choice with benzoylation of the primary amine bonded to a secondary carbon. Highly selective direct benzoylation of L-lysine methyl ester (19) at the terminal amino group formed 20 in 94% isolated yield. Scaling the reaction up to 1 g of 19·2HCl afforded 20 as the only product, which was isolated in 95% yield. Two additional substrates with primary amino groups attached to both primary and secondary carbons have been examined: selective benzoylation of 1,3-diaminopentane (21) by BCPP produced monoamide 22 in quantitative yield, and this is similar to the reported15 benzoylation of 21 by N-benzoyl-2-oxazolidinone catalysed by group IV-metals (98% yield of 3g); benzoylation of commercial isophorone diamine (23) by BCPP afforded monoamide 24 in 97% isolated yield. For di- and polyamines with selection between primary and secondary amines, benzoylation with BCPP occurred exclusively at the primary amine. Indirect benzoylation of 25 by benzoyl chloride using TMS-protection-deprotection was reported to give 26 in 94% yield,22 and the dibenzoylation of spermidine 29 was previously reported to have occurred in 92% yield using two equivalents of benzoyl cyanide (with HCN generation).14a Finally, in an extension of regioselectivity to a choice between two primary amines that are both bonded to primary carbons, 2,5-diamino-2-methylpentane (31) underwent BCPP benzoylation at the 5-position with an 86
17 using complexation with 9-BBN to supress dibenzoylation.21 Exclusive benzoylation of the primary amine bonded to a primary carbon occurred in compounds 19, 21, and 23, where there was a choice with benzoylation of the primary amine bonded to a secondary carbon. Highly selective direct benzoylation of L-lysine methyl ester (19) at the terminal amino group formed 20 in 94% isolated yield. Scaling the reaction up to 1 g of 19·2HCl afforded 20 as the only product, which was isolated in 95% yield. Two additional substrates with primary amino groups attached to both primary and secondary carbons have been examined: selective benzoylation of 1,3-diaminopentane (21) by BCPP produced monoamide 22 in quantitative yield, and this is similar to the reported15 benzoylation of 21 by N-benzoyl-2-oxazolidinone catalysed by group IV-metals (98% yield of 3g); benzoylation of commercial isophorone diamine (23) by BCPP afforded monoamide 24 in 97% isolated yield. For di- and polyamines with selection between primary and secondary amines, benzoylation with BCPP occurred exclusively at the primary amine. Indirect benzoylation of 25 by benzoyl chloride using TMS-protection-deprotection was reported to give 26 in 94% yield,22 and the dibenzoylation of spermidine 29 was previously reported to have occurred in 92% yield using two equivalents of benzoyl cyanide (with HCN generation).14a Finally, in an extension of regioselectivity to a choice between two primary amines that are both bonded to primary carbons, 2,5-diamino-2-methylpentane (31) underwent BCPP benzoylation at the 5-position with an 86![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 14 monobenzoylation selectivity.
14 monobenzoylation selectivity.
We previously reported that BCPP undergoes slow benzoylation of methanol only at 65 °C so that after 12 h methyl benzoate is obtained in 63% yield.16 Accordingly, we examined the reactivity of BCPP with other nucleophiles. Benzoyl transfer from 1a to benzyl mercaptan and aniline does not occur in dichloromethane at 20 °C; reactions at 80 °C for 24 h in 1,2-dichloroethane afforded S-benzyl benzothioate and N-benzoylaniline, but only in 22% and 11% yield, respectively, the remainder being unreacted starting materials. Obviously, the basicity of the nucleophile is an important factor in determining reactivity in reactions of 1a with nucleophiles.
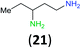
To further assess the selectivity observed for benzoyl transfer from BCPP, the kinetic rate law and rate constants were determined for a series of structurally different aliphatic mono- and diamines by UV/Vis-spectroscopy. The reactions were carried out under pseudo-first-order conditions in dichloromethane at 24 °C and were determined to be second order reactions from reactions performed for each amine with five different amine:BCPP ratios using standard kinetic methods.23,24Fig. 1 provides a comparison of second order rate constants obtained for the reaction of BCPP with 2-amino-2-methylpropane, diethylamine, 2-aminobutane, 1-aminobutane, 2,5-diamino-2-methylpentane, 1,2-diaminopropane, 1,3-diaminopropane, and 1,3-diaminopentane. Their rate constants indicate that steric factors are the major determinants of selectivity in benzoyl transfer from BCPP; there is no correlation with nucleophilicity or basicity of the amine. Among the monoamines the fastest reaction rate was observed for 1-aminobutane (k = 0.86 ± 0.04 L mol−1 s−1) as the least sterically hindered amine; the introduction of an α-methyl substituent into the aliphatic chain (2-aminobutane) decreases the reaction rate by a factor of 18 (k = 0.049 ± 0.002 L mol−1 s−1). The presence of two methyl substituents in the α-position of a primary amine (2-amino-2-methylpropane) makes the reaction about 200 times slower (k = 0.0042 ± 0.0002 L mol−1 s−1) than that for 1-aminobutane. Although diethylamine is the most nucleophilic amine in the series of monoamines tested, its rate constant for reaction with BCPP (k = 0.025 ± 0.001 L mol−1 s−1) is 34 times slower than that for 1-aminobutane, and two times slower than that for 2-aminobutane.
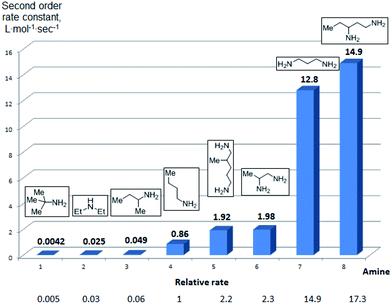 | ||
| Fig. 1 Second-order rate constants for the reaction of BCPP with mono- and diamines in DCM at 24 °C. | ||
Aliphatic diamines are more reactive than monoamines. While 1,2-diaminopropane (5) (k = 1.98 ± 0.05 L mol−1 s−1) and 2,5-diamino-2-methylpentane (31) (k = 1.92 ± 0.04 L mol−1 s−1) react with BCPP about two times faster than 1-aminobutane, the reaction rate constants with 1,3-diaminopropane (k = 12.8 ± 0.5 L mol−1 s−1) and 1,3-diaminopentane (k = 14.9 ± 0.3 L mol−1 s−1) are 15–17 times greater. These results are in accord with the second amino group serving as a base to remove a proton from nitrogen in the process of acyl transfer.
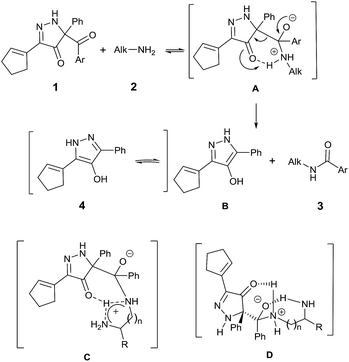
Consistent with established mechanisms for acyl transfer,25 BCPP reacts with monoamine 2 to form the classic tetrahedral zwitterionic intermediate A, which then undergoes intramolecular proton transfer to the Lewis basic carbonyl oxygen concurrent with C–C bond cleavage yielding amide 3 and 4-hydroxypyrazole 4 (Fig. 2).
This mechanism fits the model that describes initial reversible formation of A followed by rate-determining product formation.26
Reactions of BCPP with 1,(n + 1)-diamines add another possible dimension to this mechanism of reaction with intramolecular proton transfer to the second amine (C) and/or intramolecular stabilization of the tetrahedral intermediate (D) to account for their dramatic changes in reaction rate. The large rate increases observed with 1,3-diaminopropane and 1,3-diaminopentane are consistent with intramolecular stabilization of D.
Evaluation of kinetic substituent effects for benzoyl transfer was made with 5-aroyl-3-(cyclopent-1-en-1-yl)-5-phenyl-1,5-dihydro-4H-pyrazol-4-ones having different substituents in the para-position of the aromatic ring for reactions with 1-aminobutane. Second order rate constants for these reactions with three para-substituted acyl transfer reagents 1a–d have been determined. Electron-withdrawing substituents increased the rate of reaction, whereas electron-donating substituents decreased the rate of reaction. A Hammett plot using σ-values gave ρ = +1.42 (Fig. 3).
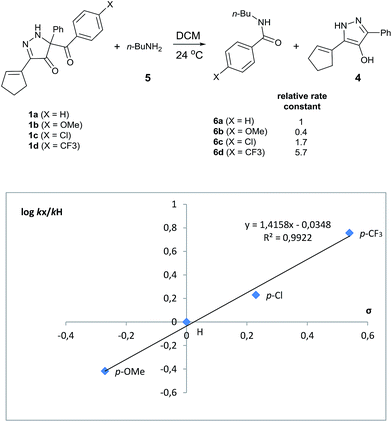 | ||
| Fig. 3 Hammett plot for the reaction of para-substituted acyl transfer reagents 1a–d with 1-aminobutane in DCM at 24 °C. | ||
Site selective O-acylation of amphotericin by para-substituted benzoylpyridinium chlorides was reported to occur with ρ = +1.69, and a calculated ρ = −0.395 projected the kinetic selectivity of acyl transfer from the corresponding acylpyridinium complexes to one hydroxyl group versus the four others in the same molecule (site selectivity = 48% with p-Cl and 64% with p-OMe).27 However, substituent effects by the BCPP acylating reagent do not have a significant influence on selectivity between two primary amino groups. As reported in Table 1, 2,5-diamino-2-methylpentane (31) underwent BCPP benzoylation at the 5-position with 86![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 14 selectivity.
14 selectivity.
Although kinetic studies showed significant differences in rates for a series of para-substituents in the benzoyl group of 1 (Fig. 3), the ratios 32a/32b were similar: 88![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12 (±0.6%) for 31 with the MeO-substituent (yield 32a + 32b 92%) and 85
12 (±0.6%) for 31 with the MeO-substituent (yield 32a + 32b 92%) and 85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15 (±0.4%) for 31 with the Cl-substituent (yield 32a + 32b 90%).
15 (±0.4%) for 31 with the Cl-substituent (yield 32a + 32b 90%).
Selective acylation of aminoglycosides as free bases has been particularly challenging.28 Previous methods for the preparation of monoacyl derivatives have required either protecting groups or the acidification of the amino group(s) in aminoglycosides.28,29 Moreover, those methods afforded pure products in only 20–40% isolated yields. Although modern methods for acylation of 6′-position of kanamycin, tobramycin and amikacin as sulfate salts using N-hydroxysuccinymyl (NHS) esters have been reported30 with yields up to 91%, the scope of this methodology is limited to substituted acetyl derivatives. Selective introduction of acetyl groups into aminoglycosides using chemoenzymatic methods31 or RNA-based aptameric groups13 have been also reported. However, to our knowledge, there have not been reports of direct selective benzoylation of aminoglycoside antibiotics. Reported direct benzoylations of amikacin by 4-vinylbenzoylating reagents having chloride, p-nitrophenoxy, and O-(N-succinimidyl) leaving groups were not selective, giving a mixture of three products.32
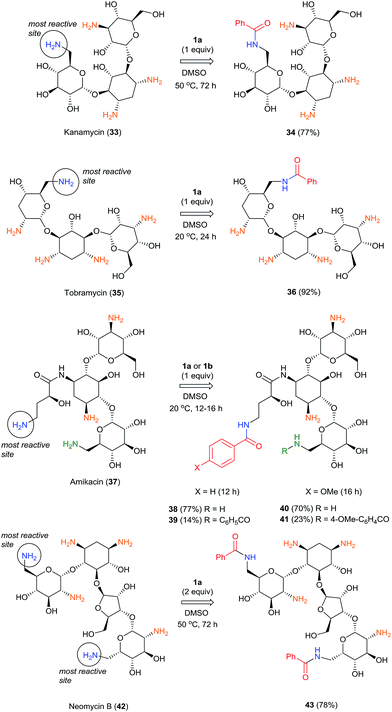
The highly selective reactions of kanamycin (33), tobramycin (35) and amikacin (37) as free bases with one equivalent of BCPP have been successfully performed in dimethylsulfoxide solutions (Scheme 3).
While the reactions with 35 and 37 occur at room temperature for 12–24 h, full conversion of 33 was observed only after 72 h at 50 °C, apparently because of low solubility of 33 in DMSO. Monobenzoylated 34 and 36 were obtained as single regioisomers in 77% and 92% isolated yields, respectively. The more complex and challenging molecule of amikacin (37) has two amino groups attached to primary carbon atoms. However, the γ-amino-β-hydroxybutyryl group (AHB) is much more reactive with BCPP than is the competitive aminomethylglycoside. As the result, with 1.0 equiv. of BCPP 37 affords monobenzoyl derivative 38 as a single monobenzoyl regioisomer in 77% isolated yield, however 14% of dibenzoylated product 39 was also isolated. The use of 2.0 equiv. of BCPP with 37 forms only dibenzoylated 39 in 90% isolated yield; however, the reaction goes to completion only after 20 h. To study the substituent effect in the benzoylation of 37, the less reactive 4-OMe-BCPP 1b was used. Although the reaction was slower (16 h), selectivity toward monoacylation (70% of mono- 40 and 23% of dibenzoyl product 41) was lower than with BCPP 1a. Neomycin B (42), which has two amino groups attached to primary carbon atoms that are predicted to have the same reactivity, reacted with 2.0 equiv. of BCPP to afford the dibenzoylated product 43 in 78% isolated yield, but only after 72 h at 50 °C. The same reaction with 1.0 equiv. of BCPP led to an inseparable mixture of two monobenzoylated products and 43. The structures of all benzoylated aminoglycosides were confirmed by 1D- and 2D-NMR experiments.24
In all benzoylation reactions of aminoglycosides with BCPP unreacted starting materials were the only residue and no benzoylated regioisomers were detected in the reaction mixtures by NMR or HPLC analyses. A reason for incomplete benzoylation is that is decomposition of BCPP, caused by proton transfer, occurs. In protic solvents BCPP undergoes irreversible intramolecular acyl transfer to form aromatic compound 44 (Fig. 4). X-Ray analysis showed that the benzoyl group in 44 is attached to the oxygen atom. The kinetics of the transformation 1a → 44 gave first order rate constants: k = 7.1×10−5 ± 0.3×10−5 s−1 (in MeOH/DCM 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and k = 2.45×10−4 ± 0.05×10−4 s−1 (in THF/H2O 1
1) and k = 2.45×10−4 ± 0.05×10−4 s−1 (in THF/H2O 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1).24 To confirm that 44 is not an active benzoylating reagent the reaction of 1,2-diaminopropane with 1.0 equiv. of 44 was carried out in DCM at r.t. for 24 h, and there was no reaction occurred.
1).24 To confirm that 44 is not an active benzoylating reagent the reaction of 1,2-diaminopropane with 1.0 equiv. of 44 was carried out in DCM at r.t. for 24 h, and there was no reaction occurred.
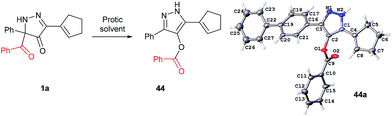 | ||
| Fig. 4 Intramolecular benzoyl transfer of BCPP in protic solvents. X-ray structure of 44a with 50% thermal ellipsoid probability. | ||
Selective benzoylation of aminoglycosides by BCPP also occurs in methanol/water or THF/water solvent mixtures. The reactions occur faster (2–3 h); however, they occur with lower product yields caused by the competing reaction of 1a → 44 (Fig. 4).
Tobramycin (35) showed only 12% conversion with BCPP in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 THF/water to form mono benzoylated tobramycin (36) exclusively in 2 h; amikacin (37) underwent 23% conversion in THF/water 1
1 THF/water to form mono benzoylated tobramycin (36) exclusively in 2 h; amikacin (37) underwent 23% conversion in THF/water 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to give a mono- to di-benzoylated product ratio 38/39 = 7
1 to give a mono- to di-benzoylated product ratio 38/39 = 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 (determined by HPLC). Reactions in methanol/water 1
2 (determined by HPLC). Reactions in methanol/water 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 went to completion in 3 h and gave higher conversions of aminoglycosides: 35 → 36 (42% yield), 37 → 38 + 39 (58% yield, 38/39 = 5
1 went to completion in 3 h and gave higher conversions of aminoglycosides: 35 → 36 (42% yield), 37 → 38 + 39 (58% yield, 38/39 = 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 by HPLC). The use of 2.0 equiv. of BCPP in 1
2 by HPLC). The use of 2.0 equiv. of BCPP in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 methanol/water afforded 38 + 39 in 83% yield in 3 h (38/39 = 5
1 methanol/water afforded 38 + 39 in 83% yield in 3 h (38/39 = 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 by HPLC). The reduced percent conversions also indicate that compound 44, which is formed competitively under these conditions, is not a benzoyl transfer reagent.
2 by HPLC). The reduced percent conversions also indicate that compound 44, which is formed competitively under these conditions, is not a benzoyl transfer reagent.
Conclusions
In summary, we have discovered a new, efficient and broadly applicable carbon-based heterocyclic acyl transfer reagent, 5-acyl-5-phenyl-1,5-dihydro-4H-pyrazol-4-one (BCPP), that is highly selective for the synthesis of N-monobenzoyl (or substituted N-monobenzoyl) derivatives of di- and polyamines, as well as aminoalcohols and aminothiols. The BCPP reagent generally achieves levels of selectivity that significantly exceed those of previously reported acylating reagents or provide access to new compounds that are inaccessible by alternative acylation methods. Moreover, 5-acyl-5-phenyl-1,5-dihydro-4H-pyrazol-4-ones are rare examples of small molecule acylating reagents that are able to achieve very high levels of selectivity for the monoacylation of polyamines. Kinetic studies of a series of mono- and diamines, as well as the low reactivity of alcohols and thiols with 1a, are consistent with the selectivities that have been obtained. Site-specific selectivity in direct benzoylation of aminoglycoside antibiotics has been demonstrated.Experimental
General procedure for benzoyl transfer using BCPP (1a)
To a stirred solution of an amine 2 (0.50 mmol) in DCM (5 mL) a solution of BCPP (1a) (0.50 mmol) in DCM (5 mL) was added at 20 °C via syringe pump over 20 min, and the reaction mixture was stirred for 0.5–12 h. A white precipitate of 4-hydroxypyrazole 4 formed during the benzoyl transfer process was filtered, the solvent was evaporated, and the monoamide 3 was purified by flash chromatography on silica gel (with a 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 gradient of DCM/methanol to pure methanol as eluents), unless specified otherwise. Products were characterized by NMR spectroscopy, and their purity was determined by HPLC and/or NMR analyses.
1 gradient of DCM/methanol to pure methanol as eluents), unless specified otherwise. Products were characterized by NMR spectroscopy, and their purity was determined by HPLC and/or NMR analyses.
Synthetic procedure for the benzoylation of aminoglycosides by BCPP
To a screw-capped 8 mL vial an aminoglycoside (0.2 mmol), BCPP (0.2 mmol), and DMSO (1.5 mL) were added sequentially. The mixture was stirred for 12–24 h at 20 °C (for 35 and 37) and 72 h at 50 °C (for 34 and 42). The completion of the reaction was monitored by TLC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 hexane/ethyl acetate as eluent, monitoring the conversion of BCPP to 4). Benzoylated aminoglycosides were purified by column chromatography on silica gel (with a gradient of methanol/28% aqueous ammonium hydroxide 100/0–90/10–80/20 as eluents). Products were characterized by NMR spectroscopy, and their purity was determined by HPLC and/or NMR analyses.
1 hexane/ethyl acetate as eluent, monitoring the conversion of BCPP to 4). Benzoylated aminoglycosides were purified by column chromatography on silica gel (with a gradient of methanol/28% aqueous ammonium hydroxide 100/0–90/10–80/20 as eluents). Products were characterized by NMR spectroscopy, and their purity was determined by HPLC and/or NMR analyses.
Kinetic determinations for benzoyl transfer by BCPP24
Rate constants for the reaction of BCPP with aliphatic amines were determined using UV/Vis-spectroscopy at 24 °C. Kinetic runs were performed for the reaction of BCPP with a series of amine solutions of different concentrations (Camine ≥ 10CBCPP). Considering that the extinction coefficient determined for BCPP ε = 2920 at 380 nm, BCPP solutions were prepared to have an absorbance at 380 nm A = 0.4–0.8. The concentrations of amine solutions (Camine ≥ 10CBCPP) were those that allowed reaction times less than 1 h. Second order constants were calculated from the slopes of the linear plot of k (pseudo-first order) vs. C (amine).Kinetic determination for the intramolecular benzoyl transfer of BCPP24
A series of BCPP solutions of different concentrations were prepared in 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 MeOH/DCM or THF/H2O, and the reaction course was monitored by UV/Vis-spectroscopy from the consumption of BCPP at 400 nm. First order rate constants were determined from the slopes of the linear plots ln
1 MeOH/DCM or THF/H2O, and the reaction course was monitored by UV/Vis-spectroscopy from the consumption of BCPP at 400 nm. First order rate constants were determined from the slopes of the linear plots ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) A vs. time.
A vs. time.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
Support for this research from the Welch Foundation (AX-1871) is gratefully acknowledged. K. C. B. thanks University Grants Commission, Government of India, Raman Foundation for fellowship. The 500 MHz NMR instrument used in this study is supported by a grant from the National Science Foundation (CHE-1625963).Notes and references
- (a) C. Schotten, Ber. Dtsch. Chem. Ges., 1884, 17, 2544 CrossRef; (b) E. Baumann, Ber. Dtsch. Chem. Ges., 1886, 19, 3218 CrossRef; (c) E. Fischer, Ber. Dtsch. Chem. Ges., 1903, 36, 2982 CrossRef CAS.
- (a) A. Williams and K. T. Douglas, Chem. Rev., 1975, 75, 627 CrossRef CAS; (b) V. R. Pattabiraman and J. W. Bode, Nature, 2011, 480, 471 CrossRef CAS PubMed.
- (a) P. R. Schreiner and C. E. Müller, Angew. Chem., Int. Ed., 2011, 50, 6012 CrossRef PubMed; (b) I. Kreituss and J. W. Bode, Acc. Chem. Res., 2016, 49, 2807 CrossRef CAS PubMed.
- N-Acylimidazoles: (a) N. Ikemoto, L.-C. Lo and K. Nakanishi, Angew. Chem., Int. Ed., 1992, 31, 890 CrossRef; (b) T. F. Molinski, T. N. Makarieva and V. A. Stonik, Angew. Chem., Int. Ed., 2000, 39, 4076 CrossRef CAS; (c) A. V. Karnik and S. S. Kamath, J. Org. Chem., 2007, 72, 7435 CrossRef CAS PubMed; (d) V. Percec, J. G. Rudick, M. Peterca, M. E. Yurchenko, J. Smidrkal and P. A. Heiney, Chem.–Eur. J., 2008, 14, 3355 CrossRef CAS PubMed; (e) S. T. Heller, T. Fu and R. Sarpong, Org. Lett., 2012, 14, 1970 CrossRef CAS PubMed; (f) S. T. Heller, E. E. Schultz and R. Sarpong, Angew. Chem., Int. Ed., 2012, 51, 8304 CrossRef CAS PubMed; (g) T.-X. Métro, J. Bonnamour, T. Reidon, A. Duprez, J. Sarpoulet, J. Martinez and F. Lamaty, Chem.–Eur. J., 2015, 21, 12787 CrossRef PubMed; N-acylbenzotriazoles: (h) A. R. Katritzky, H.-Y. He and K. Suzuki, J. Org. Chem., 2000, 65, 8210 CrossRef CAS PubMed; (i) A. R. Katritzky, N. Kirichenko and B. V. Rogovoy, Synthesis, 2003, 2777 CrossRef CAS; (j) X. Wang, H. Yu, P. Xu and R. Zheng, J. Chem. Res., 2005, 6, 595 CrossRef; (k) A. R. Katritzky, C. Cai and S. K. Singh, J. Org. Chem., 2006, 71, 3375 CrossRef CAS PubMed; (l) D. Hur, S. F. Ekti and R. Say, Lett. Org. Chem., 2007, 4, 585 CrossRef CAS; (m) I. O. Lebedyeva, S. Biswas, K. Goncalves, S. M. Sileno, A. R. Jackson, K. Patel, P. J. Steel and A. R. Katritzky, Chem.–Eur. J., 2014, 20, 11695 CrossRef CAS PubMed.
- (a) G. W. Cline and S. B. Hanna, J. Am. Chem. Soc., 1987, 109, 3087 CrossRef CAS; (b) N. Abello, H. A. M. Kerstjens, D. S. Potsma and R. Bischoff, J. Proteome Res., 2007, 6, 4770 CrossRef CAS PubMed; (c) H. Li, Z.-B. Pang, Z.-F. Jiao and F. Lin, J. Comb. Chem., 2010, 12, 255 CrossRef CAS PubMed; (d) G. Wang, Q.-Y. Yu, J. Wang, S. Wang, S.-Y. Chen and X.-Q. Yu, RSC Adv., 2013, 3, 21306 RSC.
- (a) A. Dornow and H. Theidel, Angew. Chem., 1954, 66, 605 CrossRef CAS; (b) S.-I. Murahashi, T. Naota and N. Nakajima, Tetrahedron Lett., 1985, 26, 925 CrossRef CAS; (c) S. I. Murahashi, T. Naota and N. Nakajima, Tetrahedron Lett., 1987, 28, 879 Search PubMed; (d) S.-I. Murahashi and T. Naota, Synthesis, 1993, 433 CrossRef CAS.
- B. Tan, N. Toda and C. F. Barbas III, Angew. Chem., Int. Ed., 2012, 51, 12538 CrossRef CAS PubMed.
- (a) J. Druzian, C. Zucco, M. C. Rezende and F. Nome, J. Org. Chem., 1989, 54, 4767 CrossRef CAS; (b) R. N. Ram, V. Kumar Soni and D. Kumar Gupta, Tetrahedron, 2012, 68, 9068 CrossRef CAS.
- (a) Y. Kita, H. Maeda, K. Omori, T. Okuno and Y. Tamura, Synlett, 1993, 273 CrossRef CAS; (b) I. Degani, S. Dughera, R. Fochi and E. Serra, Synthesis, 1999, 1200 CrossRef CAS; (c) C. E. Humphrey, M. A. M. Easson, J. P. Tierney and N. J. Turner, Org. Lett., 2003, 5, 849 CrossRef CAS PubMed; (d) A. Saito, M. Tojo, H. Yanai, F. Wada, M. Nakagawa, M. Okada, A. Sato, R. Okatani and T. Taguchi, J. Fluorine Chem., 2012, 133, 38 CrossRef CAS.
- (a) H. Noda and J. W. Bode, Chem. Sci., 2014, 5, 4328 RSC; (b) A. O. Gálvez, C. P. Schaack, H. Noda and J. W. Bode, J. Am. Chem. Soc., 2017, 139, 1826 CrossRef PubMed.
- N-Selectivity: (a) K. A. Watanabe and J. J. Fox, Angew. Chem., 1966, 5, 579 CrossRef CAS; (b) T. Sasaki and Y. Mizuno, Chem. Pharm. Bull., 1967, 15, 894 CrossRef CAS PubMed; (c) B. C. Ranu, P. Dutta and A. Sarkar, J. Chem. Soc., Perkin Trans. 1, 2000, 2223 RSC; (d) M. J. Petersson, I. D. Jenkins and W. A. Loughlin, Org. Biomol. Chem., 2009, 7, 739 RSC; O-selectivity: (e) T. Ohshima, T. Iwasaki, Y. Maegawa, A. Yoshiyama and K. Mashima, J. Am. Chem. Soc., 2008, 130, 2944 CrossRef CAS PubMed; (f) R. C. Samanta, S. De Sarkar, R. Fröhlich, S. Grimme and A. Studer, Chem. Sci., 2013, 4, 2177 RSC; (g) Y. Hayashi, S. Santoro, Y. Azuma, F. Himo, T. Ohshima and K. Mashima, J. Am. Chem. Soc., 2013, 135, 6192 CrossRef CAS PubMed; (h) R. Horikawa, C. Fujimoto, R. Yazaki and T. Ohshima, Chem.–Eur. J., 2016, 22, 12278 CrossRef CAS PubMed; S-selectivity: (i) K. Fu, Q.-F. Wang, F.-X. Zhan, L. Yang, Q. Yang and G.-X. Zheng, Org. Process Res. Dev., 2015, 19, 590 CrossRef CAS.
- (a) J. González-Sabín, R. Morán-Ramallal and F. Rebolledo, Chem. Soc. Rev., 2011, 40, 5321 RSC; (b) S. Schmelz, C. H. Botting, L. Song, N. F. Kadi, G. L. Challis and J. H. Naismith, J. Mol. Biol., 2011, 412, 495 CrossRef CAS PubMed; (c) F. Le Joubioux, Y. B. Henda, N. Bridiau, O. Achour, M. Graber and T. Maugard, J. Mol. Catal. B: Enzym., 2013, 85–86, 193 CrossRef CAS.
- A. A. Bastian, A. Marcozzi and A. Herrmann, Nat. Chem., 2012, 4, 789 CrossRef CAS PubMed.
- (a) S.-I. Murahashi, T. Naota and N. Nakajima, Chem. Lett., 1987, 5, 879 CrossRef; (b) S. P. Rannard and N. J. Davis, Org. Lett., 2000, 2, 2117 CrossRef CAS PubMed; (c) I. H. Chung, K. S. Cha, J. H. Seo, J. H. Kim, B. Y. Chung and C. S. Kim, Heterocycles, 2000, 53, 529 CrossRef CAS; (d) N. Nishiwaki, D. Nishida, T. Ohnishi, F. Hidaka, S. Shimizu, M. Tamura, K. Hori, Y. Tohda and M. Ariga, J. Org. Chem., 2003, 68, 8650 CrossRef CAS PubMed; (e) C. S. Song, J. N. Kim and T. H. Kim, Bull. Kor. Chem. Soc., 2005, 26, 983 CrossRef CAS; (f) C. Ferroud, M. Godart, S. Ung, H. Borderies and A. Guy, Tetrahedron Lett., 2008, 49, 3004 CrossRef CAS; (g) K. Pappas, X. Zhang, W. Tang and S. Fang, Tetrahedron Lett., 2009, 50, 5741 CrossRef CAS; (h) S. K. Verma, R. Ghorpade, A. Pratap and M. P. Kaushik, Green Chem., 2012, 14, 326 RSC.
- T. Yokomatsu, A. Arakawa and S. Shibuya, J. Org. Chem., 1994, 59, 3506 CrossRef CAS.
- K. O. Marichev, H. Qiu, A. C. Offield, H. Arman and M. P. Doyle, J. Org. Chem., 2016, 81, 9235 CrossRef CAS PubMed.
- (a) S. Ohta, S. Hayakawa and M. Okamoto, Tetrahedron Lett., 1984, 25, 5681 CrossRef CAS; (b) S. Ohta, S. Hayakawa, H. Moriwaki, S.-I. Tsuboi and M. Okamoto, Heterocycles, 1985, 23, 1759 CrossRef CAS; (c) R. C. F. Jones and J. R. Nichols, Org. Biomol. Chem., 2013, 11, 5926 RSC.
- (a) K. Kociołek and M. T. Leplawy, Synthesis, 1977, 778 CrossRef; (b) R. Ballini, G. Bosica and D. Fiorini, Tetrahedron, 2003, 59, 1143 CrossRef CAS; (c) L. El Kaïm, L. Grimaud and P. Pravin, Eur. J. Org. Chem., 2013, 4752 CrossRef; (d) S. Wang, Y. Yu, X. Chen, H. Zhu, P. Du, G. Liu, L. Lou, H. Li and W. Wang, Tetrahedron Lett., 2015, 56, 3093 CrossRef CAS; (e) R. Guo, C. Zhu, Z. Sheng, Y. Li, W. Yin and C. Chu, Tetrahedron Lett., 2015, 56, 6223 CrossRef CAS; (f) S. U. Dighe, S. Mukhopadhyay, K. Priyanka and S. Batra, Org. Lett., 2016, 18, 4190 CrossRef CAS PubMed.
- (a) J.-N. Denis, A. Correa and A. E. Greene, J. Org. Chem., 1991, 56, 6939 CrossRef CAS; (b) T. Matsui, T. Kondo, Y. Nishita, S. Itadani, S. Nakatani, N. Omawari, M. Sakai, S. Nakazawa, A. Ogata, H. Mori, K. Terai, W. Kamoshima, H. Ohno, T. Obata, H. Nakai and M. Toda, Bioorg. Med. Chem., 2002, 10, 3757 CrossRef CAS PubMed; (c) L. Araújo de Souza, E. Teixeira da Silva, M. C. S. Lourenço and M. V. Nora de Souza, Mediterranean Journal of Chemistry, 2014, 2, 648 CrossRef.
- A. Morcuende, M. Ors, S. Valverde and B. Herradón, J. Org. Chem., 1996, 61, 5264 CrossRef CAS.
- Z. Zhang, Z. Yin, N. A. Meanwell, J. F. Kadow and T. Wang, Org. Lett., 2003, 5, 3399 CrossRef CAS PubMed.
- T. Wang, Z. Zhang and N. A. Meanwell, Tetrahedron Lett., 1999, 40, 6745 CrossRef CAS.
- (a) J. Polster, Talanta, 1992, 39, 1355 CrossRef CAS PubMed; (b) D. G. Blackmond, Angew. Chem., Int. Ed., 2005, 44, 4302 CrossRef CAS PubMed; (c) P. W. Atkins and J. De Paula, Physical Chemistry for the Life Sciences, W. H. Freeman and Company, New York, 2006 Search PubMed.
- For details see: the ESI.†.
- (a) L. Claisen, Chem. Ber., 1887, 20, 646 CrossRef; (b) M. L. Bender, J. Am. Chem. Soc., 1951, 73, 1626 CrossRef CAS; (c) V. V. Vasilyev, J. Mol. Struct.: THEOCHEM, 1994, 304, 129 CrossRef.
- (a) A. C. Satterthwail and W. P. Jencks, J. Am. Chem. Soc., 1974, 96, 7018 CrossRef; (b) G. W. Cline and S. B. Hanna, J. Am. Chem. Soc., 1987, 109, 3087 CrossRef CAS.
- B. C. Wilcock, B. E. Uno, G. L. Bromann, M. J. Clark, T. M. Anderson and M. D. Burke, Nat. Chem., 2012, 4, 996 CrossRef CAS PubMed.
- (a) J. J. Wright, A. Cooper, P. J. L. Daniels, T. L. Nagabhushan, D. Rane, W. N. Turner and J. Weinstein, J. Antibiot., 1976, 29, 714 CrossRef CAS PubMed; (b) S. Horii, H. Fukase, Y. Kameda and N. Mizokami, Carbohydr. Res., 1978, 60, 275 CrossRef CAS; (c) S. Hanessian and G. Patil, Tetrahedron Lett., 1978, 12, 1035 CrossRef; (d) M. J. Cron, J. G. Keil, J.-S. Lin, M. V. Ruggeri and D. Walker, J. Chem. Soc., Chem. Commun., 1979, 266 RSC; (e) B. S. Iyengar, V. Kumar, T. P. Wunz and W. A. Remers, J. Med. Chem., 1986, 29, 611 CrossRef PubMed; (f) I. Grapsas, Y. J. Cho and S. Mobashery, J. Org. Chem., 1994, 59, 1918 CrossRef CAS; (g) M. Sainlos, P. Belmont, J.-P. Vigneron, P. Lehn and J.-M. Lehn, Eur. J. Org. Chem., 2003, 2764 CrossRef CAS; (h) J. B. Aggen, E. S. Armstrong, A. A. Goldblum, P. Dozzo, M. S. Linsell, M. J. Gliedt, D. J. Hildebrandt, L. A. Feeney, A. Kubo, R. D. Matias, S. Lopez, M. Gomez, K. B. Wlasichuk, R. Diokno, G. H. Miller and H. E. Moser, Antimicrob. Agents Chemother., 2010, 54, 4636 CrossRef CAS PubMed.
- (a) G. Theodoridis, Tetrahedron, 2000, 56, 2339 CrossRef CAS; (b) Y. Berkov-Zrihen and M. Fridman, Synthesis of Aminoglycosides, in Modern Synthetic Methods in Carbohydrate Chemistry: From Monosaccharides to Complex Glycoconjugates, ed. D. B. Werz and S. Vidal, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, Germany, 2013 Search PubMed.
- (a) P. Shaul, K. D. Green, R. Rutenberg, M. Kramer, Y. Berkov-Zrihen, E. Breiner-Goldstein, S. Garneau-Tsodikova and M. Fridman, Org. Biomol. Chem., 2011, 9, 4057 RSC; (b) N. T. Chandrika, K. D. Green, J. L. Houghton and S. Garneau-Tsodikova, ACS Med. Chem. Lett., 2015, 6, 1134 CrossRef PubMed.
- (a) N. M. Llewellyn and J. B. Spencer, Chem. Commun., 2008, 3786 RSC; (b) K. D. Green, W. Chen, J. L. Houghton, M. Fridman and S. Garneau-Tsodikova, ChemBioChem, 2010, 11, 119 CrossRef CAS PubMed.
- H. Tanaka, Y. Nishida, Y. Furuta and K. Kobayashi, Bioorg. Med. Chem. Lett., 2002, 12, 1723 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures, kinetic data, UV/Vis spectra, NMR spectra, and HPLC traces. X-ray data for 44. CCDC 1563600. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c7sc03184j |
| This journal is © The Royal Society of Chemistry 2017 |