Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Hexathioalkyl sumanenes: an electron-donating buckybowl as a building block for supramolecular materials†
Yoshiaki
Shoji‡
 ab,
Takashi
Kajitani‡
ab,
Fumitaka
Ishiwari‡
ab,
Qiang
Ding
a,
Hiroyasu
Sato
c,
Hayato
Anetai
d,
Tomoyuki
Akutagawa
ab,
Takashi
Kajitani‡
ab,
Fumitaka
Ishiwari‡
ab,
Qiang
Ding
a,
Hiroyasu
Sato
c,
Hayato
Anetai
d,
Tomoyuki
Akutagawa
 de,
Hidehiro
Sakurai
f and
Takanori
Fukushima
de,
Hidehiro
Sakurai
f and
Takanori
Fukushima
 *a
*a
aLaboratory for Chemistry and Life Science, Institute of Innovative Research, Tokyo Institute of Technology, 4259 Nagatsuta, Midori-ku, Yokohama 226-8503, Japan. E-mail: fukushima@res.titech.ac.jp
bRIKEN SPring-8 Center, 1-1-1 Kouto, Sayo, Hyogo 679-5148, Japan
cRigaku Corporation, Matsubara-cho 3-9-12, Akishima, Tokyo 196-8666, Japan
dGraduate School of Engineering, Tohoku University, Sendai 980-8579, Japan
eInstitute of Multidisciplinary Research for Advanced Materials (IMRAM), Tohoku University, 2-1-1 Katahira, Aoba-ku, Sendai, 980-8577, Japan
fDivision of Applied Chemistry, Graduate School of Engineering, Osaka University, 2-1 Yamada-oka, Suita, Osaka 565-0871, Japan
First published on 18th October 2017
Abstract
Unlike planar aromatic compounds, bowl-shaped sumanene, which has concave and convex faces with different electrostatic potentials, tends to form a one-dimensional columnar assembly without causing slip-stacking in the crystal. Here we report the first successful synthesis of liquid-crystalline (LC) sumanenes, which was brought about by the incorporation of six thioalkyl groups (R = SC6H13 or SC12H25) into the aromatic part of sumanene. In contrast to the case of the mesophase formation of corannulene, which requires the presence of many dendritic side chains, sumanene derivatives with simple alkyl chains can exhibit a remarkably high-order columnar LC mesophase over a wide temperature range. While non-substituted sumanene inherently behaves as an electron acceptor, hexathioalkyl versions, such as hexathiomethyl sumanene, show electron-donating properties, resulting in complexation with C60. Considering its unique shape, electronic properties, and self-assembly behavior, the electron-donating sumanene may represent a new building block for constructing supramolecular materials, both by itself and in combination with fullerene derivatives.
Introduction
Buckybowls, which exhibit a concave–convex geometry, are promising molecular building blocks for the development of functional organic materials and devices.1–4 Sumanene C21H12 (1H, Fig. 1) is a representative buckybowl and is characterized by a C3v-symmetric subunit of C60, i.e., a curved methylene-bridged triphenylene framework.1 In the crystal, 1H assembles through a concave–convex interaction5 to form a one-dimensional (1D) bowl-stack column without slip-stacking geometry.1b In contrast, this structural pattern is not observed in the crystal of corannulene C20H10, which is another type of buckybowl.2 According to previous reports, the construction of bowl-stacked 1D assemblies of corannulene should require proper structural extensions of its π-conjugated framework2d,e,i,j or elaborate side chains with strong hydrogen-bonding capability.2f,g Based on the difference between these two buckybowls with respect to self-assembly behavior, sumanene, with a strong preference for bowl stacking, is advantageous for the design of a highly ordered 1D columnar architecture.Another interesting feature of sumanene is that it exhibits bowl-to-bowl inversion, which is associated with dipole inversion.1 If a collective bowl-to-bowl inversion could be induced for columnarly assembled sumanene through the application of electric fields, dielectric responsive properties such as ferroelectricity could be realized.6 For this purpose, liquid-crystalline (LC) assemblies are expected to have great advantage over crystalline assemblies. However, LC sumanene has not yet been developed.
Here we report the first sumanene derivatives that can form LC mesophases with a highly ordered hexagonally arranged columnar structure over a wide temperature range. The LC sumanenes carry six simple thioalkyl side chains at the peripheral aromatic positions (1C6 and 1C12, Fig. 1), which is in striking contrast to LC corannulenes.2f,g The single-crystal X-ray analysis of 1C1 with six thiomethyl groups (Fig. 1) showed a 1D bowl-stacking structure. Theoretical calculations suggest that hexathioalkyl sumanenes have an inversed and enhanced concave–convex polarization of the sumanene core, compared to that of non-substituted 1H. We also demonstrate that 1C1 behaves as an electron donor due to the six thioalkyl substituents and forms a complex with C60, which has never been achieved with non-substituted sumanene (1H) or previously reported sumanene derivatives.
Results and discussion
Synthesis of hexathioalkyl sumanenes
Mainly due to the lack of a synthetic method for the full peripheral functionalization of sumanene,1 a sumanene derivative that can form an LC assembly has not yet been reported. Recently, we reported the successful synthesis of 2,3,5,6,8,9-hexabromosumanene (1Br, Fig. 1), which can readily be converted into hexa-arylated sumanene derivatives through Pd-catalyzed cross-coupling such as Suzuki–Miyaura reaction.1i We found that compound 1Br also allows aromatic nucleophilic substitution with thioalkoxides. Typically, 1Br was reacted with 18 equivalents of sodium thiomethoxide (NaSCH3) in 1,3-dimethyl-2-imidazolidinone (DMI) at 100 °C under argon to give 1C1 in 35% yield (ESI†). Similarly, 1C6, 1C12 and 1EH (Fig. 1) were obtained in 39–43% yields using the corresponding sodium thioalkoxide in place of NaSCH3 (ESI†). All of these hexathioalkyl sumanenes were unambiguously characterized by 1H and 13C NMR spectroscopy, IR spectroscopy, and high-resolution APCI-TOF mass spectrometry (ESI†). For instance, the 1H NMR spectrum of 1C1 in toluene-d8 at 25 °C showed two doublet signals at δ = 4.50 and 3.54 ppm arising from the benzylic protons at the exo- and endo-positions, respectively (Fig. S1, ESI†). Note that these signals were not coalesced, even at elevated temperatures (e.g., 100 °C, Fig. S1, ESI†). Thus, it is likely that the rate of bowl-to-bowl inversion of 1C1 in solution is sufficiently slow relative to the timescale of 1H NMR spectroscopy.X-ray crystal structure of hexathiomethyl sumanene (1C1)
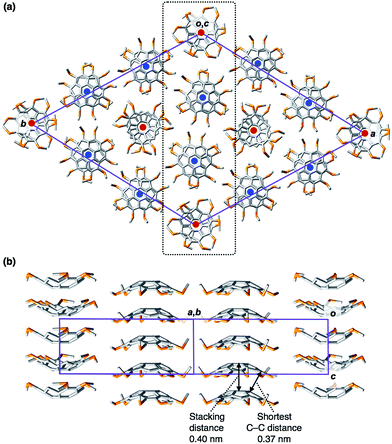
We successfully obtained needle-shaped pale-yellow single crystals of 1C1, suitable for X-ray diffraction analysis, by the slow diffusion of a hexane vapor into a dichloromethane solution of 1C1 (ESI†). Single-crystal X-ray analysis revealed detailed molecular and assembled structures of 1C1. The crystal of 1C1 belongs to the P3 space group, and the asymmetric unit in the unit cell contains four entire 1C1 molecules and six fragments of one-third of 1C1 (Z = 18). Overall, each crystallographically independent molecule is very similar in terms of bond lengths and angles. The mean bowl-depth of 1C1 (1.04 Å, Fig. 2) at the peripheral aromatic carbons is slightly shallower than those observed for the crystal structures of 1H (1.11 Å)1a and 1Br (1.08 Å).1i In the crystal of 1C1, 1D columns are formed in a bowl-stack manner with a quasi-staggered stacking geometry, where the mean stacking distance (4.00 Å) is comparable to that observed for 1Br (3.94 Å),1i while it is longer than that observed for 1H (3.86 Å).1a Considering the observed stacking geometry of 1C1, along with the previously reported simulation for 1H,7 an intermolecular electrostatic force is likely responsible for the formation of the 1D column. Meanwhile, the 1D columns assemble laterally to form a 2D quasi-hexagonal structure with an inter-columnar distance of approximately 12 Å (Fig. 2). When viewed along the c-axis, six columns with a convex upward geometry surround a column with a convex downward geometry, so that the dipole moments generated in the column can be partially cancelled (Fig. 2a). The 2D quasi-hexagonal structure developed in the crystal of 1C1 is reminiscent of the structure of a hexagonal columnar (Colh) mesophase that is typically observed for discotic LC assemblies.8DFT calculations of the molecular and electronic structures of hexathiomethyl sumanene (1C1)
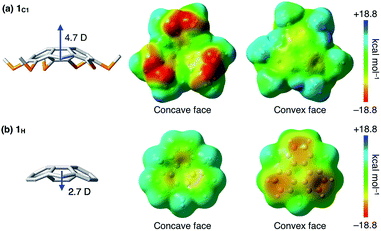
To gain insight into how the thioalkyl groups affect the electronic structure of sumanene, we performed density functional theory (DFT) calculations on the isolated molecule of 1C1 in vacuum at the w97BD/6-311G++(d,p) level (Fig. 3 and Table S1, ESI†). The molecular structure of 1C1 obtained by the single-crystal X-ray analysis was used as the initial geometry for geometry optimization. The calculated bond lengths and angles in the optimized geometries were in good agreement with those observed for the crystal structure of 1C1 (Fig. 2). Because of the electron-rich sulfur atoms, the calculated dipole moment of 1C1 along its C3-symmetric axis [4.7 Debye (D)] was much greater than that calculated for non-substituted sumanene 1H (2.7 D) at the same level (Table S2, ESI†), and the directions of the dipole moments of 1C1 and 1H were opposite to one another (Fig. 3). The surface electrostatic potential (ESP) diagram of 1C1 showed negative and positive ESP values for the concave and convex faces, respectively (Fig. 3). We suppose that intermolecular electrostatic attractive interactions between positive convex and negative concave faces of 1C1 could compensate for the unfavorable parallel dipole alignment, leading to the formation of the columnar structure in the crystal (Fig. 2). Note that, since the ESP diagram of 1H illustrates negative convex and relatively positive concave faces (Fig. 3),1l the six thioalkyl groups in 1C1 invert and enhance the concave–convex polarization of the sumanene skeleton.Characterization of liquid-crystalline (LC) mesophases of sumanene derivatives
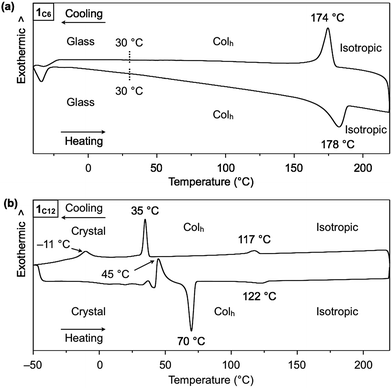
Hexathioalkyl sumanenes with long alkyl side chains (1C6 and 1C12, Fig. 1) exhibit bowl-stacking to form a highly ordered columnar LC assembly. In differential scanning calorimetry (DSC), 1C6 exhibited an LC mesophase over a wide temperature range (<174 °C), while 1C12 displayed a phase sequence involving an LC mesophase (35–117 °C) and two crystal phases (−11 to 35 °C and <−11 °C), upon cooling from the corresponding isotropic liquid phases (Fig. 4). Polarized optical microscopy (POM) images of the LC mesophases of 1C6 and 1C12 showed a fan-shaped texture, which is typically observed for hexagonal columnar (Colh) LC assemblies (Fig. 5a and b).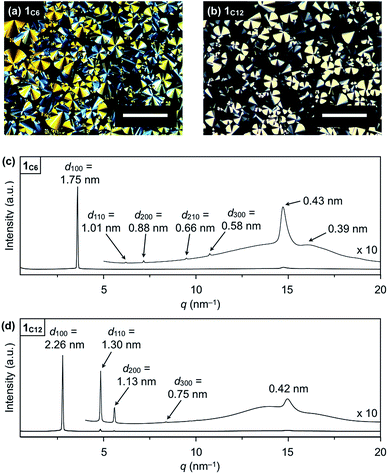 | ||
| Fig. 5 POM images of (a) 1C6 at 140 °C on cooling and (b) 1C12 at 80 °C on cooling (scale bar = 200 μm). XRD patterns of (c) 1C6 and (b) 1C12 in a glass capillary at 100 °C on heating. | ||
The powder X-ray diffraction (XRD) pattern of a bulk sample of 1C6 at 100 °C upon heating displayed five diffraction peaks with d-spacings of 1.75, 1.01, 0.88, 0.66 and 0.58 nm. The ratio of the d-spacings (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) √3
√3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) √7
√7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) completely agrees with the values expected for diffractions from the (100), (110), (200), (210) and (300) planes of a 2D hexagonal lattice (Fig. 5c and S2, ESI†). The hexagonal lattice parameter (a), given by 2d100/√3, was determined to be 2.02 nm. The diffraction peak at scattering vector q = 14.6 nm−1 (d-spacing = 0.43 nm) corresponds to the core-to-core separation of the mesogen along the columnar axis, which is comparable to the bowl-stacking distance in the 1D columns of crystalline 1C1 (4.0 Å, Fig. 2). A very broad diffraction peak at approximately q = 16 nm−1 (d-spacing = 0.39 nm) likely arises from the shortest C–C distance in the 1D column, judging from the crystal structure of 1C1 (Fig. 2). The diffraction peak corresponding to the core-to-core separation is exceptionally strong and sharp (Fig. 5c and S2, ESI†), compared to those observed for usual discotic columnar LC assemblies.8 This observation suggests that the structural order of the columnar arrays of the bowl-shaped mesogen is remarkably high. Although no phase-transition feature was observed below 30 °C in the DSC analysis, temperature-dependent XRD measurements revealed that 1C6 undergoes a phase transition from the LC mesophase to a glassy solid phase at approximately 30 °C. Accordingly, the XRD pattern of 1C6 at e.g., 20 °C upon cooling (Fig. S2, ESI†) displayed three sharp peaks at a wide-angle region (q > 16 nm−1). In addition to these peaks, some periodic peaks arising from the ordered Colh structure remained, indicating that the structural feature of the LC mesophase is maintained to some extent in the glassy solid phase.
3) completely agrees with the values expected for diffractions from the (100), (110), (200), (210) and (300) planes of a 2D hexagonal lattice (Fig. 5c and S2, ESI†). The hexagonal lattice parameter (a), given by 2d100/√3, was determined to be 2.02 nm. The diffraction peak at scattering vector q = 14.6 nm−1 (d-spacing = 0.43 nm) corresponds to the core-to-core separation of the mesogen along the columnar axis, which is comparable to the bowl-stacking distance in the 1D columns of crystalline 1C1 (4.0 Å, Fig. 2). A very broad diffraction peak at approximately q = 16 nm−1 (d-spacing = 0.39 nm) likely arises from the shortest C–C distance in the 1D column, judging from the crystal structure of 1C1 (Fig. 2). The diffraction peak corresponding to the core-to-core separation is exceptionally strong and sharp (Fig. 5c and S2, ESI†), compared to those observed for usual discotic columnar LC assemblies.8 This observation suggests that the structural order of the columnar arrays of the bowl-shaped mesogen is remarkably high. Although no phase-transition feature was observed below 30 °C in the DSC analysis, temperature-dependent XRD measurements revealed that 1C6 undergoes a phase transition from the LC mesophase to a glassy solid phase at approximately 30 °C. Accordingly, the XRD pattern of 1C6 at e.g., 20 °C upon cooling (Fig. S2, ESI†) displayed three sharp peaks at a wide-angle region (q > 16 nm−1). In addition to these peaks, some periodic peaks arising from the ordered Colh structure remained, indicating that the structural feature of the LC mesophase is maintained to some extent in the glassy solid phase.
The structure of 1C12 in the LC mesophase was essentially identical to that of 1C6, except for a larger hexagonal lattice parameter (a = 2.60 nm) due to the longer alkyl side chains. As shown in Fig. 5d and S3 (ESI†), the XRD pattern of a bulk sample of 1C12 at 100 °C upon heating displayed five diffraction peaks with d-spacings of 2.26, 1.30, 1.13, 0.75, and 0.42 nm. The last peak, which is assignable to the core-to-core separation of the mesogen, was not affected by the difference in the side-chain lengths. We also note that when branched alkyl chains are attached to the sumanene core, formation of the LC mesophase would be suppressed, by analogy to discotic LC assemblies with a planar aromatic mesogen. For instance, compound 1EH (Fig. 1) only exhibited a phase transition from crystal to isotropic liquid, as revealed by DSC, POM and XRD analyses (Fig. S4, ESI†).
Since molecules in the LC state can behave dynamically, we supposed that the LC sumanenes such as 1C12 and 1C6 might exhibit a collective bowl-to-bowl inversion in response to electric fields. To explore this possibility, we measured the dielectric properties of bulk samples of 1C12 and 1C6 (Fig. S5, ESI†). Since dielectric properties are sensitive to the motions of polar molecular units,9 dielectric relaxation measurements should detect a thermally activated bowl-to-bowl inversion of 1C12 and 1C6, if it occurs. However, the dielectric constants of 1C12 and 1C6 did not change significantly over a temperature range of crystalline and Colh phases as well as a frequency range of 103–106 Hz (Fig. S5, ESI†), indicating that the LC materials have antiferroelectric properties. Based on this observation, the bowl-stacked 1D columns of 1C12 and 1C6, in the Colh mesophases do not respond to the electric fields, but rather statically align in an antiparallel manner to cancel out the dipole generated in each column.
Complexation of hexathiomethyl sumanene (1C1) with C60
Although sumanene derivatives capable of complexation with C60 have never been reported to date, we supposed that this would not be the case with hexathioalkyl sumanenes, since the electron-donating thioalkyl groups could change the inherent electronic properties of sumanene. Indeed, cyclic voltammetry of 1C1 in CH2Cl2 in the presence of tetrabutylammonium hexafluorophosphate as a supporting electrolyte displayed a reversible oxidation wave at E1/2 = 0.64 V (versus ferrocene/ferrocenium), while non-substituted 1H under identical conditions showed an irreversible oxidation wave at 1.03 V (Fig. S6, ESI†). Due to its improved electron-donating properties as well as its concave structure, 1C1 might show strong affinity toward electron-accepting C60.10The 1H NMR spectrum of an equimolar mixture of 1C1 and C60 ([1C1] = 1.0 mM, toluene-d8, 25 °C) showed two doublet signals and one singlet signal at δ = 4.51, 3.54, and 2.26 ppm arising from the benzylic exo- and endo-protons and S–CH3 groups of 1C1, respectively (Fig. S7, ESI†). These 1H NMR signals were shifted slightly more downfield relative to those observed for 1C1 in the absence of C60 (4.50, 3.53, and 2.24 ppm, Fig. S7, ESI†). After the NMR measurement, we noticed that a considerable amount of black precipitate formed, which contained both 1C1 and C60 as confirmed by APCI-TOF mass spectrometric analysis. Obviously, 1C1 gave a complex with C60. Although the association constant between 1C1 and C60 could not be determined because of the low solubility of a mixture 1C1 and C60 in solution, Job's plot11 of 1C1 with C60 in toluene-d8 at 25 °C (total concentration [1C1] + [C60] = 0.1 mmol), based on the 1H NMR chemical shift of the S–CH3 signal as a reference, suggested the occurrence of an approximately 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complexation (Fig. S8, ESI†), which is most likely due to a concave–convex interaction.5
1 complexation (Fig. S8, ESI†), which is most likely due to a concave–convex interaction.5
The use of 1C12 in place of 1C1 allowed the evaluation of the association constant (Ka) with C60. When C60 was added to a toluene-d8 solution of 1C12 (1.0 mM, [C60]/[1C12] = 0.0–3.7), 1H NMR signals due to the benzylic and thioalkyl protons of 1C12 gradually shifted downfield without any precipitation (Fig. S9, ESI†). From the 1H NMR spectral change, Ka was determined to be 280 M−1 (Fig. S10, ESI†), which is not very high compared to those reported for curved π-systems and C60.2,4b,f,10b Job's plot of 1C12 with C60 in toluene-d8 at 25 °C (total concentration [1C12] + [C60] = 0.1 mmol) confirmed the occurrence of a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complexation (Fig. S11, ESI†).
1 complexation (Fig. S11, ESI†).
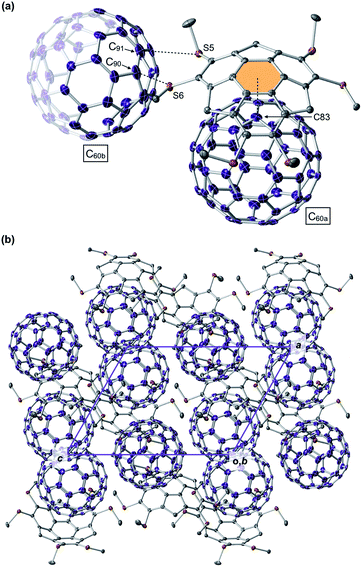
From a toluene solution of an equimolar mixture of 1C1 and C60, we successfully obtained a black-colored block single crystal suitable for single-crystal X-ray analysis (ESI†). The X-ray structure showed that 1C1 and C60 co-crystallized with a ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 to constitute a unit cell (space group: triclinic P
3 to constitute a unit cell (space group: triclinic P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) ), where the asymmetric unit contains one 1C1 and one and a half C60 molecules (Fig. 6). Thus, the complexation stoichiometry of 1C1 and C60 in the solid state (1
), where the asymmetric unit contains one 1C1 and one and a half C60 molecules (Fig. 6). Thus, the complexation stoichiometry of 1C1 and C60 in the solid state (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5) is different from that in solution (∼1
1.5) is different from that in solution (∼1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). In the crystal, the two C60 molecules are tightly packed without disordering. One of the C60 molecules (C60a) and 1C1 form a concave–convex complex, in which the shortest distance between one of the carbon atoms of C60a (C83, Fig. 6a) and the mean plane of the central six-membered ring of 1C1 is 3.370 Å. The other C60 molecule (C60b) interacts with the thiomethyl groups of 1C1 (Fig. 6a), as evidenced by the intermolecular sulfur–carbon contacts, e.g., S5–C90 (3.164 Å) and S6–C91 (3.456 Å), which are shorter than the sum of the van der Waals radii (3.50 Å).12 Accordingly, every C60b molecule interacts with a total of 12 thiomethyl groups of six neighboring 1C1 molecules, leading to the formation of a two-dimensional network (Fig. S12, ESI†). This structural feature may account for the observed low solubility (i.e., facile crystallization) of the complex of 1C1 with C60 in solution, despite relatively small association constants between hexathioalkyl sumanenes and C60.
1). In the crystal, the two C60 molecules are tightly packed without disordering. One of the C60 molecules (C60a) and 1C1 form a concave–convex complex, in which the shortest distance between one of the carbon atoms of C60a (C83, Fig. 6a) and the mean plane of the central six-membered ring of 1C1 is 3.370 Å. The other C60 molecule (C60b) interacts with the thiomethyl groups of 1C1 (Fig. 6a), as evidenced by the intermolecular sulfur–carbon contacts, e.g., S5–C90 (3.164 Å) and S6–C91 (3.456 Å), which are shorter than the sum of the van der Waals radii (3.50 Å).12 Accordingly, every C60b molecule interacts with a total of 12 thiomethyl groups of six neighboring 1C1 molecules, leading to the formation of a two-dimensional network (Fig. S12, ESI†). This structural feature may account for the observed low solubility (i.e., facile crystallization) of the complex of 1C1 with C60 in solution, despite relatively small association constants between hexathioalkyl sumanenes and C60.
Conclusions
A recently established method for full functionalization of the aromatic rings of sumanene1i allowed us to investigate the possibility that these bowl-shaped molecules could act as a mesogen for LC assemblies. As we have demonstrated in the present work, sumanene, when attached to simple thioalkyl side chains, forms a remarkably high-order columnar LC assembly, most likely due to concave–convex interactions. This result is in contrast to those with corannulene with an analogous concave–convex geometry, which requires specific side chains for the formation of a mesophase.2f,g The first successful synthesis of LC sumanenes would be a first step in the development of stimuli-responsive molecular assemblies by taking advantage of the bowl-to-bowl inversion dynamics. We have also demonstrated that, unlike non-substituted sumanene, the hexathioalkyl version behaves as an electron donor, leading to the complexation with C60. Since 1C1 shows affinity for C60, which leads to facile co-crystallization from a dilute solution, hexathioalkyl sumanenes may serve as a new building block for the construction of supramolecular materials with fullerene derivatives.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by a Grant-in-Aid for Scientific Research on Innovative Areas “π-Figuration: Control of Electron and Structural Dynamism for Innovative Functions” (JP26102008 for T. F., JP26102002 for H. S. and JP26102007 for T. A.) from the Japan Society for the Promotion of Science (JSPS). This work was also supported in part by “Dynamic Alliance for Open Innovation Bridging Human, Environment and Materials” from MEXT, Japan. The synchrotron XRD experiments were performed at the BL45XU beamline at SPring-8 with the approval of the RIKEN SPring-8 Center (proposal no. 20150068 and 20160027). Q. D. acknowledges the Program for Leading Graduate Schools “Academy for Co-Creative Education of Environment and Energy Science” from MEXT, Japan. The authors would like to thank Suzukakedai Materials Analysis Division, Technical Department, Tokyo Institute of Technology, for their support with the NMR measurements.Notes and references
- (a) H. Sakurai, T. Daiko and T. Hirao, Science, 2003, 301, 1878 CrossRef CAS PubMed; (b) H. Sakurai, T. Daiko, H. Sakane, T. Amaya and T. Hirao, J. Am. Chem. Soc., 2005, 127, 11580 CrossRef CAS PubMed; (c) T. Amaya, M. Hifumi, M. Okada, Y. Shimizu, T. Moriuchi, K. Segawa, Y. Ando and T. Hirao, J. Org. Chem., 2011, 76, 8049 CrossRef CAS PubMed; (d) S. Higashibayashi, R. B. N. Baig, Y. Morita and H. Sakurai, Chem. Lett., 2012, 41, 84 CrossRef CAS; (e) S. Higashibayashi, R. Tsuruoka, Y. Soujanya, U. Purushotham, G. N. Sastry, S. Seki, T. Ishikawa, S. Toyota and H. Sakurai, Bull. Chem. Soc. Jpn., 2012, 85, 450 CrossRef CAS; (f) B. M. Schmidt, B. Topolinski, S. Higashibayashi, T. Kojima, M. Kawano, D. Lentz and H. Sakurai, Chem.–Eur. J., 2013, 19, 3282 CrossRef CAS PubMed; (g) B. B. Shrestha, S. Karanjit, G. Panda, S. Higashibayashi and H. Sakurai, Chem. Lett., 2013, 42, 386 CrossRef CAS; (h) S. Higashibayashi, S. Onogi, H. K. Srivastava, G. N. Sastry, Y. Wu and H. Sakurai, Angew. Chem., Int. Ed., 2013, 52, 7314 CrossRef CAS PubMed; (i) H. Toda, Y. Yakiyama, Y. Shoji, F. Ishiwari, T. Fukushima and H. Sakurai, Chem. Lett., 2017, 46, 1368 CrossRef CAS; (j) B. B. Shrestha, S. Karanjit, S. Higashibayashi and H. Sakurai, Pure Appl. Chem., 2014, 86, 747 CrossRef CAS; (k) N. Ngamsomprasert, G. Panda, S. Higashibayashi and H. Sakurai, J. Org. Chem., 2016, 81, 11978 CrossRef CAS PubMed; (l) S. Mebs, M. Weber, P. Luger, B. M. Schmidt, H. Sakurai, S. Higashibayashi, S. Onogi and D. Lentz, Org. Biomol. Chem., 2012, 10, 2218 RSC.
- (a) L. T. Scott, Pure Appl. Chem., 1996, 68, 291 CAS; (b) P. W. Rabideau and A. Sygula, Acc. Chem. Res., 1996, 29, 235 CrossRef CAS; (c) Y.-T. Wu and J. S. Siegel, Chem. Rev., 2006, 106, 4843 CrossRef CAS PubMed; (d) Y.-T. Wu, D. Bandera, R. Maag, A. Linden, K. K. Baldridge and J. S. Siegel, J. Am. Chem. Soc., 2008, 130, 10729 CrossRef CAS; (e) A. S. Filatov, L. T. Scott and M. A. Petrukhina, Cryst. Growth Des., 2010, 10, 4607 CrossRef CAS; (f) D. Miyajima, K. Tashiro, F. Araoka, H. Takezoe, J. Kim, K. Kato, M. Takata and T. Aida, J. Am. Chem. Soc., 2009, 131, 44 CrossRef CAS PubMed; (g) D. Miyajima, F. Araoka, H. Takezoe, J. Kim, K. Kato, M. Takata and T. Aida, Angew. Chem., Int. Ed., 2011, 50, 7865 CrossRef CAS PubMed; (h) L. N. Dawe, T. A. AlHujran, H.-A. Tran, J. I. Mercer, E. A. Jackson, L. T. Scott and P. E. Georghiou, Chem. Commun., 2012, 48, 5563 RSC; (i) B. M. Schmidt, S. Seki, B. Topolinski, K. Ohkubo, S. Fukuzumi, H. Sakurai and D. Lentz, Angew. Chem., Int. Ed., 2012, 51, 11385 CrossRef CAS PubMed; (j) R. Chen, R.-Q. Lu, K. Shi, F. Wu, H.-X. Fang, Z.-X. Niu, X.-Y. Yan, M. Luo, X.-C. Wang, C.-Y. Yang, X.-Y. Wang, B. Xu, H. Xia, J. Peib and X.-Y. Cao, Chem. Commun., 2015, 51, 13768 RSC.
- (a) S. Furukawa, J. Kobayashi and T. Kawashima, J. Am. Chem. Soc., 2009, 131, 14192 CrossRef CAS PubMed; (b) X. Li and X. Shao, Synlett, 2014, 25, 1795 CrossRef CAS; (c) X. Li, Y. Zhu, J. Shao, B. Wang, S. Zhang, Y. Shao, X. Jin, X. Yao, R. Fang and X. Shao, Angew. Chem., Int. Ed., 2014, 53, 535 CrossRef CAS PubMed; (d) M. Saito, S. Furukawa, J. Kobayashi and T. Kawashima, Chem. Rec., 2016, 16, 64 CrossRef CAS PubMed; (e) X. Li, Y. Zhu, J. Shao, B. Wang, S. Zhang, Y. Shao, X. Jin, X. Yao, R. Fang and X. Shao, Angew. Chem., Int. Ed., 2014, 53, 535 CrossRef CAS PubMed; (f) X. Hou, Y. Zhu, Y. Qin, L. Chen, X. Li, H.-L. Zhang, W. Xu, D. Zhu and X. Shao, Chem. Commun., 2017, 53, 1546 RSC; (g) S. Furukawa, Y. Suda, J. Kobayashi, T. Kawashima, T. Tada, S. Fujii, M. Kiguchi and M. Saito, J. Am. Chem. Soc., 2017, 139, 5787 CrossRef CAS PubMed.
- (a) Z. Wang, F. Dötz, V. Enkelmann and K. Müllen, Angew. Chem., Int. Ed., 2005, 44, 1247 CrossRef CAS PubMed; (b) P. E. Georghiou, L. N. Dawe, H.-A. Tran, J. Strübe, B. Neumann, H.-G. Stammler and D. Kuck, J. Org. Chem., 2008, 73, 9040 CrossRef CAS PubMed; (c) K. Yoshida and A. Osuka, Chem.–Asian J., 2015, 10, 1526 CrossRef CAS PubMed; (d) M. Yamamura, T. Saito and T. Nabeshima, J. Am. Chem. Soc., 2014, 136, 14299 CrossRef CAS PubMed; (e) M. Yamamura, K. Sukegawa, D. Okada, Y. Yamamoto and T. Nabeshima, Chem. Commun., 2016, 52, 4585 RSC; (f) E. M. Pérez, M. Sierra, L. Sánchez, M. R. Torres, R. Viruela, P. M. Viruela, E. Ortí and N. Martín, Angew. Chem., Int. Ed., 2007, 46, 1847 CrossRef PubMed.
- (a) T. Kawase and H. Kurata, Chem. Rev., 2006, 106, 5250 CrossRef CAS PubMed; (b) E. M. Pérez and N. Martín, Chem. Soc. Rev., 2015, 44, 6425 RSC; (c) A. Sygula, F. R. Fronczek, R. Sygula, P. W. Rabideau and M. M. Olmstead, J. Am. Chem. Soc., 2007, 129, 3842 CrossRef CAS PubMed.
- (a) F. Araoka and H. Takezoe, Jpn. J. Appl. Phys., 2014, 53, 01AA01 CrossRef; (b) D. Miyajima, F. Araoka, H. Takezoe, J. Kim, K. Kato, M. Takata and T. Aida, Science, 2012, 336, 209 CrossRef CAS PubMed.
- Y. Guan and S. E. Wheeler, J. Phys. Chem. C, 2017, 121, 8541 CAS.
- (a) T. Wöhrle, I. Wurzbach, J. Kirres, A. Kostidou, N. Kapernaum, J. Litterscheidt, J. C. Haenle, P. Staffeld, A. Baro, F. Giesselmann and S. Laschat, Chem. Rev., 2016, 116, 1139 CrossRef PubMed; (b) S. K. Prasad, D. S. S. Rao, S. Chandrasekhar and S. Kumar, Mol. Cryst. Liq. Cryst., 2003, 396, 121 CrossRef CAS; (c) D. Adam, P. Schuhmacher, J. Simmerer, L. Häussling, K. Siemensmeyer, K. H. Etzbach, H. Ringsdorf and D. Haarer, Nature, 1994, 371, 141 CrossRef CAS; (d) T. Osawa, T. Kajitani, D. Hashizume, H. Ohsumi, S. Sasaki, M. Takata, Y. Koizumi, A. Saeki, S. Seki, T. Fukushima and T. Aida, Angew. Chem., Int. Ed., 2012, 51, 7990 CrossRef CAS PubMed.
- K. C. Cao, Dielectric Phenomena in Solids, Elsevier Academic Press, Boston, 2004 Search PubMed.
- (a) F. Diederich and M. Gómez-López, Chem. Soc. Rev., 1999, 28, 263 RSC; (b) S. Mizyed, P. E. Georghiou, M. Bancu, B. Cuadra, A. K. Rai, P. Cheng and L. T. Scott, J. Am. Chem. Soc., 2001, 123, 12770 CrossRef CAS PubMedK. Tashiro and T. Aida, Chem. Soc. Rev., 2007, 36, 189 RSC; (c) M.-X. Wang, Chem. Commun., 2008, 4541 RSC; (d) M. Hardouin-Lerouge, P. Hudhomme and M. Sallé, Chem. Soc. Rev., 2011, 40, 30 RSC.
- K. A. Connors, Binding Constants: The Measurement of Molecular Complex Stability, John Wiley & Sons. Inc., New York, 1987 Search PubMed.
- M. Mantina, A. C. Chamberlin, R. Valero, C. J. Cramer and D. G. Truhlar, J. Phys. Chem. A, 2009, 113, 5806 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental and crystallographic details, analytical data and theoretical calculations. CCDC 1572348 and 1572349. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c7sc03860g |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2017 |