 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Correction: A simple and traceless solid phase method simplifies the assembly of large peptides and the access to challenging proteins
N.
Ollivier
,
R.
Desmet
,
H.
Drobecq
,
A.
Blanpain
,
E.
Boll
,
B.
Leclercq
,
A.
Mougel
,
J.
Vicogne
and
O.
Melnyk
*
UMR CNRS 8161 CNRS, Université de Lille, Institut Pasteur de Lille, 1 rue du Pr Calmette, 59021 Lille Cedex, France. E-mail: oleg.melnyk@ibl.cnrs.fr
First published on 7th July 2017
Abstract
Correction for ‘A simple and traceless solid phase method simplifies the assembly of large peptides and the access to challenging proteins’ by N. Ollivier et al., Chem. Sci., 2017, DOI: 10.1039/c7sc01912b.
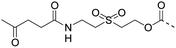
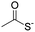
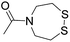
The authors regret that entries 2 and 3 in Table 1 are incorrect in the original manuscript. Entry 2 is missing the CH2CH2O group and entry 3 is missing two carbonyl groups.
A corrected version of Table 1 has been presented below:
| Entry | Functional linker (FL) | Attachment method for peptide segment 1 | Latent thioester (LT) | Cleavage | Ref. | |
|---|---|---|---|---|---|---|
| Structure | Activation method | |||||
| a The method was used for the synthesis of large protein mimetics through CuAAC ligation. | ||||||
| 1 |
|
Oximation pH 4 |
|
Alkylation with bromoacetic acid, pH 4.6 | β-Elimination, aqueous base, pH 13 | 22 |
| 2 |
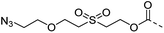
|
CuAAC pH 7 or SPAAC pH 2 |
|
Reduction (TCEP) and exchange by 3-mercaptopropionic acid, pH 4 | See entry 1 | 40 |
| 3 |
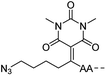
|
CuAAC | Notea | Transimination, 1 M H2NOH, pH 7–8.5 | 44 | |
| 4 (This work) |
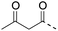
|
Oximation pH 3–4 |
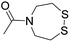
|
See entry 2 | Transoximation 0.025 M H2NOH, 3 M aniline, pH 3 | |
The Royal Society of Chemistry apologises for these errors and any consequent inconvenience to authors and readers.
| This journal is © The Royal Society of Chemistry 2017 |
