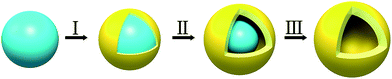Programmable synthesis of metal hydroxide/oxide hollow architectures: towards an efficient and robust photocatalyst for water remediation†
Penghui
Shao‡
,
Jiayu
Tian‡
*,
Wenxin
Shi
,
Yi
Yang
,
Xiaonan
Yang
,
Shanshan
Gao
and
Fuyi
Cui
*
State Key Laboratory of Urban Water Resource and Environment, Harbin Institute of Technology, Harbin 150090, PR China. E-mail: tjy800112@163.com; cuifuyi@hit.edu.cn
First published on 26th October 2016
Abstract
A general, one-pot, “soft” SiO2-templating strategy has been developed for the synthesis of well-defined metal hydroxide/oxide hollow nanostructures (HNs) with programmable shells. The principle of this unique strategy lies in controlling the balance of the reaction kinetics between the deposition, growth and crystallization of the shells and the simultaneous etching of the SiO2 cores by the superhot water in the hydrothermal system. As a proof-of-concept study, hydroxy iron oxide (FeOOH) HNs with tunable architecture, shape and thickness of shells have been synthesized via this proposed “soft” SiO2-templating method. Then, several important metal oxide HNs such as α-Fe2O3, CeO2, SnO2 and TiO2 have been successfully synthesized by simply altering the metal precursor and/or reaction temperature, highlighting the excellent versatility and reliability of the proposed strategy. Further, this novel method can be readily extended to the fabrication of multi-shelled HNs with tunable chemical compositions. The as-synthesized FeOOH HNs with hierarchical and thicker shells exhibited excellent photocatalytic activity and outstanding stability for sustainable degradation of organic pollutants in water.
Introduction
Hollow nanostructures (HNs) have attracted tremendous interest in various fields including energy storage, catalysis and water remediation, owing to their unique merits such as large surface area, high permeability, and low density.1–4 Engineering the shell architecture, shape and composition of HNs has been considered as one of the most promising strategies to optimize their physicochemical properties for various applications.5–12 As such, extensive research endeavors have been devoted to the development of new synthesis methods that can facilely tune the size, shape, thickness, number, and chemical composition of the shells, thereby endowing the HNs with desired properties towards specific applications.13–18 SiO2 hard template method (Si-HTM) has been regarded as one of the most popular approaches for fabricating the HNs. This is because SiO2 is easily obtainable and eco-friendly. Using the Si-HTM, Yin and co-workers synthesized well-defined TiO2 hollow spheres with controllable crystallinity, phase, and shell thickness.19–21 Despite such exciting progress, there still exist several difficulties in the programmable synthesis of HNs via the SiO2-templating method. Firstly, it is challenging to achieve homogeneous deposition of the designed materials on the non-modified surfaces of SiO2 templates in aqueous systems, especially on the high-curvature surfaces of non-spherical SiO2 templates such as SiO2 tetrahedra and polyhedra. Secondly, it is very difficult to maintain the reaction kinetics balance between the controlled growth of a shell with a specific composition and the synchronous etching of the SiO2 core. This could be the reason why the conventional Si-HTM usually involves multi-step and time-consuming synthesis procedures, including coating via the sol–gel method, annealing at high temperature and etching by a strong acid/base. Thirdly, current Si-HTMs can only succeed in obtaining single-shell hollow nanospheres with a single component, but cannot meet the needs for the synthesis of complex HNs in a programmable manner, thus limiting the opportunities for modulating the properties of HNs for many applications.Generally, SiO2 is regarded as a hard template for the fabrication of HNs, and etching of the hard SiO2 template is performed by using a strong acid or base solution.22,23 However, recent research has revealed that the SiO2 templates prepared by the classical Stöber method are not as “hard” as expected. For example, Chen et al. found that SiO2 can be disintegrated by hot water (90 °C).24 Yin and co-workers also considered that the insoluble SiO2 can be decomposed into the soluble monosilicic acid (Si(OH)4) by incubating it in the boiled water.25,26 These intriguing observations suggest that the hard SiO2 template can be softened and dissolved in heated water at a certain temperature. Therefore, based on this unique “soft” feature of SiO2, it is highly possible to achieve the growth of a desired shell and the dissolution of SiO2 core simultaneously in a hydrothermal system.
Inspired by the above considerations, a general, one-pot, “soft” SiO2-templating strategy has been developed for the synthesis of well-defined metal hydroxide/oxide HNs with programmable shells. The principle of this unique strategy lies in controlling the balance of the reaction kinetics between the deposition, growth and crystallization of the shell and the dissolution of the SiO2 core by superhot water in the hydrothermal system (Fig. 1). As a proof-of-concept study, hydroxy iron oxide (FeOOH) HNs with tunable architecture, shape and thickness of shells have been synthesized via the proposed “soft” SiO2-templating method. Then, several important metal oxide HNs such as α-Fe2O3, CeO2, SnO2 and TiO2 have been successfully synthesized by simply altering the metal precursor and/or reaction temperature, highlighting the excellent versatility and reliability of the proposed strategy. Furthermore, it is also worth noting that this strategy can be readily extended to the fabrication of some multi-shelled HNs with tunable chemical compositions. As an example to demonstrate the potential applications for water treatment, the hierarchical and thicker FeOOH shells exhibited excellent photocatalytic activity and outstanding stability for sustainable degradation of organic pollutants in water.
Results and discussion
Monodisperse SiO2 nanospheres with an average diameter of 118 nm were prepared by the classical Stöber method (Fig. S1†). The dissolution behavior of SiO2 was investigated under the hydrothermal treatment at different temperatures. It can be seen that the color of SiO2 solution exhibits a tendency to fade with the increase of reaction temperature (Fig. S2a†), which is in good agreement with the change of turbidity in the SiO2 solution (Fig. S2b†). These results indicate that the hard and insoluble SiO2 nanospheres have been transformed to the “soft” and soluble Si(OH)4 in the hydrothermal system. Meanwhile, the silicon concentration of the reaction system also increased from 0 to 411 mg L−1 with the increase of reaction temperature from 20 to 180 °C (Fig. S2b†), further supporting the above-mentioned observation. Theoretically, the as-synthesized amorphous SiO2 nanospheres are highly disordered and discontinuous. Thus, the Si–O–Si network structures in the amorphous SiO2 can be easily broken when it is incubated in water at high temperature and high pressure.24–26Based on this intriguing property of SiO2, it is considered that the growth of the designed materials against the SiO2 surface and the etching of the SiO2 template may proceed simultaneously under hydrothermal treatment, thereby giving rise to the HNs. To verify this hollowing approach, iron-based HNs were selected as the target materials due to their extensive applications in various fields, such as water remediation and energy storage.5,27,28 Iron chloride (FeCl3) and urea were respectively used as the metal precursor and alkaline source in this exploration because of their wide employment in the fabrication of iron-based nanomaterials. Unfortunately, the resulting nanomaterials are shown to be granular aggregations rather than the hollow architectures as we expected (Fig. S3†). This could be attributed to (1) the mismatch of reaction kinetics between the hydrolysis of Fe3+ ions and the dissolution of SiO2 nanospheres; (2) the insufficient adsorption capacity of Fe3+ ions on the non-modified surface of SiO2 owing to their weak electrostatic interactions (Table S1†). Therefore, ferric acetylacetonate (Fe(acac)3) was selected as the ferric precursor instead of FeCl3 because it possesses better stability and a higher electric potential (Table S1†). Eventually, highly uniform FeOOH hollow nanospheres with an average size of 162 nm (Fig. S4†) were successfully fabricated (Fig. 2a and S5†). It is also noted that no structural deformation or shell breakage is found for these hollow nanospheres, demonstrating the excellent reliability of this synthesis method. A higher magnification TEM image shows that the porous shells with a thickness of about 18 nm are composed of tiny nanoparticles (Fig. 2b). Interestingly, many ultrathin nanosheets (<3 nm) are randomly grafted onto the shell surface, thus leading to a hierarchical hollow architecture (Fig. 2b and d). The HAADF image and corresponding EDS mapping images further indicate that Fe and O are homogeneously distributed over the whole profile of hierarchical hollow nanospheres (Fig. 2c). The crystallographic structure of FeOOH hollow nanospheres was examined by X-ray diffraction (XRD). As shown in Fig. S6a,† all diffraction peaks in the patterns can be readily indexed to the phase of β-FeOOH (JCPDS card no. 341266). The selected-area electron diffraction (SAED) pattern (Fig. 2e) clearly displays the polycrystalline feature of these β-FeOOH hollow nanospheres. Although the crystallinity of the β-FeOOH hollow nanospheres is not that good, from an observation of XRD and SAED results, this instead may be conducive to their applications in the field of photochemistry and electrochemistry in view of some recent reports.29–34 The Fe 2p fine spectrum (Fig. S6b†) indicates that the levels of Fe 2p3/2 and 2p1/2 are 710.4 and 724.2 eV, respectively, with the satellite peak at ca. 719 eV, which is assigned to Fe3+ in β-FeOOH. A d spacing of 0.193 nm corresponding to the (411) plane of β-FeOOH can also be discerned in the high resolution TEM image (Fig. S7†).
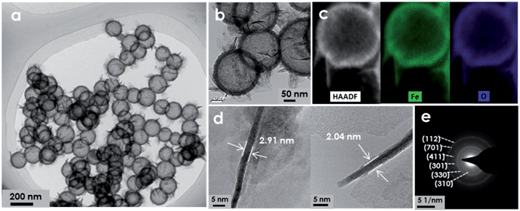 | ||
| Fig. 2 TEM images (a, b and d), HAADF image and the corresponding EDS mapping images (c), SAED pattern (e) of the FeOOH hierarchical hollow nanospheres. | ||
To gain an in-depth insight into this “soft” SiO2-assisted hollowing strategy, the effects of experimental conditions on the morphology of FeOOH hollow nanospheres have been investigated systematically. Firstly, time dependent investigation has been carried out in this work. Before starting the hydrothermal reaction, the mixed solution of SiO2 nanoparticles and Fe(acac)3 was thoroughly stirred; after that, the SiO2 was separated and characterized using a Fourier transform-infrared spectrophotometer (Fig. S8†). The result demonstrates that Fe(acac)3 can be easily adsorbed onto the surface of SiO2via the strong electrostatic interaction. When the temperature of the hydrothermal system increased from 25 to 160 °C, numerous solid SiO2@FeOOH core–shell hybrids were produced, suggesting that the SiO2 cores can maintain their structural integrity during the deposition of the FeOOH shells (Fig. S9a†). After hydrothermal treatment for only 0.5 h at 160 °C, a small amount of SiO2@FeOOH yolk–shell hybrids are observed in the system, together with large amounts of FeOOH hollow nanospheres (Fig. S9b†). The rapid morphological evolution from the solid core–shell hybrids to the yolk–shell hybrids and then to the hollow nanospheres is believed to result from the fast but mild etching of SiO2 by the superhot water under the high pressure. With the prolongation of reaction time to 6.0 h, the SiO2@FeOOH yolk–shell hybrids disappear completely and high-quality FeOOH hierarchical hollow nanospheres are obtained finally (Fig. S9c†).
Secondly, the effect of SiO2 dosage on the morphology of FeOOH hollow nanospheres has also been uncovered (Fig. S10†). When the dosage of SiO2 is 40 mg, uniform hollow FeOOH nanospheres can be obtained after 6.0 h of hydrothermal reaction. When the SiO2 dosage is increased to 80 mg, the hollow FeOOH nanospheres and SiO2@FeOOH yolk–shell hybrids are generated simultaneously. However, when SiO2 dosage is further increased to 120 mg, no hollow FeOOH nanospheres could be found in the typical TEM image. These observations illustrate that the dissolution of SiO2 has been limited by the amount of water in the reaction system. To be specific, the solubility of SiO2 in water under the given temperature and pressure should be a fixed value, and an over dosage of SiO2 could not be further dissolved in the limited amount of water in the hydrothermal system, which is further confirmed by the experimental results (Fig. S11†). This result highlights the importance of the mass ratio between the water and the SiO2 for the fabrication of HNs with this “soft” SiO2-templating strategy.
Thirdly, it is also found that the shell structure of FeOOH hollow nanospheres can be easily tuned by simply adjusting the dosage of urea and Fe(acac)3 (Fig. 3). For example, with the increase of urea dosage from 0 to 75 mg, the average thickness of the shells increased from 4.7 to 7.0 nm correspondingly. Thicker shells (∼13.2 nm) were obtained when 200 mg of urea was added. This is because urea works as the alkali source in the hydrothermal reaction, and a higher amount of urea can accelerate the hydrolysis of the ferric precursor, thus leading to the formation of thicker shells. Interestingly, the ultrathin nanosheets grafted on the shells show a growing trend in terms of amount and size with the increase of Fe(acac)3 dosage, thereby resulting in a complex and hierarchical hollow architecture. These results indicate that the ultrafine structure of the FeOOH shells can be readily regulated through this “soft” SiO2-templating method. More importantly, it provides a great opportunity to optimize the architecture of FeOOH HNs, thus developing a high-performance and robust photocatalyst for water remediation (detailed results as shown in Fig. 6).
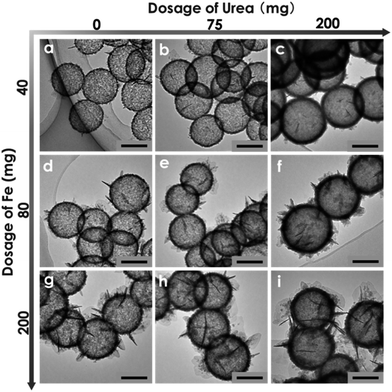 | ||
| Fig. 3 Morphological evolution of FeOOH hollow nanospheres with change in the dosage of Fe(acac)3 and urea. Scale bars: 100 nm. | ||
To further verify the reliability and versatility of this synthesis approach, SiO2 templates with various morphologies have been prepared. First, the much larger SiO2 spheres with an average diameter of 419 nm (Fig. S12a†) are selected as the templates. Fig. 4a and d show that the resultant products are the well-defined hierarchical δ-FeOOH hollow nanospheres with an average diameter of 457 nm. Encouraged by this exploration, non-spherical SiO2 with high curvature surfaces (e.g., tetrahedra, polyhedra) have been synthesized as templates to further challenge this hollowing strategy (Fig. S12b and c†). Interestingly, the appealing hollow δ-FeOOH tetrahedra and polyhedra with porous shells have been successfully fabricated (Fig. 4). To the best of our knowledge, this is the first time that a non-spherical SiO2 template has been employed for preparing anisotropic HNs.
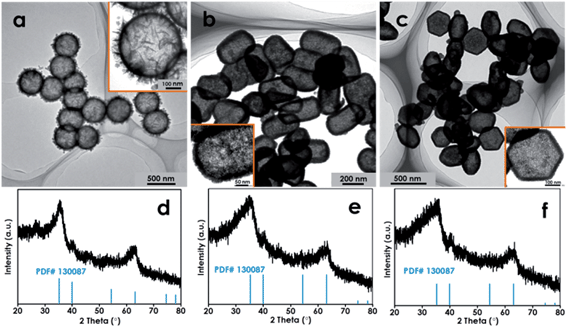 | ||
| Fig. 4 TEM images and XRD patterns of the bigger hollow FeOOH spheres (a and d), the hollow FeOOH tetrahedra (b and e) and the hollow polyhedra (c and f). | ||
In the experiments, it is also noticed that when the hydrothermal temperature increased from 160 to 200 °C, the crystallographic phase of hierarchical HNs could be readily transformed from β-FeOOH to α-Fe2O3, which is corroborated by the XRD and SAED results (Fig. 5e and S13†). This could be due to that the high reaction temperature can effectively accelerate the nucleation and growth of the oxide crystals.5,12,28 However, the unique architecture has been preserved intact during the transformation of the crystallographic structure (Fig. 5a). As a result, hierarchical α-Fe2O3 hollow nanospheres with porous shells are obtained. Additionally, several vital metal oxide hollow nanospheres such as CeO2, SnO2, and TiO2 have also been achieved by simply altering the metal precursor and reaction temperature (Fig. 5), highlighting the promising versatility of this synthesis approach. Compared to the conventional Si-HTM that usually involves the coating, annealing and etching procedures, the “soft” SiO2-templating method developed here exhibits significant advantages such as simple process procedure, mild reaction conditions, excellent reliability as well as promising versatility.
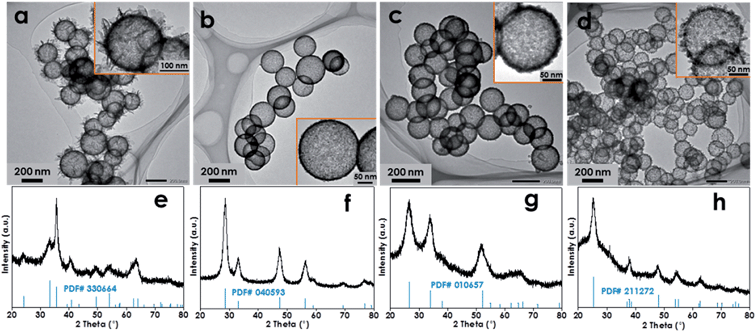 | ||
| Fig. 5 TEM images and XRD patterns of the α-Fe2O3 (a and e), CeO2 (b and f), SnO2 (c and g) and TiO2 (d and h) hollow nanospheres. | ||
More impressively, the design and synthesis of multi-shelled HNs with the desired shell composition can also be realized based on this new “soft” SiO2 template strategy. As a demonstration, double-shelled FeOOH HNs have been fabricated in this work. Firstly, single-shelled FeOOH hollow nanospheres were prepared as primary templates. Then, the surface of the primary templates are uniformly coated with the SiO2, thus producing SiO2@FeOOH shell-by-shell hollow nanospheres, which are named as secondary templates (Fig. S14†). By adding the secondary templates instead of the solid SiO2 nanospheres into the synthesis process, well-defined double-shelled FeOOH HNs can be obtained as shown in Fig. 6a and b. The elemental mapping result demonstrates that Fe and O are distributed homogeneously in both the outer shell and the inner shell (Fig. 6c). The XRD result (Fig. 6d) shows that the phase of the double-shelled FeOOH HNs is a mixture of akaganeite (JCPDS card no. 341266) and iron hydroxide (JCPDS card no. 220346). This mixed phase of the double-shelled FeOOH HNs may be conducive to their applications in the field of photochemistry, just like the commercial P25 TiO2 photocatalyst that is comprised of the anatase and rutile phases of TiO2. Also, the composition of the outer shell can be further tuned by simply employing the desired metal precursor to replace the Fe(acac)3. For example, when the acetylacetone tin(IV) dichloride salt is added, uniform SnO2@FeOOH double-shelled HNs can be achieved as shown in Fig. 6e. The elemental mapping image evidently suggests that the composition of the inner shell is Fe and O whereas that of the outer shell is Sn and O (Fig. 6f). The XRD pattern further confirms the co-existence of FeOOH and SnO2 in the products (Fig. 6g). It is noted that elaborate manipulation and integration of different materials with distinct physical/chemical properties has been rarely reported in previous studies, despite the fact that it is conceptually straightforward by a layer-by-layer templating strategy.35 Importantly, the fabrication method developed in this work can provide more opportunities for modulating and optimizing the properties of HNs to satisfy the specific requirements of various industrial applications.
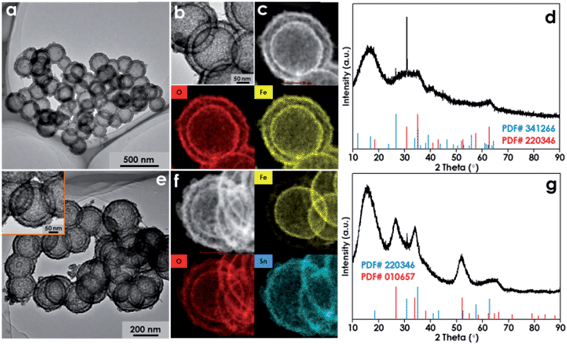 | ||
| Fig. 6 TEM images, HAADF image and the corresponding EDS mapping images, and XRD patterns: (a)–(d) for the double-shelled FeOOH HNs; (e)–(g) for the SnO2@FeOOH double-shelled HNs. | ||
Recently, photo-Fenton technology has emerged as an alternative strategy for water purification, owing to its environmental friendliness and general applicability. However, traditional photo-Fenton technology usually requires highly acidic conditions (pH 2–3) and generates large amounts of residual sludge, thus leading to a high treatment cost. To address these disadvantages, as-synthesized FeOOH HNs with different thicknesses and shell structures have been employed as heterogeneous photocatalysts for the degradation of rhodamine B (RhB) under natural pH. The blank experiment reveals that RhB is barely decomposed under visible light irradiation (Fig. 7a). The control experiments show that only limited removal efficiency (<20%) can be achieved in the presence of H2O2 and FeOOH HNs in the dark, and 24% of the RhB can be degraded with H2O2 and solid FeOOH nanoparticles under visible light irradiation (Fig. 7a and S15†). However, a significant increase in RhB removal has been observed with the co-existence of H2O2 and FeOOH HNs under visible light irradiation. This demonstrates that all the FeOOH HNs possess excellent photocatalytic activities due to their hollow features. By further comparing the degradation results of these FeOOH HNs, three obvious differences can be clearly discerned. Firstly, the removal efficiency of RhB gradually decreased from 92 to 72% with the increase of shell thickness from 4.7 to 13.2 nm (samples a–c). Coincidentally, both the surface areas and total pore volumes of these FeOOH HNs exhibit a similar declining tendency with the increase of shell thickness (Fig. 7b and Table S3†). Thus, it can be deduced that thicker shells with smaller surface areas and lower pore volumes might result in the decrease of active sites, which should be responsible for the decrease in photocatalytic performance. Secondly, the removal efficiency of RhB gradually increased from 72 to 84% with the increase of the amount and size of the ultrathin nanosheets grafted on the shells (samples c–i). This could be attributed to the increased complexity in the shell architecture, which can enhance the light absorption, reflection and scattering (Fig. 7c), and thus deliver higher photocatalytic efficiency.8,36 Thirdly, the cyclic degradation tests show that the RhB removal efficiency by the thinner shells (sample a) seriously decreased from 92 to 61% after three cycles of repeated use, which can be attributed to the severe collapse of the hollow architecture as observed by TEM (Fig. S16a†). However, the photocatalytic activity of the thicker hierarchical shells (sample i) has been well maintained in the cyclic tests owing to the excellent structural stability (Fig. S16b†). Besides, the thicker hierarchical FeOOH HNs are also found to be robust enough to withstand heat treatment upon converting to α-Fe2O3 at 700 °C, further highlighting their outstanding stability (Fig. S17†). Therefore, in view of practical application, the thicker hierarchical FeOOH shells should be a better candidate for the photocatalytic degradation of organic pollutants in water.
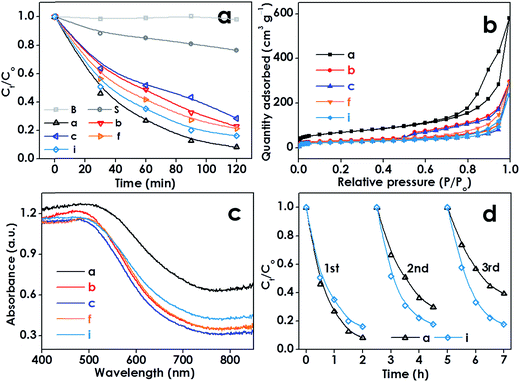 | ||
| Fig. 7 Photocatalytic efficiencies of the samples in the degradation of RhB under visible light irradiation (a). N2 adsorption–desorption isotherms (b) and diffuse reflectance spectra (c) of the samples. Cyclic photo-degradation tests for samples (a) and (i) (d). “B” and “S” denote “Blank” and “Solid”, respectively. The number of samples (a–i) was in agreement with Fig. 3 and the detailed sample description can be found in Table S2.† | ||
To elucidate the mechanism involved in the photocatalytic degradation of RhB, classical quenching tests have been carried out by employing tert-butanol as the scavenger for ˙OH radicals and ascorbic acid as the scavenger for O2˙− radicals (as shown in Fig. S18†).37 When tert-butanol was added in the reaction solution, the degradation effectiveness of RhB decreased from 92 to 51%. When ascorbic acid was added, only 70% of RhB could be degraded. Thus, it can be deduced that both ˙OH and O2˙− radicals generated by the FeOOH HNs under visible light irradiation make a certain contribution to the degradation of RhB.
The solution pH is generally considered as an important factor in the photo-Fenton system. Therefore, the effect of pH on the photo-Fenton performance of the FeOOH HNs (sample i) has been further investigated. As shown in Fig. S19,† although the photocatalytic activity of the FeOOH HNs decreased with the increase of pH value, 57% of RhB removal could still be achieved at a pH of 9.0, implying that the typical disadvantage of traditional Fenton and photo-Fenton technologies of requiring highly acidic conditions could be well overcome by employing the FeOOH HNs.
In addition, the colorless phenol has also been employed as a recalcitrant pollutant to further verify the photocatalytic activity of the FeOOH HNs (sample i). Fig. S20† shows that only very limited degradation of phenol (<3%) was achieved by the solid FeOOH nanoparticles. However, when the FeOOH HNs (sample i) were employed in the heterogeneous photo-Fenton process, the degradation efficiencies of phenol could reach up to 37%, demonstrating again the excellent photo-activity of the FeOOH hierarchical hollow nanospheres and their potential applications in water purification.
Conclusion
In summary, we have developed a general, one-pot, “soft” SiO2-templating method for the programmable synthesis of metal hydroxide/oxide HNs. The principle of this strategy lies in controlling the balance of reaction kinetics between the deposition, growth and crystallization of the shells and the simultaneous etching of SiO2 by superhot water in the hydrothermal system. The FeOOH HNs exhibited excellent photocatalytic activity and outstanding stability for water purification. We believe that the synthesis strategy proposed in this work could not only provide a novel and open-ended platform for the design and synthesis of various HNs for many applications, but also provide more possibilities for modulating and optimizing the properties of the HNs towards specific applications.Experimental section
Synthesis
Characterization
The micro-morphologies of the samples were observed by using the FEI Tecnai G2 F30 TEM, FEI Talos F200x TEM and JEOL JEM-1400 TEM. XRD patterns were collected by using a Bruker D8 X-ray diffractometer with Cu Kα radiation (λ = 0.15418 nm). The BET surface area measurements were performed by using an ASAP2020 instrument. The FTIR spectra were recorded on a Perkin-Elmer Spectrum One B spectrometer. The UV-vis diffuse spectra were obtained by using a Shimadzu UV2550 spectrophotometer. X-ray photoelectron spectroscopy measurements were performed using a Thermo ESCALAB 250 with Al Kα X-ray (hν = 1486.6 eV) radiation.Photocatalytic activities of HNs
The photocatalytic activities of HNs were evaluated by measuring the decomposition of RhB. A 300 W short-arc xenon lamp (Perkin-Elmer, PE300BF) equipped with a 400 nm cut-off filter was used as the light source. The reactor was made of open cylindrical Pyrex with diameter 4 cm and height 7 cm. The distance between the light source and the surface of the solute was approximately 20 cm. For photocatalytic degradation of RhB, 10 mg of the sample was suspended in 50 mL of RhB solution (Co = 20 mg L−1, pH = 6.1), and the mixture was stirred for 30 min to reach an adsorption equilibrium in the dark. 0.2 mL of H2O2 aqueous solution (1%) was added to the reaction solution at the beginning of irradiation. The temperature of the reactor was maintained at 25 °C by continuously circulating water. About 2.0 mL of the suspension was collected at regular intervals for the analysis of residual contaminant concentration. The concentrations of RhB were determined by the absorption peak at 554 nm, using a UV-vis spectrophotometer (Hach, DR5000).Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 51408162), Open Project of State Key Laboratory of Urban Water Resource and Environment, Harbin Institute of Technology (No. QA201524), the Heilongjiang Postdoctoral Special Fund (No. LBH-TZ0409), and the Postdoctoral Scientific Research Developmental Fund of Heilongjiang Province (No. LBH-Q14070).References
- J. Hu, M. Chen, X. Fang and L. Wu, Fabrication and Application of Inorganic Hollow Spheres, Chem. Soc. Rev., 2011, 40, 5472–5491 RSC.
- Y. Li and J. Shi, Hollow-Structured Mesoporous Materials: Chemical Synthesis, Functionalization and Applications, Adv. Mater., 2014, 26, 3176–3205 CrossRef CAS PubMed.
- Z. Wang, L. Zhou and X. W. Lou, Metal Oxide Hollow Nanostructures for Lithium-Ion Batteries, Adv. Mater., 2012, 24, 1903–1911 CrossRef CAS PubMed.
- X. Wang, J. I. Feng, Y. Bai, Q. Zhang and Y. Yin, Synthesis, Properties, and Applications of Hollow Micro-/Nanostructures, Chem. Rev., 2016, 116, 10983–11060 CrossRef CAS PubMed.
- B. Wang, H. Wu, L. Yu, R. Xu, T. Lim and X. W. Lou, Template-Free Formation of Uniform Urchin-Like α-FeOOH Hollow Spheres with Superior Capability for Water Treatment, Adv. Mater., 2012, 24, 1111–1116 CrossRef CAS PubMed.
- J. Qi, X. Lai, J. Wang, H. Tang, H. Ren, Y. Yang, Q. Jin, L. Zhang, R. Yu, G. Ma, Z. Su, H. Zhao and D. Wang, Multi-Shelled Hollow Micro-/Nanostructures, Chem. Soc. Rev., 2015, 44, 6749–6773 RSC.
- F. X. Ma, L. Yu, C. Y. Xu and X. W. Lou, Self-Supported Formation of Hierarchical NiCo2O4 Tetragonal Microtubes with Enhanced Electrochemical Properties, Energy Environ. Sci., 2016, 9, 862–866 CAS.
- Z. Dong, X. Lai, J. E. Halpert, N. Yang, L. Yi, J. Zhai, D. Wang, Z. Tang and L. Jiang, Accurate Control of Multishelled ZnO Hollow Microspheres for Dye-Sensitized Solar Cells with High Efficiency, Adv. Mater., 2012, 24, 1046–1049 CrossRef CAS PubMed.
- J. Zhu, Z. Yin, D. Yang, T. Sun, H. Yu, H. E. Hoster, H. H. Hng, H. Zhang and Q. Yan, α-Fe2O3 Multi-Shelled Hollow Microspheres for Lithium Ion Battery Anodes with Superior Capacity and Charge Retention, Energy Environ. Sci., 2014, 7, 632–637 Search PubMed.
- L. Shen, L. Yu, H. B. Wu, X. Y. Yu, X. Zhang and X. W. Lou, Formation of Nickel Cobalt Sulfide Ball-in-Ball Hollow Spheres with Enhanced Electrochemical Pseudocapacitive Properties, Nat. Commun., 2015, 6, 6694 CrossRef CAS PubMed.
- L. Zhang, L. Zhou, H. B. Wu, R. Xu and X. W. Lou, Unusual Formation of Single-Crystal Manganese Sulfide Microboxes Co-Mediated by the Cubic Crystal Structure and Shape, Angew. Chem., Int. Ed., 2012, 51, 7267–7270 CrossRef CAS PubMed.
- J. Nai, Y. Tian, X. Guan and L. Guo, Pearson's Principle Inspired Generalized Strategy for the Fabrication of Metal Hydroxide and Oxide Nanocages, J. Am. Chem. Soc., 2013, 135, 16082–16091 CrossRef CAS PubMed.
- M. H. Oh, T. Yu, S. H. Yu, B. Lim, K. T. Ko, M. G. Willinger, D. H. Seo, B. H. Kim, M. G. Cho, J. H. Park, K. Kang, Y. E. Sung, N. Pinna and T. Hyeon, Galvanic Replacement Reactions in Metal Oxide Nanocrystals, Science, 2013, 340, 964–968 CrossRef CAS PubMed.
- M. Hu, A. A. Belik, M. Imura, K. Mibu, Y. Tsujimoto and Y. Yamauchi, Synthesis of Superparamagnetic Nanoporous Iron Oxide Particles with Hollow Interiors by Using Prussian Blue Coordination Polymers, Chem. Mater., 2012, 24, 2698–2707 CrossRef CAS.
- F. X. Ma, H. Hu, H. B. Wu, C. Y. Xu, Z. Xu, L. Zhen and X. W. Lou, Formation of Uniform Fe3O4 Hollow Spheres Organized by Ultrathin Nanosheets and Their Excellent Lithium Storage Properties, Adv. Mater., 2015, 27, 4097–4101 CrossRef CAS PubMed.
- H. C. Zeng, Synthetic Architecture of Interior Space for Inorganic Nanostructures, J. Mater. Chem., 2006, 16, 649–662 RSC.
- X. Xu, Z. Zhang and X. Wang, Well-Defined Metal–Organic-Framework Hollow Nanostructures for Catalytic Reactions Involving Gases, Adv. Mater., 2015, 27, 5365–5371 CrossRef CAS PubMed.
- Z. Teng, X. Su, Y. Zheng, J. Zhang, Y. Liu, S. Wang, J. Wu, G. Chen, J. Wang, D. Zhao and G. Lu, A Facile Multi-Interface Transformation Approach to Monodisperse Multiple-Shelled Periodic Mesoporous Organosilica Hollow Spheres, J. Am. Chem. Soc., 2015, 137, 7935–7944 CrossRef CAS PubMed.
- H. Liu, J. B. Joo, M. Dahl, L. Fu, Z. Zeng and Y. Yin, Crystallinity Control of TiO2 Hollow Shells through Resin-Protected Calcination for Enhanced Photocatalytic Activity, Energy Environ. Sci., 2015, 8, 286–296 CAS.
- J. B. Joo, I. Lee, M. Dahl, G. D. Moon, F. Zaera and Y. Yin, Controllable Synthesis of Mesoporous TiO2 Hollow Shells: Toward an Efficient Photocatalyst, Adv. Funct. Mater., 2013, 23, 4246–4254 CrossRef CAS.
- J. B. Joo, M. Dahl, N. Li, F. Zaera and Y. Yin, Tailored Synthesis of Mesoporous TiO2 Hollow Nanostructures for Catalytic Applications, Energy Environ. Sci., 2013, 6, 2082–2092 CAS.
- K. Dong, Z. Liu and J. Ren, A General and Eco-Friendly Self-Etching Route to Prepare Highly Active and Stable Au@Metal Silicate Yolk–Shell Nanoreactors for Catalytic Reduction of 4-Nitrophenol, CrystEngComm, 2013, 15, 6329–6334 RSC.
- W. Li, Y. Deng, Z. Wu, X. Qian, J. Yang, Y. Wang, D. Gu, F. Zhang, B. Tu and D. Zhao, Hydrothermal Etching Assisted Crystallization: A Facile Route to Functional Yolk–Shell Titanate Microspheres with Ultrathin Nanosheets-Assembled Double Shells, J. Am. Chem. Soc., 2011, 133, 15830–15833 CrossRef CAS PubMed.
- Y. J. Wong, L. Zhu, W. S. Teo, Y. W. Tan, Y. Yang, C. Wang and H. Chen, Revisiting the Stöber Method: Inhomogeneity in Silica Shells, J. Am. Chem. Soc., 2011, 133, 11422–11425 CrossRef CAS PubMed.
- T. Zhang, J. Ge, Y. Hu, Q. Zhang, S. Aloni and Y. Yin, Formation of Hollow Silica Colloids through a Spontaneous Dissolution–Regrowth Process, Angew. Chem., 2008, 120, 5890–5895 CrossRef.
- Y. Hu, Q. Zhang, J. Goebl, T. Zhang and Y. Yin, Control over the Permeation of Silica Nanoshells by Surface-Protected Etching with Water, Phys. Chem. Chem. Phys., 2010, 12, 11836–11842 RSC.
- S. Xu, C. M. Hessel, H. Ren, R. Yu, Q. Jin, M. Yang, H. Zhao and D. Wang, α-Fe2O3 Multi-Shelled Hollow Microspheres for Lithium Ion Battery Anodes with Superior Capacity and Charge Retention, Energy Environ. Sci., 2014, 7, 632–637 CAS.
- B. Wang, J. S. Chen, H. B. Wu, Z. Wang and X. W. Lou, Quasiemulsion-Templated Formation of α-Fe2O3 Hollow Spheres with Enhanced Lithium Storage Properties, J. Am. Chem. Soc., 2011, 133, 17146–17148 CrossRef CAS PubMed.
- J. Nai, Z. Chen, H. Li, F. Li, Y. Bai, L. Li and L. Guo, Structure-Dependent Electrocatalysis of Ni(OH)2 Hourglass-Like Nanostructures Towards L-Histidine, Chem.–Eur. J., 2013, 19, 501–508 CrossRef CAS PubMed.
- H. B. Li, M. H. Yu, F. X. Wang, P. Liu, Y. Liang, J. Xiao, C. X. Wang, Y. X. Tong and G. W. Yang, Amorphous Nickel Hydroxide Nanospheres with Ultrahigh Capacitance and Energy Density as Electrochemical Pseudocapacitor Materials, Nat. Commun., 2013, 4, 1894 CrossRef CAS PubMed.
- J. Guo, Q. Liu, C. Wang and M. R. Zachariah, Interdispersed Amorphous MnOx–Carbon Nanocomposites with Superior Electrochemical Performance as Lithium-Storage Material, Adv. Funct. Mater., 2012, 22, 803–811 CrossRef CAS.
- J. W. Hall, N. Membreno, J. Wu, H. Celio, R. A. Jones and K. J. Stevenson, Low-Temperature Synthesis of Amorphous FeP2 and Its Use as Anodes for Li Ion Batteries, J. Am. Chem. Soc., 2012, 134, 5532–5535 CrossRef CAS PubMed.
- S. H. Choi, D. Hwang, D. Y. Kim, Y. Kervella, P. Maldivi, S. Y. Jang, R. Demadrille and I. D. Kim, Amorphous Zinc Stannate (Zn2SnO4) Nanofibers Networks as Photoelectrodes for Organic Dye-Sensitized Solar Cells, Adv. Funct. Mater., 2013, 23, 3146–3155 CrossRef CAS.
- P. Shao, J. Tian, Z. Zhao, W. Shi, S. Gao and F. Cui, Amorphous TiO2 Doped with Carbon for Visible Light Photodegradation of Rhodamine B and 4-Chlorophenol, Appl. Surf. Sci., 2015, 324, 35–43 CrossRef CAS.
- H. Hu, B. Guan, B. Xia and X. W. Lou, Designed Formation of Co3O4/NiCo2O4 Double-Shelled Nanocages with Enhanced Pseudocapacitive and Electrocatalytic Properties, J. Am. Chem. Soc., 2015, 137, 5590–5595 CrossRef CAS PubMed.
- J. Qi, K. Zhao, G. Li, Y. Gao, H. Zhao, R. Yu and Z. Tang, Multi-Shelled CeO2 Hollow Microspheres as Superior Photocatalysts for Water Oxidation, Nanoscale, 2014, 6, 4072–4077 RSC.
- P. Shao, J. Tian, B. Liu, W. Shi, S. Gao, Y. Song, M. Ling and F. Cui, Morphology-tunable Ultrafine Metal Oxide Nanostructures Uniformly Grown on Graphene and Their Applications in the Photo-Fenton System, Nanoscale, 2015, 7, 14254–14263 RSC.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ta08042a |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2017 |