Thermostable polymeric nanomicelles of iridium(III) complexes with aggregation-induced phosphorescence emission characteristics and their recyclable double-strand DNA monitoring†
Jingwen
Ma‡
,
Yun
Zeng‡
,
Yongchun
Liu
and
Daocheng
Wu
*
Key Laboratory of Biomedical Information Engineering of Education Ministry, School of Life Science and Technology, Xi'an Jiaotong University, Xi'an, 710049, P. R. China. E-mail: wudaocheng@mail.xjtu.edu.cn; Tel: +86 029 82663941
First published on 1st November 2016
Abstract
Using 4,7-diphenyl-1,10-phenanthroline (DIP) as a main ligand and ethyl cyanoacrylate (ECA) as both an auxiliary ligand and a polymer skeleton, polymer iridium(III) complexes (DIP)2Ir(ECA) and their nanomicelles with aggregation-induced phosphorescence emission (AIPE) activity were synthesized. The morphology, chemical structure and composition of (DIP)2Ir(ECA) nanomicelles were characterized using transmission electron microscopy, size distribution/zeta potential analysis, nuclear magnetic resonance, and Fourier transform infrared spectroscopy. Their AIPE-active effects and double-stranded DNA (dsDNA) monitoring abilities were determined using phosphorescence spectroscopy using a spectrophotofluorometer. The results showed that (DIP)2Ir(ECA) nanomicelles had a good thermostability within 0–100 °C and their size distribution was 29.14 ± 1.46 nm. These nanomicelles showed AIPE-active effects and their phosphorescence intensity increased nearly 30-fold in water compared to in acetone. These nanomicelles could be used in AIPE-active intracellular imaging and dsDNA monitoring. Owing to the specific phosphorescence quenching that occurred when dsDNA encountered (DIP)2Ir(ECA) nanomicelles, thermostable (DIP)2Ir(ECA) nanomicelles could quickly detect dsDNA with high sensitivity and could be conveniently applied not only in monitoring DNA degradation in a wider pH range (specifically in an acidic environment), but also during PCR procedures. More importantly, both (DIP)2Ir(ECA) nanomicelles and immobilized DNase I could be recycled and utilized at least four times using our novel phosphorescence “quenching-recovery” dsDNA detection procedure. The polymeric (DIP)2Ir(ECA) nanomicelles were fast, sensitive, and convenient in monitoring dsDNA and could be recycled four times owing to their thermostability, indicating their great potential in biomedical and environmental applications.
1. Introduction
Aggregation-induced emission (AIE) was first reported by Tang B. et al. in 2001.1 Traditional fluorophores self-quench at high concentrations, but AIE fluorophores have the distinguishing feature of demonstrating a significant increase in intensity and quantum yield in the solid state. Therefore, they are frequently used in the solid thin-film or aggregated form. Silole derivatives with AIE properties were previously developed,1 and their distinguishing feature was caused by the restriction of intramolecular rotation (RIR).2 These derivatives have been applied in many fields, such as fluorescence quenching, pH sensing, and biological probing. They particularly demonstrate specific selectivity and high sensitivity towards different ions, proteins, DNA, and RNA in water.3Afterwards, many transition metal complexes also displayed AIE properties. Unlike fluorophores, transition metal complexes emit phosphorescence aside from fluorescence after irradiation. This phenomenon is aggregation-induced phosphorescence emission (AIPE). Phosphorescent nanowires have been synthesized from platinum(II) complexes with AIPE properties via π–π stacking interactions.4,5 Apart from platinum(II) complexes, iridium(III) complexes with AIPE properties are also studied as an important category. Zhao Q. et al. firstly developed iridium(III) complexes with intense AIPE properties.6 Subsequently, a large number of AIPE-active iridium(III) complexes have been synthesized.7–9 Compared with fluorescent dyes, transition metal complexes have better thermostability and activity in a broader pH range. Iridium(III) complexes also have good optical properties, such as high quantum yield and appropriate and tunable spectra.
β-Diketone chemicals are the most common ligands, which are used to adjust the spectra of iridium(III) complexes. However, the water insolubility of inorganic iridium(III) complexes with β-diketone chemicals restricts their application in biological reactions.10,11 Not only can fabricating iridium(III) complexes into nanoparticles enhance their phosphorescence intensity and quantum yield, but it can also improve their solubility. In our previous research, we introduced poly(n-butyl cyanoacrylate)/chitosan nanoparticles into iridium(III) complexes to improve their solubility and tumor imaging ability.12 Their improved characteristics were partially dependent on the coordination between iridium(III) ions and cyanoacrylate with a β-diketone structure.13 Furthermore, the functions and applications of these polymeric nanoparticles could be realized by their functional chemical groups.
As a carrier of hereditary information, DNA is utilized in various applications, such as DNA scaffolding,14 drug delivery,15 analytical sensing,16 and recently anti-counterfeiting technology. The protection and de-protection of DNA are necessary in anti-counterfeiting systems.17 The most commonly used method of DNA protection and de-protection is through encapsulation in starch or silica capsules synthesized by inverse micro-emulsion.18–20 Polymerase chain reaction (PCR) is a very common method in biological fields and is frequently utilized to evaluate the efficiency of a DNA-based anti-counterfeit system. However, PCR apparatus is expensive. Fluorescent dyes, such as SYBR Green I, are required to monitor the quantity of double-stranded DNA (dsDNA) during the PCR process. In addition, these fluorescent dyes are usually susceptible to the prevailing environment. Some of these dyes are inactivated at high temperatures, at extreme (too high or too low) pH values, and in off-standard PCR environments. Photo-bleaching is also a severe challenge with these fluorescent dyes. Furthermore, although real-time PCR can monitor DNA amplification, it is time-consuming, and the apparatus and dyes required are expensive and cumbersome. Therefore, it is critical to identify an economical detection probe for the dynamic and rapid monitoring of the DNA state in a dynamic process, such as PCR. Currently, most fluorescent dyes are expensive and intolerant to heating and acidic environments, and cannot be recycled for repeated use. Thus, conventional fluorescent dyes cannot be used for the dynamic and rapid monitoring of DNA. There have also been few reports on recyclable bio-macromolecule detection, such as DNA. Furthermore, waste fluorescent materials are harmful to the environment. Recyclable fluorescent materials could alleviate their polluting effects on the environment.
Polypyridyl metal complexes with copper(II), iron(II), or ruthenium(II) as central metal ions interact with DNA and can be potentially used as anti-tumor drugs or bio-imaging agents.21–23 Polypyridyl ruthenium(II) complexes are commonly used in precision imaging of the DNA structure in living cells because they can be inserted into the grooves of dsDNA.24 Cao R. et al. synthesized 4,7-diphenyl-1,10-phenanthroline (DIP)-containing iridium(III) complexes with improved anti-tumor and imaging effects.25 Therefore, we hypothesized that iridium(III) polypyridyl complexes with AIPE properties might have excellent properties in DNA monitoring owing to their high phosphorescence efficiency and easily tunable color.
Since iridium(III) is a noble metal, using (DIP)2Ir(ECA) nanomicelles [ethyl cyanoacrylate (ECA)] repeatedly will save costs and also prevent the need for additional purification processes to remove them from waste streams, thereby making them “greener” compared with common approaches. However, very few AIE fluorescent materials have been reported as recyclable detectors. Li et al. constructed a water-soluble detector by grafting an amine-modified mesoporous SBA-15. The fluorescence of the detector was quenched by picric acid and also remained active even after five cycles. However, AIE detection by picric acid quenching cannot be used in other systems, such as DNA monitoring.26 So far, most recyclable detectors can only recycle one compound, and the recycling or activation processes are complex, requiring magnetic separation or centrifugal separation.
As described above, we synthesized and utilized AIPE iridium(III) polypyridyl complexes as bio-detectors and designed a new strategy for real-time monitoring of dsDNA. First, we synthesized polypyridyl DIP and iridium(III) to chloro-bridged dimers. Then, ECA acted as an auxiliary ligand and a polymer skeleton, and iridium(III) dimer complexes (DIP)2Ir(ECA) were fabricated into AIPE-active iridium(III) complexes by simultaneous coordination and polymerization. Finally, the complexes were self-assembled into (DIP)2Ir(ECA) nanomicelles. The (DIP)2Ir(ECA) nanomicelles showed heat and acid resistance without any adverse effects on the PCR process. More importantly, the (DIP)2Ir(ECA) nanomicelles could not only be inserted into DNA and showed phosphorescence quenching, but could also be reused without separation by degrading DNA and enzymes with the addition of DNase I and heating to 80 °C. (DIP)2Ir(ECA) nanomicellar phosphorescence could be recovered and used repeatedly at least four times without any significant loss in monitoring activity. DNase I could also be recovered four times via magnetic separation to achieve “green” detection, substantially reducing costs and pollution. The (DIP)2Ir(ECA) nanomicelles could be used in the rapid, convenient, and inexpensive monitoring of DNA degradation. Recyclable and reactivation methods are simple and rapid. The results of the current study are in agreement with our design. Therefore, thermostable polymeric iridium(III) complexes, (DIP)2Ir(ECA) nanomicelles, have great potential in biomedical and environmental applications.
2. Experimental section
2.1. Experimental reagents
IrCl3 and DIP were purchased from Sigma-Aldrich Trading Co., Ltd (Shanghai, China). ECA was purchased from Zhejiang Gold Roc chemical Co., Ltd (Taizhou, China). Bovine serum albumin (BSA), cysteine, NaCl, single-stranded RNA (ssRNA), glutathione (GSH), glycine (Gly), L-tryptophan (L-Trp), tetrahydrofuran (THF), dimethyl sulphoxide (DMSO), methyl thiazolyl tetrazolium (MTT) and calf thymus DNA were purchased from Aladdin Co., Ltd (Shanghai, China). Specific DNA sequences, DNase I, and primers were ordered from Sangon Biotech Co., Ltd (Shanghai, China). SYBR Green I and PCR reagents (loading buffer, dNTPs, Taq enzyme, and DNA target template) were purchased from Takara Co., Ltd (Dalian, China). Thermostable hollow magnetic microspheres CS(2.0)-5 were prepared via layer-by-layer (LbL) assembly on a uniform melamine-formaldehyde resin microsphere template coated with silica. Organic resin was burnt off by calcination, as previously reported by our group. The microspheres had an average diameter of 2.0 μm. Polydopamine was deposited on CS(2.0)-5 microspheres [CS(2.0)-5@PDA] to conjugate DNase I as previously described.27 Inorganic reagents and solvents were purchased from Tianjin Chemical Reagents Co., Ltd (Tianjin, China). All reagents were of analytical grade and used directly without further purification. All the cell lines (A549, PC3 and IMR-90) were provided by the School of Life Science and Technology, Xi'an Jiaotong University.2.2. Fabrication of (DIP)2Ir(ECA) and nanomicelles
First, iridium(III) chloro-bridged dimers were synthesized according to a previous study.12 Briefly, DIP (1.0 mmol, 332.40 mg) and IrCl3 (0.5 mmol, 149.28 mg) were poured into a flask containing a solvent mixture of 16.3 mL of 2-ethoxyethanol and 5.4 mL of H2O. The DIP and IrCl3 solution was heated at 106 °C with magnetic stirring and nitrogen protection for 15 h to evaporate the solvent to dryness. The powder residue was collected.Iridium(III) chloro-bridged dimers (78.0 mg) and ECA (200 μL) were added to 20 mL of 2-methoxyethanol. (DIP)2Ir(ECA) was obtained after the reaction of the entire system at 95 °C for 2 h. An ultrasound/microwave-assisted extraction apparatus (CW-2000, Xintuo, China) was utilized for coordination and polymerization.
A total of 50 μL of (DIP)2Ir(ECA) pregnant liquor was added to 4.0 mL of deionized H2O and stirred for 1 h. (DIP)2Ir(ECA) nanomicelles were then obtained and adjusted to a concentration of 0.05 mg mL−1. Additionally, two kinds of Ir(III) complex polymers with 2-(2-pyridyl)benzothiophene as a ligand and with polyethyleneimine (PEI) as a skeleton were synthesized using a similar method as for the (DIP)2Ir(ECA) polymer.
2.3. Characterization of (DIP)2Ir(ECA) and nanomicelles
Pregnant liquor (100 μL) was added to different volumes of deionized H2O to obtain phosphorescent nanomicelles. The (DIP)2Ir(ECA) nanomicelles were measured using a Malvern instrument (Nano-ZS92, Malvern, UK) to acquire their size distribution and zeta potential. The stability of the (DIP)2Ir(ECA) nanomicelles was measured at different levels of pH, temperature, and ionic strength.The morphology, structure, and composition of the (DIP)2Ir(ECA) nanomicelles were confirmed using a transmission electron microscope (TEM; H-800, Hitachi, Japan).
The chemical structure and functional groups of the (DIP)2Ir(ECA) nanomicelles were determined using a nuclear magnetic resonance (NMR) system (ADVANCED III, Bruker, Germany) and a Fourier transform infrared spectroscopy (FTIR) instrument (TENSOR27, Bruker, Germany). The samples were dissolved in DMSO-d6 and then injected into NMR tubes. The 1H NMR spectrum of each sample [including DIP, ECA and (DIP)2Ir(ECA) polymer] was obtained using an NMR instrument. The FTIR spectra of DIP, ECA, and (DIP)2Ir(ECA) were obtained as well. Phosphorescence spectra were recorded using a spectrophotofluorometer (FluoroMax-4, HORIBA Jobin Yvon, France). The excitation wavelength was set to 365 nm and the emission spectra were recorded in the 440–570 nm wavelength range. Gel permeation chromatography Gel permeation chromatography (GPC, Waters 1515, U.S.) was used to measure the molecular weight of the (DIP)2Ir(ECA) polymer.
2.4. Measurement of nanomicellar AIPE properties
(DIP)2Ir(ECA) nanomicelles were dissolved in H2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) acetone solutions of different proportions: pure H2O, 7
acetone solutions of different proportions: pure H2O, 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 3
1, 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, and pure acetone. Maximum spectral emission peaks of the spectra in different H2O:acetone proportions were obtained (excitation: 365 nm; emission: 440–570 nm). Maximum emission peaks of the spectra at different H2O-dilution factors (1.25, 2.5, 10, 30 and 60) were confirmed. Pregnant liquor was dropped onto a filter paper, excited with an ultraviolet lamp, and observed for the AIPE phenomenon.
3, and pure acetone. Maximum spectral emission peaks of the spectra in different H2O:acetone proportions were obtained (excitation: 365 nm; emission: 440–570 nm). Maximum emission peaks of the spectra at different H2O-dilution factors (1.25, 2.5, 10, 30 and 60) were confirmed. Pregnant liquor was dropped onto a filter paper, excited with an ultraviolet lamp, and observed for the AIPE phenomenon.
(DIP)2Ir(ECA) nanomicelles (0.05 mg mL−1) were added to A549 cell cultures at a final concentration of 2%. AIPE imaging was then performed using an inverted fluorescence microscope.
Calf thymus DNA, single-stranded RNA, NaCl, cysteine, or BSA were added at a concentration of 2.0 mg mL−1 to the (DIP)2Ir(ECA) nanomicellar solutions. The samples were excited with an ultraviolet lamp to observe phosphorescence quenching. All the emission spectra were recorded. The same concentrations of dsDNA or single-stranded RNA were added to the nanomicellar solutions, and the samples were heated to melt the DNA. The quenching effects were observed and the quantitative data were recorded.
The phosphorescence lifetimes of the (DIP)2Ir(ECA) nanomicelles in solvent with different water content were also measured using a time-resolved photoluminescence spectroscopy setup (QM 40, Photon Technology International, U.S.). The water![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) acetone (v/v%) ratio was set as 100%, 90%, 75%, 50%, 25%, 10% and 0%. The excitation wavelength was 366 nm, and the emission wavelength was set at 446 nm. To further investigate whether the presence of other compounds would affect the dsDNA detection properties, 50 μL of 5.0 mg mL−1 substance was added to 4.0 mL of 0.05 mg mL−1 (DIP)2Ir(ECA) nanomicelles before the addition of 50 μL of 2.0 mg mL−1 dsDNA. The phosphorescence intensity was measured to assess the effects. The involved substance included NaCl, BSA, GSH, Gly and L-Trp. To further evaluate the stability of the (DIP)2Ir(ECA) nanomicelles, the phosphorescence intensity was measured for a week. To evaluate the thermal stability, the phosphorescence intensity of the (DIP)2Ir(ECA) nanomicelles was recorded from 25–85 °C with a gradient of 10 °C. An MTT assay was carried out to evaluate the cell viability of the (DIP)2Ir(ECA) nanomicelles. 0.0, 2.5, 5.0, 7.5 and 10.0 μg mL−1 of the (DIP)2Ir(ECA) nanomicelles were added to human embryo lung fibroblast IMR-90 cells and human prostate cancer PC3 cells in a 96-well plate. After incubation at 37 °C for 24 h, the absorbance at 495 nm was measured using a microplate reader (Infinite® 200 Pro, Tecan, Switzerland). In addition, the pathological section was investigated to assess the pathological effect on female BALB/C mice. Hematoxylin–eosin (HE) stains were introduced to stain both the control and (DIP)2Ir(ECA) nanomicelle treated groups. The sections were evaluated using a microscope.
acetone (v/v%) ratio was set as 100%, 90%, 75%, 50%, 25%, 10% and 0%. The excitation wavelength was 366 nm, and the emission wavelength was set at 446 nm. To further investigate whether the presence of other compounds would affect the dsDNA detection properties, 50 μL of 5.0 mg mL−1 substance was added to 4.0 mL of 0.05 mg mL−1 (DIP)2Ir(ECA) nanomicelles before the addition of 50 μL of 2.0 mg mL−1 dsDNA. The phosphorescence intensity was measured to assess the effects. The involved substance included NaCl, BSA, GSH, Gly and L-Trp. To further evaluate the stability of the (DIP)2Ir(ECA) nanomicelles, the phosphorescence intensity was measured for a week. To evaluate the thermal stability, the phosphorescence intensity of the (DIP)2Ir(ECA) nanomicelles was recorded from 25–85 °C with a gradient of 10 °C. An MTT assay was carried out to evaluate the cell viability of the (DIP)2Ir(ECA) nanomicelles. 0.0, 2.5, 5.0, 7.5 and 10.0 μg mL−1 of the (DIP)2Ir(ECA) nanomicelles were added to human embryo lung fibroblast IMR-90 cells and human prostate cancer PC3 cells in a 96-well plate. After incubation at 37 °C for 24 h, the absorbance at 495 nm was measured using a microplate reader (Infinite® 200 Pro, Tecan, Switzerland). In addition, the pathological section was investigated to assess the pathological effect on female BALB/C mice. Hematoxylin–eosin (HE) stains were introduced to stain both the control and (DIP)2Ir(ECA) nanomicelle treated groups. The sections were evaluated using a microscope.
2.5. Determination of the analytical detection limit and range linearity
The detection limit (DL) and the range of linearity were measured and calculated as follows: a specific DNA sequence (5′-GGC AGG GAT GGC TTT TTG CCA TCC CTG CC-3′) was designed to form a hairpin structure containing at least one double-stranded helix. Eleven contrasting samples were investigated, and the standard deviation (SD) was calculated. Furthermore, the range of linearity of quenching and the slope (n = 3) were also determined. The DL was calculated using the following equation: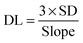 | (2.1) |
2.6. Monitoring of DNA hydrolysis and PCR procedure
A total of 50 μL of calf thymus DNA (2.0 mg mL−1) was added to 4.0 mL of the (DIP)2Ir(ECA) nanomicellar (0.05 mg mL−1) solution. Different pH environments were set at 4.49, 5.29, 5.91, 6.98, 8.04, and 9.18 in pH buffers. The effects of phosphorescence quenching were measured in these different pH environments. DNA was degraded by DNase I (50 μL, 5.0 mg mL−1) to simulate DNA hydrolysis. Phosphorescence signals were recorded per minute (n = 3).The PCR procedure followed the standard protocol and was performed using a quantitative real-time PCR instrument (Lightcycler 480II, Roche, Switzerland). A 200 μL PCR sample contained the following components: deionized H2O, 145 μL of loading buffer (10×), 20 μL of dNTPs (2.5 μmol L−1), 16 μL of the up-stream primer (5′-GGC TTC GGT CCC TTC TGT-3′, 100 μM), 4 μL of the down-stream primer (5′-CAC CAC CTG TTC AAA CTC TGC-3′, 100 μmol L−1), 4 μL of Taq enzyme (5U μL−1), and 1–2 μL of the DNA target template (λDNA, 0.35 μg μL−1). PCR progress was monitored using 10 μL of pregnant liquor or 10 μL of SYBR Green I. The samples were divided into several portions for convenient replication and incubated at 95 °C for 2 min. The PCR conditions were as follows: 95 °C for 30 s, 66 °C for 60 s, and 72 °C for 30 s. There were 16 PCR cycles.
2.7. Monitoring of DNA hydrolysis using immobilized DNase I
Phosphorescence recovery was calculated using the following formula:
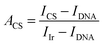 | (2.2) |
2.8. Recyclable dsDNA detection of (DIP)2Ir(ECA) nanomicelles
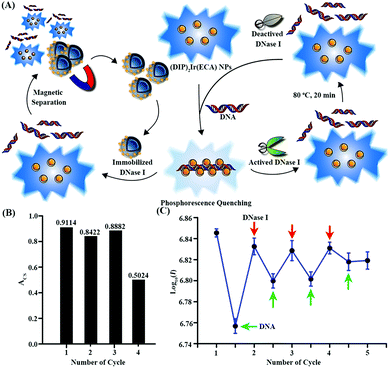
The following experiments were performed to evaluate the ability of the recyclable (DIP)2Ir(ECA) nanomicelles in the repeated detection of dsDNA. To detect dsDNA, 50 μL of calf thymus DNA (2.0 mg mL−1) was mixed with 4.0 mL of (DIP)2Ir(ECA) nanomicelles (0.05 mg mL−1). (DIP)2Ir(ECA) nanomicellar phosphorescence was quenched. Then, 50 μL of DNase I (5.0 mg mL−1) was added to hydrolyze DNA for 5 min to recover the phosphorescence of the (DIP)2Ir(ECA) nanomicelles. The system was placed in an 80 °C water bath for 20 min to deactivate DNase I. This testing procedure was performed four times to evaluate the (DIP)2Ir(ECA) nanomicelles' recyclable dsDNA detection ability. The emission phosphorescence spectrum was obtained from 385 nm to 600 nm under 365 nm excitation. Three parallel experiments were performed simultaneously.3. Results and discussion
3.1. Synthesis of (DIP)2(ECA) and (DIP)2Ir(ECA) nanomicelles
We successfully synthesized an amphipathic polymer iridium(III) complex, (DIP)2Ir(ECA), with AIPE properties. Its structure is shown in Fig. 1; DIP was the main ligand containing the functional chemical group for binding DNA. ECA was the auxiliary ligand and also polymerized as the skeleton. Subsequently, we synthesized this polymer into (DIP)2Ir(ECA) nanomicelles via self-assembly. Finally, the amphipathic polymer was dissolved in organic solvent and then dropped into water to form (DIP)2Ir(ECA) nanomicelles. Owing to the fact that (DIP)2Ir(ECA) was the amphipathic polymer, the hydrophobic portion of the iridium(III) coordination compound formed the nanomicelle core via self-assembly, allowing for the observation of its AIPE properties.28,29 Compared with the complex fabrication methods for block copolymers with phosphorescent iridium(III) complexes, fabrication of metal complexes into nanomicelles via self-assembly is a convenient method for use in certain applications.30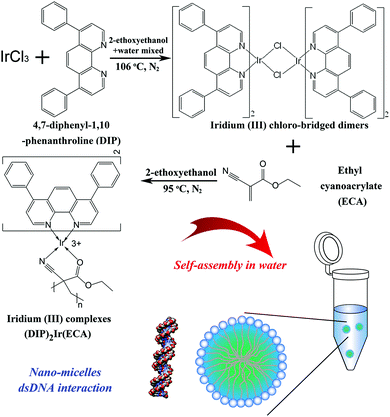 | ||
| Fig. 1 The scheme of AIPE (DIP)2Ir(ECA) complex synthesis as well as the nanomicelle fabrication processes. | ||
3.2. Characterization of (DIP)2Ir(ECA) and the nanomicelles
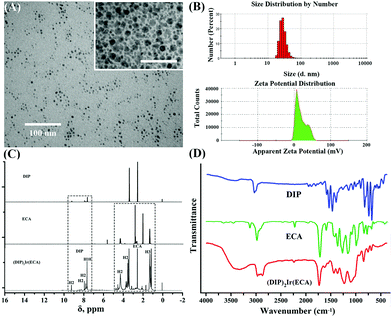
The size distribution and zeta potential of the (DIP)2Ir(ECA) nanomicelles were characterized using a TEM and a Malvern size analyzer. TEM images of the (DIP)2Ir(ECA) nanomicelle morphology (Fig. 2A) showed that the nanomicelles had a relatively narrow size distribution (approximately 10 nm). The size distribution of (DIP)2Ir(ECA) in organic solvent (acetone) was also measured and we found that the size was about 1000 nm and its polydispersity index was extremely high (1.0), which implied that the (DIP)2Ir(ECA) polymer could not form nanomicelles in acetone and that the phosphorescence was absent in acetone. Thus, the (DIP)2Ir(ECA) nanomicelles had a narrower size distribution and significantly higher phosphorescence intensity. Size analyzer results showed that the nanomicelles had a particle size of 29.14 ± 1.46 nm, a polydispersity index of 0.181, and a surface zeta potential of +18.6 ± 0.93 mV. The cationic iridium complexes contributed to the positive potential (Fig. 2B). The results obtained by the size analyzer were significantly higher than those obtained using a TEM because of the existence of the hydrated shell outside. The hydrated radius of the nanomicelles affected the dynamic light scattering (DLS) results. (DIP)2Ir(ECA) nanomicellar stability was measured. The phosphorescence intensity was stable as well, although it increased gradually and slightly, which was probably because of the evaporation of the residual organic solvent to enhance the AIPE effect (Fig. 5C). In addition, the (DIP)2Ir(ECA) nanomicelles were stable in an acidic environment (pH < 7) (Fig. 6), at a high temperature (as high as 85 °C) (Fig. 4D), and under low ionic strength (0.1 mol L−1).The chemical structure of DIP, ECA and (DIP)2Ir(ECA) was confirmed using NMR and FTIR. Both NMR and FTIR could illustrate the chemical structure of a polymer containing transition metal complex units, which we desired to synthesize (Fig. 2C and D). The 1H NMR (400 MHz, DMSO-d6) spectrum contained the following peaks: δ, ppm 9.18 (d, 2H, Phenanthroline H-2), 7.88 (s, 2H, Phenanthroline H-5), 7.75 (d, 2H, Phenanthroline H-3), 7.61 (d, 10H, Phenyl ring H), 4.23 (q, 2H, –CH2–), 3.44 (d, 2H, –CH2CH3), and 1.10 (t, 3H, –CH2CH3). δ 2.51 and 3.35 were solvent peaks, including DMSO and a small amount of water. Previous studies reported that the 1H NMR spectrum included DIP and ECA peaks.31–33 Our 1H NMR spectrum showed that we had synthesized a new compound with both DIP and ECA units. We found that the spectrum of the (DIP)2Ir(ECA) polymer contained both DIP and ECA characteristic units, indicating that the (DIP)2Ir(ECA) polymer was a new composite complex. In addition, the GPC result also demonstrated that the Mn and Mw value of the (DIP)2Ir(ECA) polymer were 32![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 109 and 56
109 and 56![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 418, respectively (Fig. S1, ESI†), in agreement with both the 1H NMR and FTIR results. Fig. 2D shows the FTIR spectra of DIP, ECA, and (DIP)2Ir(ECA). In the DIP spectrum, the peak at 3049.4 cm−1 is attributed to the stretching vibration of
418, respectively (Fig. S1, ESI†), in agreement with both the 1H NMR and FTIR results. Fig. 2D shows the FTIR spectra of DIP, ECA, and (DIP)2Ir(ECA). In the DIP spectrum, the peak at 3049.4 cm−1 is attributed to the stretching vibration of ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–H on the benzene rings. The peaks at 1668.4, 1600.9, and 1554.6 cm−1 correspond to the stretching vibrations of the C
C–H on the benzene rings. The peaks at 1668.4, 1600.9, and 1554.6 cm−1 correspond to the stretching vibrations of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C skeleton on the benzene rings. The peaks at 840.9, 785.0, and 698.2 cm−1 correspond to the C–H deformation vibrations on the benzene rings. Notably, the peaks at 1489.0 cm−1 and 1413.8 cm−1 correspond to the stretching vibrations of the skeleton on the pyridine rings, and these peaks disappeared in the final product because of nitrogen atom coordination. In the ECA spectrum, the peak at 2989.4 cm−1 corresponds to the stretching vibration of –CH3. The peak at 1737.4 cm−1 is typical of C
C skeleton on the benzene rings. The peaks at 840.9, 785.0, and 698.2 cm−1 correspond to the C–H deformation vibrations on the benzene rings. Notably, the peaks at 1489.0 cm−1 and 1413.8 cm−1 correspond to the stretching vibrations of the skeleton on the pyridine rings, and these peaks disappeared in the final product because of nitrogen atom coordination. In the ECA spectrum, the peak at 2989.4 cm−1 corresponds to the stretching vibration of –CH3. The peak at 1737.4 cm−1 is typical of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O characteristics. The peak at 2239.3 cm−1 represents –C
O characteristics. The peak at 2239.3 cm−1 represents –C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N, and this peak slightly shifted to 2249.0 cm−1 in (DIP)2Ir(ECA). The peak at 1187.7 cm−1 corresponds to the stretching vibrations of C–O–C. Finally, the peaks at 1392.6, 1291.3, 865.0, and 803.9 cm−1 represent the deformation vibration of –CH2– and –CH3. In the (DIP)2Ir(ECA) spectrum, most of the peaks were inherited from ECA. The peak at 3336.8 cm−1 is attributed to the solvent, and those in the range of 2978.0–2879.7 cm−1 are ascribed to the stretching vibrations of −CH3. The peak at 1745.5 cm−1 is the typical C
N, and this peak slightly shifted to 2249.0 cm−1 in (DIP)2Ir(ECA). The peak at 1187.7 cm−1 corresponds to the stretching vibrations of C–O–C. Finally, the peaks at 1392.6, 1291.3, 865.0, and 803.9 cm−1 represent the deformation vibration of –CH2– and –CH3. In the (DIP)2Ir(ECA) spectrum, most of the peaks were inherited from ECA. The peak at 3336.8 cm−1 is attributed to the solvent, and those in the range of 2978.0–2879.7 cm−1 are ascribed to the stretching vibrations of −CH3. The peak at 1745.5 cm−1 is the typical C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O feature. The 1244.1 cm−1 to 1116.8 cm−1 peaks correspond to the stretching vibrations of C–O–C. The other peaks are similar to those ascribed to the –CH2– and –CH3 deformation vibrations in ECA. The locations of the functional groups could also be compared with those in a previous study and an online spectral database.34,35 Overall, the 1H NMR and FTIR results demonstrate that a new compound has been successfully synthesized.
O feature. The 1244.1 cm−1 to 1116.8 cm−1 peaks correspond to the stretching vibrations of C–O–C. The other peaks are similar to those ascribed to the –CH2– and –CH3 deformation vibrations in ECA. The locations of the functional groups could also be compared with those in a previous study and an online spectral database.34,35 Overall, the 1H NMR and FTIR results demonstrate that a new compound has been successfully synthesized.
3.3. Phosphorescence character of (DIP)2Ir(ECA) and the nanomicelles
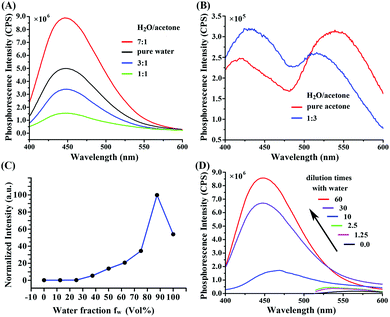
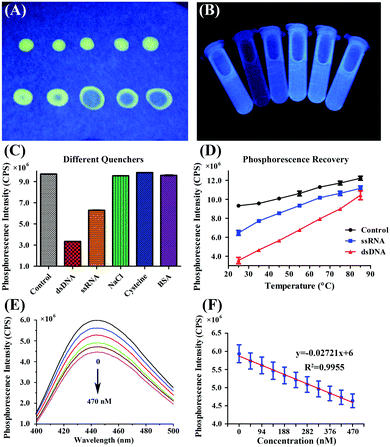
The location of the light emission spanned the yellow to blue regions with a phosphorescence spectrum of 600–400 nm. The phosphorescence spectra show different emission locations in different H2O:acetone mixed solvents (Fig. 3A and B), in contrast to the general principles of solvent polarity effects on fluorophores.36 The quantitative data are presented in Table S1 (ESI†). The phosphorescence wavelength blue-shifted as the water fraction was increased, and the phosphorescence intensity correspondingly increased 30-fold (Fig. 3C). Therefore, the phosphorescence mechanism was not due to solvent polarity action. The distinguishing feature of AIE is a significant increase in the intensity and quantum yield of fluorophores in a solid state, given that the fluorophores were originally from the benzene rings in DIP where the rotation is restricted. These RIR effects were eliminated when the fluorophores were dissolved in acetone, but were enhanced in water; thus, acetone is frequently used in dissolving cyanoacrylate. Herein, the intensity of phosphorescence increased 30-fold when the water fraction increased. However, the phosphorescence intensity decreased in pure water. This phenomenon might be due to enhanced scattered light with decreased solubility. This result is in agreement with a previous report.37The peak wavelength of the phosphorescence spectrum in 2-ethoxyethanol was 566 nm when the (DIP)2Ir(ECA) pregnant liquor was dissolved in pure water (sample 14 in Table S2, ESI†), as partially illustrated in Fig. 3D. This result indicates that the RIR effects of the benzene rings consequently showed AIPE-active effects. This change in effects could be proved through ultraviolet lamp excitation. When 50 μL of pregnant liquor was placed in 4.0 mL of deionized H2O to form nanomicelles, the living cell imaging results showed that green phosphorescence was the highest among the three channels (Fig. S2, ESI†), indicating that the RIR effects of the benzene rings in the intracellular membrane had AIPE effects. When pregnant liquor was dropped onto filter paper, the emitted light was weak, but it was intensely enhanced by drying. Fig. 4A illustrates that the phosphorescence was intense along the edge but weak in the center. Fig. 4C and Table S3 (ESI†) show that the (DIP)2Ir(ECA) nanomicellar phosphorescence could be strongly quenched by dsDNA in a concentration-dependent manner as shown in Fig. 4C and Table S3 (ESI†), and the direct effects are illustrated in Fig. 4B. The phosphorescence of the samples mixed with dsDNA was specifically quenched among the samples in which calf thymus DNA, single-stranded RNA, NaCl, cysteine, and BSA were added. When the (DIP)2Ir(ECA) nanomicelles were mixed with the same amount of RNA or dsDNA, the phosphorescence quenching effect of dsDNA was double times than that of RNA. This result might be due to mispairing within the RNA molecule, although the RNA was considered to be single-stranded (Fig. 4B). To prove our spectra including wavelength and intensity with the pregnant liquor hypothesis, we heated and melted quenched samples containing single-stranded RNA or dsDNA. As a result, the phosphorescence intensity recovered but did not achieve its original level at the same temperature because only a partially formed, double-stranded structure could be melted at the melting temperature used (65 °C for RNA and 85 °C for DNA) (Fig. 4D).
The bimolecular quenching effects of double-stranded nucleic acid might be caused by both the electrostatic interaction between the negatively charged nucleic acid and the positively charged cationic iridium(III) complexes, and the insertion of DIP ligands into the double-stranded structure.14–17,38
To prove our hypothesis, we synthesized similar iridium(III) complex nanomicelles with 2-(2-pyridyl)benzothiophene as their main ligand, which could not be inserted into the grooves of DNA. The experimental results demonstrated that the AIPE effects still occurred, but that quenching by dsDNA was absent. However, the polyethyleneimine-coated nanomicelles did have either AIPE nor dsDNA quenching effects. According to these definite results, the dsDNA quenching effects were directly correlated with DIP ligand properties, and the RIR effects of the benzene rings on DIP were required to induce AIPE. The synthesis routes and the structure are shown in Fig. S3 (ESI†).
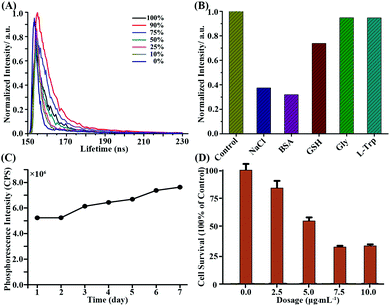
The phosphorescence lifetimes in different water contents were measured and the lifetimes were 5.106, 5.886, 5.274, 3.286, 2.213, 1.928 and 1.725 ns, respectively, for different water contents of 100%, 90%, 75%, 50%, 25%, 10% and 0%, which are shown in Fig. 5A. The lifetime decreased along with the decrease in water content, which coincided with previous research studies and demonstrated that the composite (DIP)2Ir(ECA) polymer has AIPE properties.4,7,9,39,40
According to the Stern–Volmer equation (3.1), the phosphorescence intensity was proportional to the phosphorescence lifetime, in which F is the phosphorescence intensity, τ is the lifetime, kq is the bimolecular quenching rate constant and [Q] is the quencher concentration. Furthermore, the quantum efficiency was calculated to be 0.06787 with quinine sulfate dehydrate as a standard. The phosphorescence lifetime results coincide with phosphorescence intensity (Fig. 3C).
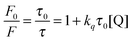 | (3.1) |
The influence of five compounds on (DIP)2Ir(ECA) nanomicelle dsDNA detection was investigated, including NaCl, BSA, GSH, Gly and L-Trp. Compared with the control, it was found that the sensitivity of the (DIP)2Ir(ECA) nanomicelles to dsDNA decreased to about 40% in the presence of NaCl and BSA and by about 70% in the presence of GSH. Furthermore, there seemed to be no effects in the presence of Gly and L-Trp. The corresponding results are shown in Fig. 5B.
In Fig. 5C, it can be seen that the phosphorescence intensity of the (DIP)2Ir(ECA) nanomicelles was stable for a week, although it increased gradually and slightly which was probably because of the evaporation of the residual organic solvent to enhance the AIPE effect.
An MTT assay was carried out to evaluate the cytotoxicity towards the IMR-90 cell line, and the results are shown in Fig. 5D. We found that the cell viability remained about 50% in the presence of 5.0 μg mL−1 of (DIP)2Ir(ECA) nanomicelles and about 25% in the presence of 7.5 and 10.0 μg mL−1 of (DIP)2Ir(ECA) nanomicelles. (Fig. 5D) These nanomicelles showed similar levels of cytotoxicity towards PC3 cells, which is not shown. The tissue section of the (DIP)2Ir(ECA) nanomicelles was also investigated. There was no obvious necrosis in the heart, liver, spleen and kidneys, as shown in Fig. S4 (ESI†), which indicates that these nanomicelles might possess prospects of imaging in vivo.
3.4. Analytical detection limit and range of linearity
Fig. 4E and Table S3 (ESI†) show the concentration-dependent phosphorescence quenching by DNA. The range of linearity is shown in Fig. 4F. The DL was 1.865 μg mL−1 or 210 nmol L−1. The range of linearity was from 47 nmol L−1 to 423 nmol L−1, indicating the rapid and sensitive detection of dsDNA. The linear regression equation was y = −0.02721x + 6 (the unit of x was nM) and the regression coefficient was R2 = 0.9955, which are shown in Fig. 4E.3.5. Monitoring of DNA hydrolysis and PCR procedure
DNA is easily hydrolyzed in an acidic environment and fluorescent dyes are only effective within a narrow pH range. The results of DNA hydrolysis are illustrated in Fig. 6A. The results indicate that the (DIP)2Ir(ECA) nanomicelles were stable in weakly acidic and neutral pH ranges (from 5.91 to 9.18), whereas traditional SYBR Green I was only effective within a narrow pH range of 7.0–8.5. Thus, the (DIP)2Ir(ECA) nanomicelles allowed the real-time monitoring of DNA acidic hydrolysis using a simple ultraviolet lamp. As DNA hydrolysis is a time-consuming process, we performed a DNA destruction experiment. Fig. 6B shows the results for the four measured samples, including 1-control, 2-control + DNase I, 3-control + DNA, and 4-control + DNA + DNase I. Compared with the control, the phosphorescence intensity decreased to 40% when DNA was added. DNA hydrolysis was monitored after adding DNase I to DNA. Phosphorescence intensity increased sharply at the start of hydrolysis, steadily increased within 7 min, and finally recovered to 80%. This trend could be attributed to the presence of short fragments containing double-stranded structures, even if DNase I catalyzed the hydrolytic cleavage of the phosphodiester linkages. Furthermore, the PCR procedure could also be monitored using (DIP)2Ir(ECA) nanomicelles, even if the PCR system's initial pH value was slightly higher, because the pH value decreased to an appropriate level when the PCR system was heated. The SYBR Green I fluorescence intensity increased and exhibited an “S” curve as the dNTPs were depleted and the amount of target DNA increased exponentially. However, the (DIP)2Ir(ECA) nanomicelles showed different behaviors because of linear quenching effects. The phosphorescence intensity decreased sharply during the first cycles (<6), and stabilized to almost zero (Fig. 6C), probably benefiting from a low initial dose of target DNA in the PCR system.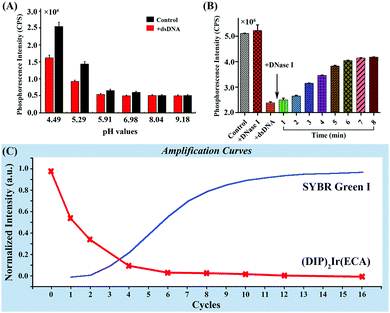 | ||
| Fig. 6 (A) Monitoring of DNA at different pH values; (B) monitoring of the DNA destructive process; (C) the amplification curve of PCR monitored using SYBR Green I and (DIP)2Ir(ECA). | ||
As indicated by the above results, iridium(III) complexes (DIP)2Ir(ECA) nanomicelles allowed for an inexpensive, real-time, and rapid method for monitoring quantity changes in dsDNA. As transition metal complexes, (DIP)2Ir(ECA) nanomicelles were more stable than fluorescent dyes, and could be excited by an ultraviolet lamp; thus, (DIP)2Ir(ECA) nanomicelles could be promising candidates for use in DNA anti-counterfeiting technologies. If DNA was degraded, it could still be detected even without using a PCR process. Moreover, the (DIP)2Ir(ECA) nanomicelles did not negatively influence the PCR process and could monitor it in real time.
3.6. Monitoring of DNA hydrolysis by immobilized DNase I
Fig. S5 (ESI†) shows the SEM and TEM images of CS(2.0)-5. CS(2.0)-5 was approximately 2.0 μm in size. Its hollow structure is evident in the case of broken microspheres in the SEM images in Fig. S5A (ESI†). From the SEM image, it can be seen that the size distribution of CS(2.0)-5 was narrow and its coefficient of variation was lower than 4% after investigating more than 200 microspheres. The TEM image also demonstrates the hollow structure of the CS(2.0)-5 microspheres in Fig. S5B (ESI†). The CS(2.0)-5 microspheres had a saturation magnetization of 25.41 emu g−1.27 DNase I was immobilized on CS(2.0)-5@PDA via a Schiff base reaction, as previously reported by our group. The amount of DNase I immobilized on CS(2.0)-5@PDA was measured using the Bradford method. The amount of DNase I immobilized on CS(2.0)-5@PDA was 69.276 μg of DNase I per mg of microspheres.The main experimental result indicates that the DNA-quenched phosphorescence intensity could be recovered by adding free DNase I to hydrolyze the DNA. Thus, we hypothesized that immobilized DNase I could probably also recover the phosphorescence intensity. A certain amount of CS(2.0)-5@DNase I was added to the quenched system to hydrolyze the DNA for 40 min. The process is displayed in Fig. 7A in detail. The supernatant's relative phosphorescence intensity was measured and evaluated.
The relative phosphorescence recovery intensity was calculated to evaluate its recovery using formula (2.2).
The recovered relative phosphorescence intensities were 0.9114, 0.8422, 0.8882, and 0.5024 for each cycle (Fig. 7B). The results showed that CS(2.0)-5@DNase I attenuated the quenching effect of DNA at least 4 times, although this phenomenon was not dramatic during the fourth time. Recovery might be induced by the deactivation of DNase I immobilized on CS(2.0)-5@PDA.
3.7. Recyclable DNA detection of (DIP)2Ir(ECA) nanomicelles
AIE fluorescent probes have been developed for DNA detection for many years. Wang et al. synthesized a compound, a silole with an ammonium group, for application in turn-on DNA detection based on its AIE feature. The fluorescence of it increased, when mixed with DNA, promoted by the electrostatic interaction which contributed to the aggregation of the positively charged compound on negatively charged DNA. It could also be utilized in both DNA cleavage by nucleases and inhibitor screening of nucleases.41 Hong et al. developed two kinds of AIE fluorescent bio-probes of tetraphenylethene (TPE) salts, which showed high affinity with the G-quadruplex in the presence of K+.42,43 Although DNA detecting probes based on AIE fluorescent dyes have been investigated for a long time, there are few reports on recyclable DNA detection based on AIE fluorescent dyes. In addition, there are few reports on DNA detection based on AIPE effects, especially on recyclable DNA detection because of their poor water-solubility and complex separation process.We found that (DIP)2Ir(ECA) nanomicelles were sensitive to DNA molecules in a turn-down manner and that the phosphorescence of the (DIP)2Ir(ECA) nanomicelles could be quenched by DNA. Moreover, this quenching effect could be attenuated by the addition of DNase I, which was speculated to be the cause of the hydrolyzation of DNA. Furthermore, the phosphorescence properties of the (DIP)2Ir(ECA) nanomicelles still remained at high temperatures, providing an opportunity to deactivate DNase I by hyperthermia without the introduction of other denaturing agents. This thermostable property enables the application of (DIP)2Ir(ECA) nanomicelles in PCR monitoring and recycling.
To further investigate the (DIP)2Ir(ECA) nanomicellar recyclable DNA detection ability, the system was placed in an 80 °C water bath for 20 min to deactivate DNase I. The system was applied in DNA detection for a second time. (DIP)2Ir(ECA) nanomicellar phosphorescence was quenched again by DNA, indicating that the (DIP)2Ir(ECA) nanomicelles were sensitive to DNA and could still be used for DNA detection (Fig. 7A). The recyclable DNA detection of the (DIP)2Ir(ECA) nanomicelles was tested four times and the results are shown in Fig. 7C. It was found that the (DIP)2Ir(ECA) nanomicelles remained sensitive to DNA throughout four detection tests, although sensitivity gradually decreased. Decreased DNA sensitivity might be caused by increased content of hydrolyzed DNA fragments and deactivated DNase I. The recovery rate of the (DIP)2Ir(ECA) nanomicelles after dsDNA monitoring during this recyclable detection was 91.77%, 41.78%, 34.37% and 16.83% for each cycle, respectively.
Fluorescent/phosphorescent materials have been widely used in various applications, especially in detection, because they are highly sensitive and easily measured. However, they were infrequently applied as recyclable sensors. Fluorescent/phosphorescent materials are currently difficult to reutilize because they usually work as small molecules. There are only a few reports on recyclable fluorescent materials for detecting bioactive substances. Zhang et al. immobilized naphthalimide–DPA–Cu(II) (MSIND–Cu) complexes in mesoporous silica. This fluorescent chemosensor showed a response to pyrophosphate sensitively, rapidly and stably within a wide pH range. It showed good reusability eight times without losing sensitivity.44 Quantum dots have also been frequently used in recyclable fluorescence detection. Carbon nanoparticles (CNPs) were synthesized using a microwave-assisted hydrothermal method, in which the fluorescence of the CNPs could be quenched by Hg2+. This quenching effect could be blocked by mercapto biomolecules. This Hg2+-quenched CNP system was developed to detect mercapto biomolecules and used repeatedly ten times.45 Hg2+ detection using silicon nanocrystals was reported on the basis of a similar mechanism. The detecting ability of the nanocrystals was still available after five cycles.46 Besides, Lin et al. also fabricated a fluorescent gold nanocluster membrane (FGM) with high sensitivity to copper(II) ions. FGM fluorescence was quenched by the addition of Cu2+ and recovered by the addition of histidine. This “quenching-recovery” ability of FGM was still obvious after five cycles.47 The reports above did not focus on the recyclable detection of bio-macromolecules, such as DNA, which is important in biomedical applications. Most of the recyclable detection systems can only recycle one compound. The recycling and activation processes are also complex, requiring magnetic separation and centrifugal separation. In addition, the current fluorescent/phosphorescent materials have several drawbacks, such as high toxicity and non-reutilization. Waste fluorescent/phosphorescent materials are harmful to both humans and the environment. Therefore, the recyclability of fluorescent/phosphorescent materials is very significant not only for their own application in detection, but also in alleviating their polluting effects on the environment.
Fluorescent/phosphorescent nanoparticles or bulk materials are more preferable than small fluorescent molecules due to their better stability and water solubility. Current recyclable detection systems usually involve complex procedures or heavy metal ions, which are not convenient for detection and are not environmentally friendly. Although iridium(III) is an expensive metal, using (DIP)2Ir(ECA) nanomicelles repeatedly could save costs and prevent the need for additional purification processes to remove them from the waste stream, making them a “greener” monitoring system compared with conventional approaches. The use of (DIP)2Ir(ECA) nanomicelles is also a rapid and sensitive method for monitoring DNA degradation. Compared with previously reported recyclable detection systems, our recyclable detection system can be reactivated simply and rapidly because of the thermostable properties of the (DIP)2Ir(ECA) nanomicelles. The recycling or activation process only involves heating the (DIP)2Ir(ECA) nanomicellar solution after DNase I addition. This reactivation of (DIP)2Ir(ECA) nanomicelles can be repeated four times. Meanwhile, excess DNase I can also be recycled four times via magnetic separation. Moreover, the small sizes (approximately 10 nm) of the (DIP)2Ir(ECA) nanomicelles are convenient for cellular or subcellular organelle imaging. Furthermore, most of the current recyclable fluorescent/phosphorescent nanoparticles can only recycle one compound. Therefore, our recyclable detection systems are convenient and economical for DNA detection.
4. Conclusions
We successfully synthesized AIPE-active thermostable polymeric iridium(III) complexes (DIP)2Ir(ECA) containing DIP as a main ligand and ECA as both an auxiliary ligand and a polymer skeleton. (DIP)2Ir(ECA) nanomicelles with aggregation-induced phosphorescence emission characteristics were fabricated in water, which displayed AIPE effects with different emission wavelengths and intensities in diverse solvents. The phosphorescence intensity of the nanomicelles was enhanced 30-fold in a H2O:acetone solution by increasing the water fraction. Both (DIP)2Ir(ECA) nanomicelles and immobilized DNase I were recyclable and could be utilized at least four times in the phosphorescence “quenching-recovery” dsDNA detection procedure. The recycling and reactivation of the nanomicelles can be achieved simply and rapidly by heating the solution. Thus, (DIP)2Ir(ECA) nanomicelles can be used as a rapid, sensitive, convenient, and economical means for monitoring DNA, indicating their great potential in biomedical and environmental applications.Acknowledgements
This work was sponsored in part by the National Natural Science Foundation of China (81271686, 81228011 and 81471771), and the grants of the National Key Research and Development Program (NO. 2016YFC0100701).Notes and references
- Y. Hong, J. W. Lam and B. Tang, Chem. Soc. Rev., 2011, 40, 5361 RSC.
- Y. Hong, J. W. Lam and B. Tang, Chem. Commun., 2009, 4332 RSC.
- Z. Li, Y. Dong, J. W. Lam, J. Sun, A. Qin, M. Häußler, Y. Dong, H. H. Sung, I. D. Williams, H. Kwok and B. Tang, Adv. Funct. Mater., 2009, 19, 905 CrossRef CAS.
- S. Liu, H. Sun, Y. Ma, S. Ye, X. Liu, X. Zhou, X. Mou, L. Wang, Q. Zhao and W. Huang, J. Mater. Chem., 2012, 22, 22167 RSC.
- Y. Sun, K. Ye, H. Zhang, J. Zhang, L. Zhao, B. Li, G. Yang, B. Yang, Y. Wang. S. Lai and C. Che, Angew. Chem., Int. Ed., 2006, 118, 5738 CrossRef.
- Q. Zhao, L. Li, F. Li, M. Yu, Z. Liu, T. Yi and C. Huang, Chem. Commun., 2008, 685 RSC.
- G. Shan, D. Zhu, H. Li, P. Li, Z. Su and Y. Liao, Dalton Trans., 2011, 40, 2947 RSC.
- M. Mauro, G. De Paoli, M. Otter, D. Donghi, G. D'Alfonso and L. De Cola, Dalton Trans., 2011, 40, 12106 RSC.
- G. Shan, L. Zhang, H. Li, S. Wang, D. Zhu, P. Li, C. Wang, Z. Su and Y. Liao, Dalton Trans., 2012, 41, 523 RSC.
- C. Shin, J. Huh, S. Baek, S. Kim, M. Lee and Y. Do, Eur. J. Inorg. Chem., 2010, 3642 CrossRef CAS.
- Y. Zeng, Y. Liu, J. Shang, J. Ma, R. Wang, L. Deng, Y. Guo, F. Zhong, M. Bai, S. Zhang and D. Wu, PLoS One, 2015, 10, e0121293 Search PubMed.
- S. Zhang, M. Hosaka, T. Yoshihara, K. Negishi, Y. Iida, S. Tobita and T. Takeuchi, Cancer Res., 2010, 7, 4490 CrossRef PubMed.
- Y. Zeng, S. Zhang, M. Jia, Y. Liu, J. Shang, Y. Guo, J. Xu and D. Wu, Nanoscale, 2013, 5, 12633 RSC.
- R. Wang, C. Nuckolls and S. J. Wind, Angew. Chem., Int. Ed., 2012, 51, 11325 CrossRef CAS PubMed.
- Q. Zhang, Q. Jiang, N. Li, L. Dai, Q. Liu, L. Song, J. Wang, Y. Li, J. Tian, B. Ding and Y. Du, ACS Nano, 2014, 8, 6633 CrossRef CAS PubMed.
- Y. Zhao, F. Chen, Q. Li, L. Wang and C. Fan, Chem. Rev., 2015, 115, 12491 CrossRef CAS PubMed.
- D. Paunescu, R. Fuhrer and R. N. Grass, Angew. Chem., Int. Ed., 2013, 52, 4269 CrossRef CAS PubMed.
- M. Puddu, D. Paunescu, W. J. Stark and R. N. Grass, ACS Nano, 2014, 8, 2677 CrossRef CAS PubMed.
- D. Paunescu, M. Puddu, J. O. Soellner, P. R. Stoessel and R. N. Grass, Nat. Protoc., 2013, 8, 2440 CrossRef CAS PubMed.
- G. Baier, A. Musyanovych, M. Dass, S. Theisinger and K. Landfester, Biomacromolecules, 2010, 11, 960 CrossRef CAS PubMed.
- N. Shahabadi, M. Falsafi and N. H. Moghadam, J. Photochem. Photobiol., B, 2013, 122, 45 CrossRef CAS PubMed.
- K. Wijaya, N. Yoshioka and H. Inoue, J. Inorg. Biochem., 2003, 94, 263 CrossRef.
- M. R. Gill and J. A. Thomas, Chem. Soc. Rev., 2012, 41, 3179 RSC.
- M. R. Gill, J. Garcia-Lara, S. J. Foster, C. Smythe, G. Battaglia and J. A. Thomas, Nat. Chem., 2009, 1, 662 CrossRef CAS PubMed.
- R. Cao, J. Jia, X. Ma, M. Zhou and H. Fei, J. Med. Chem., 2013, 56, 3636 CrossRef CAS PubMed.
- D. Li, J. Liu, R. Kwok, Z. Liang, B. Tang and J. Yu, Chem. Commun., 2012, 48, 7167 RSC.
- J. Ma, Y. Wu, Y. Zeng, Y. Li and D. Wu, J. Mater. Chem. A, 2015, 3, 16762 CAS.
- J. Wang, J. Mei, R. Hu, J. Sun, A. Qin and B. Tang, J. Am. Chem. Soc., 2012, 134, 9956 CrossRef CAS PubMed.
- K. M. Wong and V. W. Yam, Acc. Chem. Res., 2011, 44, 424 CrossRef CAS PubMed.
- K. L. Metera, K. D. Hanni, G. Zhou, M. K. Nayak and H. S. Bazzi, ACS Macro Lett., 2012, 1, 954 CrossRef CAS.
- R. Caspar, C. Cordier, J. B. Waern, C. Guyard-Duhayon, M. Gruselle, P. Le Floch and H. Amouri, Inorg. Chem., 2006, 45, 4071 CrossRef CAS PubMed.
- N. Shahabadi and A. Fatahi, J. Mol. Struct., 2010, 970, 90 CrossRef CAS.
- P. Klemarczyk, Polymer, 2001, 42, 2837 CrossRef CAS.
- S. K. Tomlinson, O. R. Ghita, R. M. Hooper and K. E. Evans, Vib. Spectrosc., 2016, 40, 133 CrossRef.
- Spectral Database for Organic Compounds, http://sdbs.db.aist.go.jp/sdbs/cgi-bin/cre_index.cgi?lang=eng, accessed: May, 2016.
- J. R. Lakowicz, Solvent and Environmental Effects, in Principles of Fluorescence Spectroscopy, Springer-Verlag, Berlin, 3rd edn, 2006, p. 205 Search PubMed.
- P. Alam, G. Kaur, V. Kachwal, A. Gupta, A. R. Choudhury and I. R. Laskar, J. Mater. Chem. C, 2015, 3, 5450 RSC.
- J. R. Lakowicz Jr., Quenching of Fluorescence, in Principles of Fluorescence Spectroscopy, Springer-Verlag, Berlin, 3rd edn, 2006, p. 278 Search PubMed.
- Y. Chen, L. Qiao, B. Yu, G. Li, C. Liu, L. Jia and H. Chao, Chem. Commun., 2013, 49, 11095 RSC.
- K. S. Bejoymohandas, T. M. George, S. Bhattacharya, S. Natarajanb and M. L. P. Reddy, J. Mater. Chem. C, 2013, 2, 515 RSC.
- M. Wang, D. Zhang, G. Zhang, Y. Tang, S. Wang and D. Zhu, Anal. Chem., 2008, 80, 6443 CrossRef CAS PubMed.
- Y. Hong, M. Häußler, J. Lam, Z. Li, K. Sin, Y. Dong, H. Tong, J. Liu, A. Qin, R. Renneberg and B. Tang, Chem. – Eur. J., 2008, 14, 6428 CrossRef CAS PubMed.
- Y. Hong, H. Xiong, J. Lam, M. Häußler, J. Liu, Y. Yu, Y. Zhong, H. Williams, K. Wong and B. Tang, Chem. – Eur. J., 2010, 16, 1232 CrossRef CAS PubMed.
- J. Zhang, M. Park, W. Ren, Y. Kim, S. Kim, J. Jung and J. Kim, Chem. Commun., 2011, 47, 3568 RSC.
- M. Lan, J. Zhang, Y. Chui, H. Wang, Q. Yang, X. Zhu, H. Wei, W. Liu, J. Ge, P. Wang, X. Chen, C. Lee and W. Zhang, J. Mater. Chem. B, 2015, 3, 127 RSC.
- J. Zhang and S. Yu, Nanoscale, 2014, 6, 4096 RSC.
- Z. Lin, F. Luo, T. Dong, L. Zheng, Y. Wang, Y. Chi and G. Chen, Analyst, 2012, 137, 2394 RSC.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6tb02336c |
| ‡ These authors contributed equally to this study. |
| This journal is © The Royal Society of Chemistry 2017 |