Application of a bilayer tubular scaffold based on electrospun poly(L-lactide-co-caprolactone)/collagen fibers and yarns for tracheal tissue engineering†
Tong
Wu‡
a,
Hui
Zheng‡
b,
Jianfeng
Chen
c,
Yuanfei
Wang
d,
Binbin
Sun
a,
Yosry
Morsi
 e,
Hany
El-Hamshary
fg,
Salem S.
Al-Deyab
f,
Chang
Chen
*b and
Xiumei
Mo
*a
e,
Hany
El-Hamshary
fg,
Salem S.
Al-Deyab
f,
Chang
Chen
*b and
Xiumei
Mo
*a
aState Key Lab for Modification of Chemical Fibers and Polymer Materials, College of Chemistry, Chemical Engineering and Biotechnology, Donghua University, 2999 North Renmin Road, Shanghai 201620, China. E-mail: xmm@dhu.edu.cn
bTongji University Affiliated Shanghai Pulmonary Hospital, Shanghai 200433, China. E-mail: changchenc@hotmail.com
cCollege of Material Science and Engineering, Donghua University, Shanghai 201620, China
dState Key Laboratory of Bioreactor Engineering, School of Resources and Environmental Engineering, East China University of Science and Technology, Shanghai 200237, China
eFaculty of Engineering and Industrial Sciences, Swinburne University of Technology, Hawthorn, Vic 3122, Australia
fDepartment of Chemistry, College of Science, King Saud University, Riyadh 11451, Kingdom of Saudi Arabia
gDepartment of Chemistry, Faculty of Science, Tanta University, Tanta 31527, Egypt
First published on 22nd November 2016
Abstract
A bilayer tubular scaffold (BLTS) consisting of poly(L-lactide-co-caprolactone) (P(LLA–CL))/collagen submicron sized fibers and micron sized yarns, was prepared via electrospinning. Then, autologous tracheal epithelial cells and chondrocytes were separately seeded onto the two layers of the BLTS. After culturing for 7 days, the cell-seeded BLTS (CS-BLTS) was implanted and wrapped in rat tracheal fascia for pre-vascularization. The pre-vascularized BLTS (PV-BLTS) was subjected to an in situ trachea regeneration study using a rat trachea injury model, along with CS-BLTS and bare BLTS for comparison. The results presented the bilayer structure of the BLTS, and the two layers were arranged conterminously. The porosity of the outer layer (collagen/P(LLA–CL) yarns) was found to be significantly higher (P < 0.05) than that of the inner layer (collagen/P(LLA–CL) fibers). In vitro biological analysis demonstrated that the collagen/P(LLA–CL) showed good biocompatibility, which promoted tracheal epithelial cell initial adhesion and proliferation with a highly significant difference (P < 0.001) or significant difference (P < 0.05) compared to those of pure P(LLA–CL) materials respectively. Chondrocyte activity and proliferation were also enhanced on collagen/P(LLA–CL) yarns with a significant difference (P < 0.05) compared to those of pure P(LLA–CL). Chondrocyte penetration was promoted as well, due to the loose and porous structure of the electrospun collagen/P(LLA–CL) yarns. The in vivo evaluation results of immune response analysis and histological investigation demonstrated that the PV-BLTS performed better in new capillary regeneration, reducing immunogenicity and improving tracheal tissue regeneration compared to the CS-BLTS and bare BLTS, indicating its promising potential as a new tissue engineered alternative for trachea repair and regeneration.
1. Introduction
The use of autogenous tissues, allografts, prosthetic materials, or a combination of these approaches, has been utilized for tracheal replacement in recent years.1 However, a suitable replacement has not been found so far because of the shortage of appropriate autologous donors, the restricted source of immune-inhibited allogenous trachea, as well as the substandard biofunction of synthetic tracheal grafts.2–7 Hence, the development of an alternative which could be biocompatible and bio-resorbable is urgently required. Furthermore, the substitute should maintain mechanical strength and accommodate the natural trachea structure for trachea damage repair.8 Tissue engineering approaches based on a cellular or acellular graft with the necessary properties have been proposed and promisingly applied to tracheal tissue regeneration.9In the last few years, considerable progress has been made in tissue engineered organ replacements, using scaffolds repopulated with cells or stem cells into the clinic.2,10 Decellularized scaffolds derived from the tracheal tissue with or without recellularization by autologous cells have been investigated for use in tracheal replacement due to their biochemical similarity to the native trachea,11 but it is possible that the decellularization process may affect the composition, ultrastructure, mechanical properties and surface topography of the resulting scaffold.12,13 Therefore, the long-term graft properties would be affected. In comparison, tissue-engineered tracheas based on biodegradable scaffolds are advantageous to construct and regenerate functional tissues, because ideal scaffolds can be independently designed using synthetic and natural polymers in consideration of the scaffold composition, structure and function. Such a favorable scaffold aims at replacing the native trachea with specific environmental signals (cell/stem cell mobilization) to allow for tissue regeneration, including cell proliferation, migration and high quality remodeling of the trachea structure.2,14 Vacanti et al. have successfully engineered new tissue equivalents, including cartilage, by utilizing synthetic biodegradable polymer scaffolds seeded in vitro with cells. Then, nonwoven meshes of polyglycolic acid fibers–chondrocytes constructs were used to repair defects in the tracheas.1 This study advanced the progress that it should be underway to develop composite tubular structures which are lined by tracheal epithelium and infiltrated with flexible cartilage as a support. Hence, a bilayer tubular scaffold, especially one with dense fibers as an inner layer and a porous structure as an outer layer, has the advantage as a tissue-engineered trachea, due to the promotion of covering epithelium on the reconstructed lumen and the three-dimensional penetration of chondrocytes into the outer layer.8,15
Electrospinning is in widespread use for fabricating numerous tissue engineering scaffolds, especially for fiber-reinforced and multilayered composite scaffolds.16–21 The multilayered tubular structures based on composites can be expectedly designed and constructed due to a wide range of material selection including both synthetic and natural polymers.16,21 Furthermore, the fibrous scaffolds are beneficial to tissue regeneration because they have a similar size-scale to the native extracellular matrix (ECM) and are able to control cellular behavior by regulating their physical and chemical performances.16,22 Generally, natural biomaterials like collagen, chitosan or silk fibroin have good biocompatibility, while synthetic biomaterials such as polyurethane (PU), poly(caprolactone) (PCL), poly(L-lactic acid) (PLA) and poly(L-lactide-co-caprolactone) (P(LLA–CL)) can provide suitable mechanical strength.23–28 1,1,1,3,3,3-Hexafluoro-2-propanol (HFIP) is a commonly used solvent for electrospinning in various tissue engineering applications, because it is easy and stable to fabricate fibers from HFIP solvent during the electrospinning process.29–31 Although the use of HFIP as the solvent led to partially denatured collagen,32,33 it was found that electrospun collagen fibers experienced a lower inflammatory response than electrospun gelatin (denatured collagen) through subcutaneous implantation evaluation.33,34 Therefore, electrospun collagen retains some advantageous properties differentiating from gelatin.33 Besides, scaffolds fabricated from synthetic polymer and collagen composites showed better properties than the pure synthetic materials when applied to tissue engineering. For example, P(LLA–CL) and collagen composites were popularly selected for fabricating tissue engineered scaffolds, due to the optimal combination of mechanical properties, biodegradability, and biocompatibility.29,35–40 Hence, the composites based on P(LLA–CL) and collagen have been utilized as raw materials in our research.
In the preliminary study, we tested the feasibility of fabricating a bilayer tubular scaffold (BLTS) based on electrospun P(LLA–CL)/collagen fibers and yarns via traditional electrospinning and following dynamic liquid electrospinning methods. The bilayer tubular scaffold consisted of dense fibers in the inner layer and porous micron sized yarns as the outer layer, which had better porosity, pore diameter and mechanical properties than the single inner layer. The FTIR results confirm the existence of P(LLA–CL) and collagen, indicating the feasibility of fabricating the BLTS. Furthermore, the dense fibers and porous yarns based on P(LLA–CL)/collagen had better in vitro biocompatibility than the single P(LLA–CL) components. The P(LLA–CL)/collagen fibers displayed good tracheal epithelial cell (RTEC) proliferation and morphology. P(LLA–CL)/collagen yarns showed a significant advantage in rat tracheal chondrocyte (RTC) penetration. The in vitro biological results indicated the encouraging potential and necessity of the BLTS for epithelium formation and chondrocyte infiltration in three-dimensional tracheal tissue reconstruction.
Therefore, we proceeded to use the bilayer tubular scaffold and pre-seeded RTECs in the lumen and RTCs in the outer layer, resulting in cellular tissue engineered scaffolds. Then, the cell-seeded scaffolds were implanted and wrapped in rat tracheal fascia and processed by pre-vascularization to obtain an immunogenicity-reduced tissue engineered scaffold. The potential of the bare BLTS, cell-seeded BLTS (CS-BLTS), and pre-vascularized BLTS (PV-BLTS) for tracheal tissue regeneration was evaluated in situ in rat tracheal defect models.
2. Materials and methods
2.1 Materials
P(LLA–CL) (300 kDa, LA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CL = 50
CL = 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50, Nara Medical University, Japan) and fish skin derived collagen type I (Shanghai Fisheries Research Institute) were blended (w/w, 75
50, Nara Medical University, Japan) and fish skin derived collagen type I (Shanghai Fisheries Research Institute) were blended (w/w, 75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25) and dissolved in 1,1,1,3,3,3-hexafluoro-2-propanol (HFIP, Shanghai Darui Fine Chemicals, China) at a concentration of 8%. All reagents provided for cell culture were obtained from Gibco Life Technologies, USA, unless otherwise specified.
25) and dissolved in 1,1,1,3,3,3-hexafluoro-2-propanol (HFIP, Shanghai Darui Fine Chemicals, China) at a concentration of 8%. All reagents provided for cell culture were obtained from Gibco Life Technologies, USA, unless otherwise specified.
2.2 Fabrication
Collagen/P(LLA–CL) fibers were fabricated via a general electrospinning method at a high voltage of 14 kV, a flow rate of 1 mL h−1 and a collecting distance of 15 cm. Collagen/P(LLA–CL) yarns were obtained with a customized dynamic liquid electrospinning setup26,41 and subsequently freeze dried for final use. For comparison, P(LLA–CL) fibers and yarns were also electrospun. The collagen/P(LLA–CL) bilayer tubular scaffold (BLTS) with dense fibers as the inner layer and porous yarns as the outer layer was fabricated via continuous electrospinning of the above two methods. Equally, the fabricated BLTS was processed next by freeze drying for utilization. The scaffolds with collagen were crosslinked by glutaraldehyde vapor (GA, 25%, Sinopharm Chemical Reagent, China) for 24 h. The scaffolds were dried in a vacuum for 48 h before further evaluation.2.3 Physical characterization
The scaffold morphology was observed using a scanning electronic microscope (SEM, JSM-5600, Japan). The fiber and yarn diameters were measured using image analysis software (Image-J, National Institutes of Health, USA). The BLTS was immersed in liquid nitrogen and rapidly cut with a scalpel to obtain a cross-section. Then, the bilayer structure was observed using SEM. The porosity of the electrospun fibers and yarns was assessed using a liquid substitution method using the formula p(%) = (V1 − V3)/(V2 − V3), where p is the porosity, V1 is the original volume of ethanol, V2 is the volume after the samples were immersed in ethanol for 10 min and V3 is the residual volume after removing the wet samples.26 The pore diameter was measured using a CFP-1100-AI capillary flow porometer (PMI Porous Materials Int.) as previously described, and Calwick with a surface tension of 21 dyn cm−1 was selected as the wetting agent for the porometry measurements.422.4 Biodegradability
The biodegradation of the collagen/P(LLA–CL) fibers, collagen/P(LLA–CL) yarns, P(LLA–CL) fibers and P(LLA–CL) yarns was evaluated for 15 weeks. Briefly, 2 cm × 2 cm samples (n = 3) were immersed in 10 mL of phosphate-buffered solution (PBS) containing 2.0 mg of sodium azide in a continuous horizontal shaker with 120 cycles per min at 37 °C. At predetermined periods of time, the samples were processed with vacuum freeze drying for weighing. The mass loss ratio was estimated using the formula of mass loss ratio (%) = (W1 − W2)/W1 × 100%, where W1 is the primary weight and W2 is the dry weight after biodegradation. The pH value of degradation medium at different time points was tested using a digital pH meter (PHS-3C, China).2.5 Preparation of the cell-seeded BLTS and pre-vascularized BLTS
The experimental procedures for cell extraction were carried out under Institutional guidelines for animal care and approved by the Animal Ethics Committee of the Tongji University affiliated Shanghai Pulmonary Hospital (Shanghai, China). The rat tracheal epithelial cells (RTECs) were extracted from the SD rat and incubated in Dulbecco's modified Eagle's medium (DMEM)-F12 complete medium with 10% fetal bovine serum and 1% antibiotic–antimycotic in an atmosphere of 5% CO2 at 37 °C. The rat tracheal chondrocytes (RTCs) were extracted from new born SD rats as well. The RTCs were cultured in the mixed medium of DMEM-F12 and cartilage cell complete culture medium (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v). The BLTS (inner diameter of 4 mm and length of 1 cm) was sterilized under 75% ethanol vapor for 12 hours followed by UV irradiation for 30 min. Then, the BLTS was rinsed with a sterilized PBS solution three times and immersed in culture medium for 24 hours. After one end of the BLTS was fastened with 3-0 nylon wires, 1 × 105 mL−1 RTECs were injected into the lumen of the BLTS through the other end. Then, this end of the BLTS was fastened as well. The RTECs-BLTS construct was incubated in DMEM-F12 complete medium with 10% fetal bovine serum and 1% antibiotic–antimycotic in an atmosphere of 5% CO2 at 37 °C for 24 hours. The RTECs were seeded repeatedly three times. After the RTECs were seeded, the BLTS was rinsed with PBS solution. 1 × 105 mL−1 RTCs were subsequently seeded on the outside surface and the cells–BLTS construct was cultured in the DMEM-F12 complete medium under the same conditions. Chondrocytes were repetitively seeded three times as well. The RTEC and RTC seeded BLTS was cultured for 7 days and the obtained scaffold was designated as cell-seeded BLTS (CS-BLTS, Group B). Furthermore, the rat trachea was cut and exposed, and the CS-BLTS was embedded in the fascia beside the sternocleidomastoid muscle. The length of the BLTS was kept parallel to the native muscle bundles for pre-vascularization. After incubation in the rat for two weeks, the pre-vascularized BLTS was explanted and called PV-BLTS (Group A). For comparison, the bare BLTS was used as a control (Group C).
1, v/v). The BLTS (inner diameter of 4 mm and length of 1 cm) was sterilized under 75% ethanol vapor for 12 hours followed by UV irradiation for 30 min. Then, the BLTS was rinsed with a sterilized PBS solution three times and immersed in culture medium for 24 hours. After one end of the BLTS was fastened with 3-0 nylon wires, 1 × 105 mL−1 RTECs were injected into the lumen of the BLTS through the other end. Then, this end of the BLTS was fastened as well. The RTECs-BLTS construct was incubated in DMEM-F12 complete medium with 10% fetal bovine serum and 1% antibiotic–antimycotic in an atmosphere of 5% CO2 at 37 °C for 24 hours. The RTECs were seeded repeatedly three times. After the RTECs were seeded, the BLTS was rinsed with PBS solution. 1 × 105 mL−1 RTCs were subsequently seeded on the outside surface and the cells–BLTS construct was cultured in the DMEM-F12 complete medium under the same conditions. Chondrocytes were repetitively seeded three times as well. The RTEC and RTC seeded BLTS was cultured for 7 days and the obtained scaffold was designated as cell-seeded BLTS (CS-BLTS, Group B). Furthermore, the rat trachea was cut and exposed, and the CS-BLTS was embedded in the fascia beside the sternocleidomastoid muscle. The length of the BLTS was kept parallel to the native muscle bundles for pre-vascularization. After incubation in the rat for two weeks, the pre-vascularized BLTS was explanted and called PV-BLTS (Group A). For comparison, the bare BLTS was used as a control (Group C).
2.6 Implantation
All experimental procedures relating to animals in this study were carried out under Institutional guidelines for animal care and approved by the Animal Ethics Committee of the Tongji University affiliated Shanghai Pulmonary Hospital (Shanghai, China). 27 female SD rats weighing around 200 g were randomly divided into 3 groups and tracheal defects were created that served as tracheal replacement models (each group had 9 models). The PV-BLTS (Group A), CS-BLTS (Group B) and bare BLTS were, respectively, in situ implanted into the tracheal gap and sutured at the proximal and distal ends of the native trachea. All of the rats were intraperitoneally injected with penicillin at a dosage of 2 × 105 units per day for three days. At 1, 2 and 4 weeks after surgery, the rats were euthanized and the scaffolds were taken out. The explanted grafts were washed with PBS solution and fixed in 10% neutral formalin liquid.2.7 Evaluation of the explanted BLTS grafts
To assess the immune response of each group after implantation, the CD4T and CD8T lymphocytes in the peripheral blood of the rats after in situ implanting for 2 weeks were detected using flow cytometry. The effects of pre-vascularization on the BLTS, PV-BLTS (Group A) and CS-BTS (Group B) were evaluated using hematoxylin and eosin (H&E) staining using conventional procedures after implantation for 1 and 2 weeks. The newly regenerated capillaries were counted and summarized under three random H&E images (200×). Histological investigations based on cross-section slices of the implanted grafts were further conducted. Cell growth and lumen morphology of the in situ implanted grafts after implanting for 2 and 4 weeks were assessed using an immunofluorescence assay (with anti-cytokeratin antibody) and H&E staining. The explanted BLTSs of Group A and Group B at 4 weeks were performed with Masson trichrome and Verhoeff–Van Gieson elastic staining for collagen and elastic tissue detection.2.8 Statistical analysis
Statistical analysis was performed using Origin 9.0 (Origin lab Inc., USA). All the values were averaged at least in triplicate and expressed as mean ± standard deviation (SD). Statistical differences were determined by the analysis of one-way ANOVA and differences were considered to be statistically significant (P < 0.05) or highly significant (P < 0.001).3. Results
3.1 Morphology and structure of the BLTS
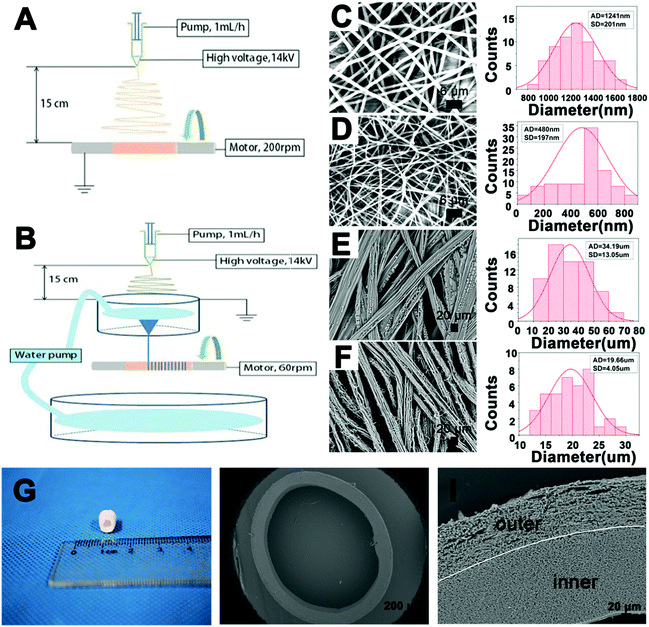
Fig. 1A and B shows the schematic diagrams of the fabricated collagen/P(LLA–CL) fibers and yarns. The random fibers were fabricated using a general eletrospinning method and the yarns were fabricated by twisting the fibers using a dynamic liquid system. Fig. 1C and D show that the P(LLA–CL) fibers (FS-P) and collagen/P(LLA–CL) fibers (FS-CP) had a dense fibrous structure, consisting of smooth and homogeneous submicron sized fibers (FS-CP, 480 ± 192 nm) or microscale fibers (FS-P, 1241 ± 201 nm) respectively. In comparison, the P(LLA–CL) yarns (YS-P) and collagen/P(LLA–CL) yarns (YS-CP) in Fig. 1E and F display a loose and porous structure, having an average yarn diameter of 34.19 ± 13.05 μm and 19.66 ± 4.05 μm respectively. Then, the general electrospinning and dynamic liquid electrospinning methods were continuously performed (Fig. 1A and B) and the bilayer tubular scaffold (BLTS) was obtained. Fig. 1G shows the optical image of the BLTS. SEM images (Fig. 1H and I) display the integral cross-section and the differentiated bilayer structure of the BLTS.3.2 Porosity
The porosities of the P(LLA–CL) fibers, collagen/P(LLA–CL) fibers, P(LLA–CL) yarns and collagen/P(LLA–CL) yarns are demonstrated in Fig. 2. It can be seen that the porosity of the P(LLA–CL) yarns and collagen/P(LLA–CL) yarns was separately 84.6 ± 2.7% and 82.2 ± 4.6%, which were significantly higher (P < 0.05) than the P(LLA–CL) fibers and collagen/P(LLA–CL) fibers (at 64.8 ± 9.0% and 68.7 ± 4.0% respectively).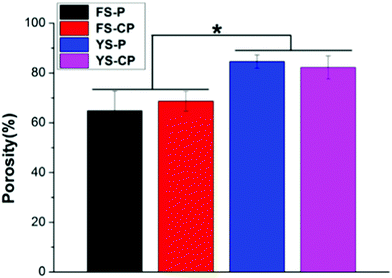 | ||
| Fig. 2 Porosity of the P(LLA–CL) fibers (FS-P), collagen/P(LLA–CL) fibers (FS-CP), P(LLA–CL) yarns (YS-P) and collagen/P(LLA–CL) yarns (YS-CP) (* indicates a significant difference when P < 0.05). | ||
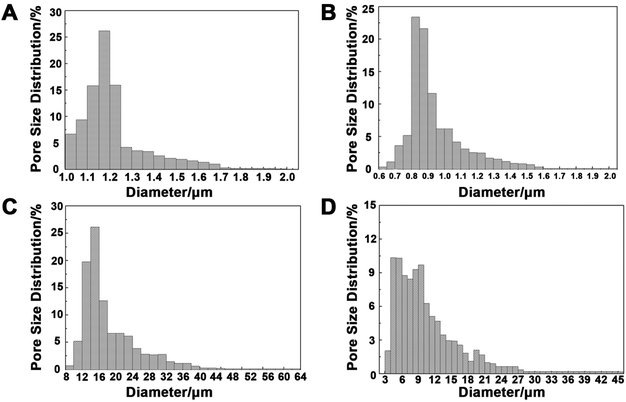
3.3 Pore size distribution of the double layers in the BLTS
The pore diameters of the electrospun collagen/P(LLA–CL) fibers and yarns were measured before and after cross-linking. As Table 1 presents, the electrospun yarn layer had a much larger pore diameter (15.95 μm and 9.12 μm before and after crosslinking) than that of the fiber layer (1.18 μm and 0.88 μm before and after crosslinking). Fig. 3 displays the pore diameter distribution of the electrospun fibers and yarns. The pore diameters of the fibers were around 1 μm, while the pore diameters of the yarns were in the range from several micrometers to dozens of micrometers.| Smallest pore diameter/μm | Largest pore diameter/μm | Average diameter/μm | |
|---|---|---|---|
| Inner layer before crosslinking | 1.01 | 1.98 | 1.18 |
| Inner layer after crosslinking | 0.69 | 2.06 | 0.88 |
| Outer layer before crosslinking | 9.83 | 64.48 | 15.95 |
| Outer layer after crosslinking | 3.87 | 45.82 | 9.12 |
3.4 Biodegradability
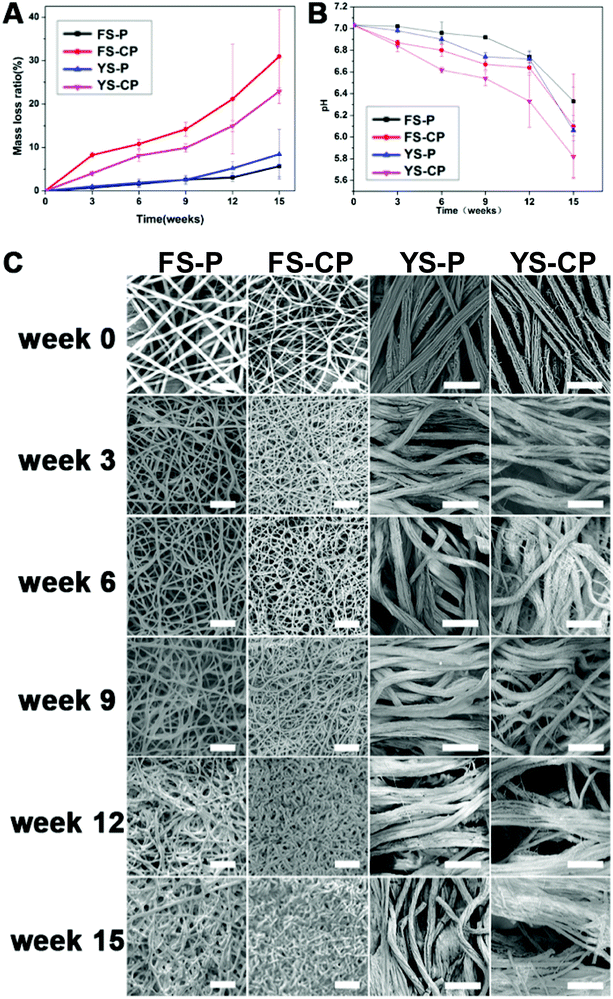
The degradation behaviors of the electrospun fibers and yarns were evaluated for 15 weeks in PBS solution under the conditions of 37 °C and 120 rpm oscillation. Fig. 4A shows the mass loss ratio of different electrospun scaffolds during the degradation. The mass loss ratios of FS-P, FS-CP, YS-P and YS-CP were, respectively, up to 5.78 ± 2.41%, 30.98 ± 10.79%, 8.52 ± 5.77% and 22.86 ± 0.82% in 15 weeks. Based on the mass loss ratio results during the degradation, it can be theoretically found that almost all the collagen components have been degraded in 15 weeks. In Fig. 4B, the pH value of all the electrospun scaffolds kept reducing during the degradation. After degradation for 15 weeks, the pH value of the P(LLA–CL) fibers and collagen/P(LLA–CL) fibers severally reduced to 6.33 ± 0.13 and 6.10 ± 0.48, while that of the P(LLA–CL) yarns and collagen/P(LLA–CL) yarns declined to 6.06 ± 0.09 and 5.82 ± 0.19 respectively.The morphology changes of the fibers and yarns during the degradation are observed in the SEM images in Fig. 4C. During the first 9 months, all of the electrospun samples maintained the fiber or yarn morphology and no significant difference was observed. However, the morphology changes with slight dissolution and break were observed in the collagen/P(LLA–CL) fibers and collagen/P(LLA–CL) yarns at 12 weeks. At 15 weeks, the collagen/P(LLA–CL) yarns had a visible fiber break. Several yarns were dissolved together and the collagen/P(LLA–CL) fibers were partly dissolved as well.
3.5 In vivo evaluation
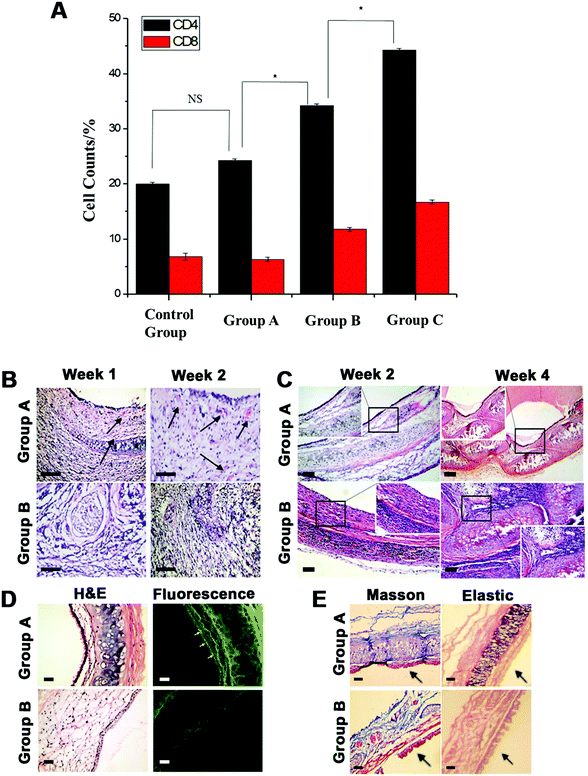
The cell-seeded BLTS (CS-BLTS) and the pre-vascularized BLTS (PV-BLTS) were obtained as demonstrated in Fig. 5. The digital photos of CS-BLTS are shown in Fig. S10 (ESI†), and the pre-vascularization process is displayed in Fig. S11 (ESI†). Then, the BLTS grafts were implanted in a rat tracheal transplant model (Fig. S12, ESI†). At the pre-determined time, the grafts were explanted and evaluated for tracheal tissue regeneration. To assess the cellular immune response in vivo, immunological indicators (CD4T and CD8T lymphocytes) were detected after Group A, Group B and the bare BLTS were implanted for 2 weeks. Flow cytometry analysis of CD4T and CD8T in rat peripheral blood after implantation is presented in Fig. S13, ESI†. CD4T and CD8T grouping and cell numbers of the PV-BLTS (Group A) were close to those of normal rats (Control group), while those of the CS-BLTS (Group B) and bare BLTS were significantly higher than those of the control group (Fig. 6A). Typical H&E images show new regeneration capillaries for the PV-BLTS (Group A) and CS-BLTS (Group B) after implantation for 1 and 2 weeks (Fig. 6B). It is clearly observed that more capillaries were regenerated in the PV-BLTS (as marked by black arrows) in comparison to the CS-BLTS at the same time point. By counting, the average regeneration capillary numbers of the PV-BLTS at 1 week and 2 weeks were, respectively, 4.33 ± 0.58 and 8.67 ± 1.53, which was separately greater than that of CS-BLTS (1.67 ± 0.58 at 1 week and 2.67 ± 0.58 at 2 weeks) (Table 2).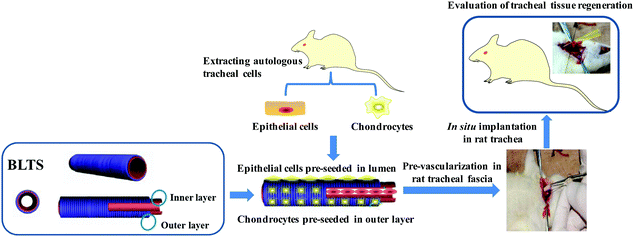 | ||
| Fig. 5 A schematic diagram of the preparation of the cell-seeded BLTS and pre-vascularized BLTS, and in situ implantation in a rat trachea transplant model. | ||
| Newly regenerated capillary numbers at 1 week | Newly regenerated capillary numbers at 2 weeks | |||||||
|---|---|---|---|---|---|---|---|---|
| View 1 | View 2 | View 3 | Average | View 1 | View 2 | View 3 | Average | |
| Group A | 4 | 5 | 4 | 4.33 | 7 | 9 | 10 | 8.67 |
| Group B | 1 | 2 | 2 | 1.67 | 3 | 2 | 3 | 2.67 |
Fig. 6C displays the cell growth and lumen morphology change of the BLTS after in situ implantation for 2 and 4 weeks. The H&E images indicate that the PV-BLTS (Group A) maintained an integrated and patent lumen structure at 2 weeks. A complete layer of columnar epithelium was visible and a few chondrocytes in the submucosa were observed as well. At 4 weeks, simple ciliated columnar epithelium was formed and a continuous cartilage cell layer was generated in the submucosa without noticeable inflammatory cell infiltration in Group A. In comparison, flat epithelial cells grew on the lumen of the CS-BLTS (Group B) and no evident chondrocytes formed in the submucosa after implantation for 2 weeks. At 4 weeks, inflammatory cell infiltration was more visible in the CS-BLTS and irregular chondrocytes were formed in the submucosa with hyperplasia of the fibrous tissue. For the bare BLTS, no covered epithelial tissue was produced and abundant inflammatory cells infiltrated into the submucosa (Fig. S14A, ESI†). After 4 weeks, the lumen showed significant stenosis with vast desmoplasia in the bare BLTS (Fig. S14B, ESI†).
To further verify epithelial cell regeneration on the different BLTSs, immunofluorescence images with the anti-cytokeratin antibody are displayed in Fig. 6D and the same slide was utilized for H&E staining after implantation for 4 weeks. The immunofluorescence image of the PV-BLTS (Group A) presented a better fluorescence signal on the mucous epithelium (as the yellow arrows indicate), while no significant CK expression was observed on the CS-BLTS (Group B). The BLTSs were observed as dye colored as backgrounds because of the collagen components. The bare BLTS had severe stenosis and no visible fluorescence was observed (Fig. S14C, ESI†). After implanting the scaffolds in situ for 4 weeks, Masson trichrome and Verhoeff–Van Gieson elastic fiber staining was, respectively, performed for collagen and elastic tissue detection. From Fig. 6E, submucous collagen production (blue collagen fibers) surrounding the chondrocytes was more obviously presented in the PV-BLTS (Group A) than that in the CS-BLTS (Group B). Similarly, more pink elastic fibers were observed in the PV-BLTS (Group A) than in the CS-BLTS (Group B).
4. Discussion
Collagen/P(LLA–CL) composites with a weight ratio of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 were selected for this study. Due to the addition of collagen components, the collagen/P(LLA–CL) composites were crosslinked with glutaraldehyde vapor. SEM images show the structures of the collagen/P(LLA–CL) fibers and yarns after crosslinking (Fig. S1, ESI†). The fiber diameter of the collagen/P(LLA–CL) composite was decreased in comparison to pure P(LLA–CL), because of the blending with collagen.43 Similarly, the yarn diameter of P(LLA–CL) was greater than that of collagen/P(LLA–CL). Compared with pure P(LLA–CL) yarns, the collagen/P(LLA–CL) yarns had more random fibers on the surface or between the neighbors of the yarns, mainly because of the hydrophilicity of collagen during the twisting by the water vortex. The bilayer tubular scaffold (BLTS) consisted of collagen/P(LLA–CL) fibers (inner layer) and collagen/P(LLA–CL) yarns (outer layer). The whole thickness of the double layers was about 200 to 300 μm. The thickness of the outer layer was around 100 μm, which was responsible for chondrocyte migration, while the wall of the inner layer was mildly thicker than the outer layer, which mainly helped to maintain the tubular structure. The cross-sectional view (Fig. 1H and I) proved that the fibers and yarns were arranged conterminously, with the fiber/yarn diameter in the range from hundreds of nanometers to dozens of micrometers.
1 were selected for this study. Due to the addition of collagen components, the collagen/P(LLA–CL) composites were crosslinked with glutaraldehyde vapor. SEM images show the structures of the collagen/P(LLA–CL) fibers and yarns after crosslinking (Fig. S1, ESI†). The fiber diameter of the collagen/P(LLA–CL) composite was decreased in comparison to pure P(LLA–CL), because of the blending with collagen.43 Similarly, the yarn diameter of P(LLA–CL) was greater than that of collagen/P(LLA–CL). Compared with pure P(LLA–CL) yarns, the collagen/P(LLA–CL) yarns had more random fibers on the surface or between the neighbors of the yarns, mainly because of the hydrophilicity of collagen during the twisting by the water vortex. The bilayer tubular scaffold (BLTS) consisted of collagen/P(LLA–CL) fibers (inner layer) and collagen/P(LLA–CL) yarns (outer layer). The whole thickness of the double layers was about 200 to 300 μm. The thickness of the outer layer was around 100 μm, which was responsible for chondrocyte migration, while the wall of the inner layer was mildly thicker than the outer layer, which mainly helped to maintain the tubular structure. The cross-sectional view (Fig. 1H and I) proved that the fibers and yarns were arranged conterminously, with the fiber/yarn diameter in the range from hundreds of nanometers to dozens of micrometers.
The Fourier transform infrared (FTIR) spectral results show a peak at 3319 cm−1, which was attributed to the N–H stretch coupled with the hydrogen bond, while the peak at 2946 cm−1 was assigned to the CH3 stretching vibration absorption (Fig. S2, ESI†).44 The absorption peaks at 1652 cm−1 (amide I) and 1558 cm−1 (amide II) represent the existence of collagen, and the representative absorption bands at 1759 cm−1 correspond to the C–O stretching in P(LLA–CL).39 Compared with pure collagen and P(LLA–CL) materials, it was found that the BLTS maintained the original components via general electrospinning and subsequent dynamic liquid electrospinning.
The mechanical properties of the bilayer scaffold were also measured in our previous study.44 The ultimate tensile stress and strain of the inner layer were 14.27 ± 1.39 MPa and 101.69 ± 1.87%, respectively (Fig. S3A, ESI†). The outer layer had 14.09 ± 1.15 MPa in tensile strength and 281.31 ± 16.22% in elongation in the perpendicular direction, and those in the perpendicular direction were 2.51 ± 0.60 MPa and 266.94 ± 30.23%, respectively (Fig. S3B, ESI†). It can be seen that the fiber alignment had a positive effect on the elongation of the outer layer. Besides, the ultimate tensile strength of the bilayer scaffold in the parallel and perpendicular directions was 6.92 ± 1.70 MPa and 5.15 ± 0.16 MPa, while the elongation was 276.22 ± 23.43% and 267.85 ± 59.61%, respectively (Fig. S3C, ESI†). The stress–strain curve of the bilayer scaffold showed an elastic behavior at the initial stage, while the strength decreased drastically and then increased gradually after the strain reached 100%. Finally, the elongation at the break of the bilayer scaffold was found to be up to 300%, attributed to the first break of the inner layer and the subsequent break of the outer layer. The overall mechanical results demonstrate that the BLTS not only had good tensile strength from the inner layer, but also maintained the better strain of the outer layer.44
Collagen/P(LLA–CL) fibers and yarns were the inner and outer layers of the BLTS respectively. Because of the loose and porous structure of the yarns, the outer layer of the BLTS showed enhanced porosity compared with the inner layer. Besides, due to the addition of soluble collagen and the crosslinking process,43 the pore sizes of the collagen/P(LLA–CL) fibers and yarns were both decreased compared with the untreated samples. Generally, the pore size of the submicron fiber structure was unsatisfactory for cell penetration, while the performance of micron sized yarns was better for the three-dimensional growth of cells.
Samples with collagen components were crosslinked with glutaraldehyde vapor and the crosslinking process could help the composite scaffold maintain the structure and integrality during the degradation for 3 to 6 months in vitro.43,45 In the degradation process, the weight loss was mainly generated from the dissolution of soluble oligomeric compounds in the degradation medium through the hydrolysis scission of polymer chains.46 The scaffolds having collagen components showed quicker degradation than the pure P(LLA–CL) regardless of the scaffold structure, because collagen is water-soluble and can be hydrolyzed more easily. Hence, the collagen/P(LLA–CL) fibers presented the fastest degradation rate among all the samples and the moss loss ratio at 15 weeks was up to 30%. The degradation rate of the collagen/P(LLA–CL) yarns was slower than the collagen/P(LLA–CL) fibers, because parts of the collagen components were dissolved during the dynamic liquid electrospinning and the collagen proportion in the collagen/P(LLA–CL) fibers was greater than that in the collagen/P(LLA–CL) yarns. In comparison, the P(LLA–CL) fibers and yarns had a similar weight change at the beginning of 9 months, but the P(LLA–CL) yarns showed faster mass loss at 12 and 15 weeks. This was attributed to the larger porosity and pore diameter provided by the yarn structure, which contributed to water infiltration and hydrolysis. The decrease in the pH value in the degradation medium was mainly affected by the P(LLA–CL) components, because P(LLA–CL) accounted for 75% in the composites. The soluble oligomers in P(LLA–CL) diffused and dissolved in the degradation medium and the carboxyl group (–COOH) of the oligomers contributed to the pH decrease.46 The morphology changes in the different scaffolds were observed using SEM. In comparison to pure P(LLA–CL), the collagen/P(LLA–CL) fibers and yarns had more melting and fusion fibers, mainly because of the rapid hydrolysis of collagen in the composites. The overall results indicate that the collagen/P(LLA–CL) composites or pure P(LLA–CL) fibers and yarns could maintain a relatively stable degradation during 15 weeks in vitro.
The in vitro biocompatibility of the collagen/P(LLA–CL) fibers and yarns was assessed before in vivo evaluation. The methyl thiazolyl tetrazolium (MTT) assay results of rat tracheal epithelial cell (RTEC) adhesion to and proliferation on the collagen/P(LLA–CL) fibers are displayed in Fig. S4 (ESI†). The results show that the initial RTEC adhesion (after culturing for 0.5 hour) to collagen/P(LLA–CL) fibers was highly significant (P < 0.001) compared with the pure P(LLA–CL) fibers and the tissue culture plate (TCP). RTEC adhesion after culturing for 2 and 6 hours showed a significant difference (P < 0.05) compared with the pure P(LLA–CL) samples as well. The RTEC proliferation results indicate that the RTEC numbers on the collagen/P(LLA–CL) fibers increased very quickly from day 1 to day 7 and the proliferated cell numbers showed a highly significant difference (P < 0.001) compared with those at the previous time point. After culturing RTECs for 14 days, the collagen/P(LLA–CL) fibers still had a significant difference (P < 0.05) compared to the pure P(LLA–CL) sample. The SEM observation of RTEC morphology on the collagen/P(LLA–CL) fibers (Fig. S5, ESI†) showed the attachment and stretching of the RTECs, indicating the interaction between RTECs and the fibers. After growing for 5 days, RTECs proliferated to a continuous cell monolayer on the collagen/P(LLA–CL) fibers, showing the collaboration between RTECs on the scaffold. Green fluorescent protein (GFP) expression of the active RTECs based on gene transfection was measured to confirm RTEC activity (Fig. S6, ESI†) after culturing for 1, 3 and 5 days. H&E images presented the RTEC morphology and density on collagen/P(LLA–CL) fibers at the same periods. GFP expression on collagen/P(LLA–CL) fibers showed increased fluorescence intensity from day 1 to day 5 and the fluorescence intensity showed consistency with the cell density in the H&E images. The results confirm the positive contribution of the collagen/P(LLA–CL) fibers on RTEC growth and activity.
Rat tracheal chondrocyte (RTC) proliferation on the collagen/P(LLA–CL) yarns is displayed in Fig. S7 (ESI†). Similarly, the RTC numbers on the collagen/P(LLA–CL) yarns showed the most increase at the first week and the RTC proliferation on the collagen/P(LLA–CL) yarns showed a significant difference (P < 0.05) compared with pure P(LLA–CL) at 14 days as well. H&E images of the cross-sections showed sufficient RTC infiltration throughout the collagen/P(LLA–CL) yarns after culturing for 14 days (Fig. S8, ESI†). Red fluorescent protein (RFP) expression of active RTCs on the collagen/P(LLA–CL) yarns showed increased fluorescence intensity from day 1 to day 14, confirming the positive effect of the collagen/P(LLA–CL) yarns on RTC proliferation and activity (Fig. S8, ESI†). H&E and Masson staining images of the collagen/P(LLA–CL) yarns after being subcutaneously embedded for 2 and 4 weeks further confirmed the advantages of the collagen/P(LLA–CL) yarns towards cell penetration (Fig. S9, ESI†). The overall in vitro biological results demonstrate that collagen/P(LLA–CL) shows good biocompatibility and an enhanced RTEC/RTC activity and proliferation. RTC penetration into the scaffold was promoted due to the loose and porous structure of the collagen/P(LLA–CL) yarns.
The cell-seeded BLTS (CS-BLTS), the pre-vascularized BLTS (PV-BLTS) and the bare BLTS were implanted in a rat tracheal transplant model for in vivo evaluation. Immune response experiments indicated that the PV-BLTS had lower immunogenicity than the CS-BLTS and bare BLTS. According to the study, it was speculated that the lack of vascularization in the transplanted tissue engineered scaffold was the critical reason resulting in the cartilage softening and epithelialization delay.3,47 Hence, pre-vascularization played a more effective role in epithelialization formation and cartilage maturation. The H&E staining results (Fig. 6B) indicate that the pre-vascularization process was effective for new capillary regeneration on the BLTS.
The H&E results in Fig. 6C demonstrate that the epithelial tissues on the CS-BLTS and PV-BLTS were mainly produced from the pre-seeding of the epithelial cells and their proliferation. Besides, the lumen morphology of the PV-BLTS (Group A) was better than that of the CS-BLTS (Group B). This was mainly because of the advantage of pre-vascularization, which played an important role in structure preservation.3 Both the PV-BLTS and CS-BLTS preserved better lumen morphology than the bare BLTS, because the pre-seeded chondrocytes performed better than chondrocyte penetration from the surroundings. Hence, the cell-seeding and pre-vascularization processes were of great importance for cell maturation, functioning and scaffold structure preservation. Besides, it demonstrated epithelial tissue regeneration and cytokeratin expression on the PV-BLTS as shown in Fig. 6D. Significant ciliated columnar epithelium was observed in the PV-BLTS (Group A) in the H&E images, showing that epithelial tissue regeneration on the PV-BLTS was obviously better than the other two BLTS scaffolds. The results of Masson trichrome and Verhoeff–Van Gieson elastic fiber staining demonstrate better collagen and elastic tissue regeneration in the PV-BLTS than in the CS-BLTS (Fig. 6E). Hence, the in vivo results indicate that the PV-BLTS better promoted new capillary regeneration, reduced immunogenicity and improved tracheal tissue regeneration than the CS-BLTS and bare BLTS.
5. Conclusion
The bilayer tubular scaffold (BLTS) was prepared from collagen/P(LLA–CL) dense fibers and porous yarns via general electrospinning and dynamic liquid electrospinning methods. The BLTS had a coherent bilayer structure and showed a good performance in biodegradation and in vitro biocompatibility. After preprocessing of cellularization with autologous tracheal epithelial cells and chondrocytes and pre-vascularization in rat tracheal fascia, the BLTS showed significantly lower immunological rejection and considerable development in rat trachea tissue regeneration. This is mainly due to the improved biological function and enhanced production of epithelium, cartilage maturation and capillary neogenesis on the pre-vascularized BLTS. Our study demonstrated that the bilayer tubular scaffold, consisting of collagen/P(LLA–CL) fibers/yarns and preprocessing with autologous tracheal cells and vascularization, was beneficial to trachea tissue regeneration and reconstruction. Therefore, the pre-vascularized BLTS based on the collagen/P(LLA–CL) composite provides a promising alternative for trachea tissue repair.Acknowledgements
This research was supported by the National Natural Science Foundation of China (31470941 and 31271035), the Science and Technology Commission of Shanghai Municipality (15JC1490100 and 15441905100), the National Major Research Program of China (2016YFC1100200), the PhD Programs Foundation of Ministry of Education of China (20130075110005) and the light of textile project (J201404), The authors extend their appreciation to the International Scientific Partnership Program ISPP at King Saud University for its funding research through (ISPP# 0049).References
- C. A. Vacanti, K. T. Paige and W. S. Kim, J. Pediatr. Surg., 1994, 29, 204–205 CrossRef.
- S. Baiguera, P. Jungebluth, A. Burns, C. Mavilia, J. Haag, P. De Coppi and P. Macchiarini, Biomaterials, 2010, 31, 8931–8938 CrossRef CAS PubMed.
- X. Luo, Y. Liu, Z. Zhang, R. Tao, Y. Liu, A. He, Z. Yin, D. Li, W. Zhang, W. Liu, Y. Cao and G. Zhou, Biomaterials, 2013, 34, 3336–3344 CrossRef CAS PubMed.
- H. C. Grillo, Ann. Thorac. Surg., 2002, 73, 1995–2004 CrossRef PubMed.
- H. C. Grillo, Ann. Surg., 1970, 7, 3–59 Search PubMed.
- E. J. Propst, J. D. Prager, J. Meinzen-Derr, S. L. Clark, R. T. Cotton and M. J. Rutter, Arch. Otolaryngol., Head Neck Surg., 2011, 137, 583–590 CrossRef PubMed.
- M. Friedman and A. D. Mayer, Ann. Otol., Rhinol., Laryngol., 1992, 101, 897–908 CAS.
- C. H. Kim, J. H. Bae, S. Son, J. H. Kim, J. G. Lee and J. H. Yoon, Acta Otolaryngol., 2009, 126, 594–599 CrossRef PubMed.
- L. Song, D. Sengupta and C. Shu, Wiley Interdiscip. Rev.: Syst. Biol. Med., 2014, 6, 61–76 CrossRef PubMed.
- H. Siba, T. K. Waddell and S. O. P. Hofer, J. Cell. Mol. Med., 2011, 15, 24–25 CrossRef PubMed.
- N. T. Remlinger, C. A. Czajka, M. E. Juhas, D. A. Vorp, D. B. Stolz, S. F. Badylak, S. Gilbert and T. W. Gilbert, Biomaterials, 2010, 31, 3520–3526 CrossRef CAS PubMed.
- J. Haag, S. Baiguera, P. Jungebluth, D. Barale, C. Del Gaudio, F. Castiglione, A. Bianco, C. E. Comin, D. Ribatti and P. Macchiarini, Biomaterials, 2012, 33, 780–789 CrossRef CAS PubMed.
- T. W. Gilbert, T. L. Sellaro and S. F. Badylak, Biomaterials, 2006, 27, 3675–3683 CAS.
- F. Ajalloueian, M. L. Lim, G. Lemon, J. C. Haag, Y. Gustafsson, S. Sjoqvist, A. Beltran-Rodriguez, C. Del Gaudio, S. Baiguera, A. Bianco, P. Jungebluth and P. Macchiarini, Biomaterials, 2014, 35, 5307–5315 CrossRef CAS PubMed.
- Y. S. Jang, C. H. Jang, Y. B. Cho, M. Kim and G. H. Kim, Int. J. Pediatr. Otorhinolaryngol., 2014, 78, 2237–2243 CrossRef PubMed.
- R. B. Metter, J. L. Ifkovits, K. Hou, L. Vincent, B. Hsu, L. Wang, R. L. Mauck and J. A. Burdick, Acta Biomater., 2010, 6, 1219–1226 CrossRef CAS PubMed.
- R. M. Nezarati, M. B. Eifert, D. K. Dempsey and E. Cosgriff-Hernandez, J. Biomed. Mater. Res., Part B, 2015, 103, 313–323 CrossRef PubMed.
- W. Liu, C. Ni, D. B. Chase and J. F. Rabolt, ACS Macro Lett., 2013, 2, 466–468 CrossRef CAS.
- J. A. Matthews, G. E. Wnek, D. G. Simpson and G. L. Bowlin, Biomacromolecules, 2002, 3, 232–238 CrossRef CAS PubMed.
- M. Pakravan, M.-C. Heuzey and A. Ajji, Biomacromolecules, 2012, 13, 412–421 CrossRef CAS PubMed.
- A. Tamayol, M. Akbari, N. Annabi, A. Paul, A. Khademhosseini and D. Juncker, Biotechnol. Adv., 2013, 31, 669–687 CrossRef CAS PubMed.
- C. Mahoney, D. Conklin, J. Waterman, J. Sankar and N. Bhattarai, J. Biomater. Sci., Polym. Ed., 2016, 27, 611–625 CrossRef CAS PubMed.
- S. Liu, C. Dong, G. Lu, Q. Lu, Z. Li, D. L. Kaplan and H. Zhu, Acta Biomater., 2013, 9, 8991–9003 CrossRef CAS PubMed.
- A. Yin, K. Zhang, M. J. McClure, C. Huang, J. Wu, J. Fang, X. Mo, G. L. Bowlin, S. S. Al-Deyab and M. El-Newehy, J. Biomed. Mater. Res., Part A, 2013, 101, 1292–1301 CrossRef PubMed.
- K. Zhang, H. Wang, C. Huang, Y. Su, X. Mo and Y. Ikada, J. Biomed. Mater. Res., Part A, 2010, 93, 984–993 Search PubMed.
- J. Wu, C. Huang, W. Liu, A. Yin, W. Chen, C. He, H. Wang, S. Liu, C. Fan and G. L. Bowlin, J. Biomed. Nanotechnol., 2014, 10, 603–614 CrossRef CAS PubMed.
- K. T. Shalumon, K. H. Anulekha, C. M. Girish, R. Prasanth, S. V. Nair and R. Jayakumar, Carbohydr. Polym., 2010, 80, 413–419 CrossRef CAS.
- J. Xu, J. Zhang, W. Gao, H. Liang, H. Wang and J. Li, Mater. Lett., 2009, 63, 658–660 CrossRef CAS.
- E. Kijenska, M. P. Prabhakaran, W. Swieszkowski, K. J. Kurzydlowski and S. Ramakrishna, J. Biomed. Mater. Res., Part B, 2012, 100, 1093–1102 CrossRef PubMed.
- A. Hasan, A. Memic, N. Annabi, M. Hossain, A. Paul, M. R. Dokmeci, F. Dehghani and A. Khademhosseini, Acta Biomater., 2014, 10, 11–25 CrossRef CAS PubMed.
- C. P. Barnes, S. A. Sell, E. D. Boland, D. G. Simpson and G. L. Bowlin, Adv. Drug Delivery Rev., 2007, 59, 1413–1433 CrossRef CAS PubMed.
- D. I. Zeugolis, S. T. Khew, E. S. Yew, A. K. Ekaputra, Y. W. Tong, L. Y. Yung, D. W. Hutmacher, C. Sheppard and M. Raghunath, Biomaterials, 2008, 29, 2293–2305 CrossRef CAS PubMed.
- M. J. McClure, D. G. Simpson and G. L. Bowlin, J. Mech. Behav. Biomed. Mater., 2012, 10, 48–61 CrossRef CAS PubMed.
- D. G. Simpson, Expert Rev. Med. Devices, 2006, 3, 471–484 CrossRef PubMed.
- I. K. Kwon and T. Matsuda, Biomacromolecules, 2005, 6, 2096–2105 CrossRef CAS PubMed.
- W. Fu, Z. Liu, B. Feng, R. Hu, X. He, H. Wang, M. Yin, H. Huang, H. Zhang and W. Wang, Int. J. Nanomed., 2014, 9, 2335–2344 CrossRef PubMed.
- S. A. Sell, M. J. McClure, K. Garg, P. S. Wolfe and G. L. Bowlin, Adv. Drug Delivery Rev., 2009, 61, 1007–1019 CrossRef CAS PubMed.
- Y. Su, Q. Su, W. Liu, M. Lim, J. R. Venugopal, X. Mo, S. Ramakrishna, S. S. Al-Deyab and M. El-Newehy, Acta Biomater., 2012, 8, 763–771 CrossRef CAS PubMed.
- Y. Xu, J. Wu, H. Wang, H. Li, N. Di, L. Song, S. Li, D. Li, Y. Xiang, W. Liu, X. Mo and Q. Zhou, Tissue Eng., Part C, 2013, 19, 925–936 CrossRef CAS PubMed.
- Y. Xu, S. Dong, Q. Zhou, X. Mo, L. Song, T. Hou, J. Wu, S. Li, Y. Li, P. Li, Y. Gan and J. Xu, Biomaterials, 2014, 35, 2760–2772 CrossRef CAS PubMed.
- W. E. Teo, R. Gopal, R. Ramaseshan, K. Fujihara and S. Ramakrishna, Polymer, 2007, 48, 3400–3405 CrossRef CAS.
- C. Huang, R. Chen, Q. Ke, Y. Morsi, K. Zhang and X. Mo, Colloids Surf., B, 2011, 82, 307–315 CrossRef CAS PubMed.
- T. Wu, C. Huang, D. Li, A. Yin, W. Liu, J. Wang, J. Chen, H. Ei-Hamshary, S. S. Al-Deyab and X. Mo, Colloids Surf., B, 2015, 133, 179–188 CrossRef CAS PubMed.
- J. Chen, W. Liu, T. Wu, D. Li, J. Zhang, N. Wang and X. Mo, J. Donghua Univ., 2014, 31, 718–722 Search PubMed.
- T. Wu, B. Jiang, Y. Wang, A. Yin, C. Huang, S. Wang and X. Mo, J. Mater. Chem. B, 2015, 3, 5760–5768 RSC.
- K. Zhang, A. Yin, C. Huang, C. Wang, X. Mo, S. S. Al-Deyab and M. El-Newehy, Polym. Degrad. Stab., 2011, 96, 2266–2275 CrossRef CAS.
- Q. Tan, R. Steiner, S. P. Hoerstrup and W. Weder, Eur. J. Cardiothorac. Surg., 2006, 30, 782–786 CrossRef PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: FTIR spectra of collagen/P(LLA–CL) fibers and yarns; SEM images of collagen/P(LLA–CL) fibers and yarns after crosslinking; in vitro biocompatibility analysis (including tracheal epithelial cell (RTEC) adhesion to and proliferation on collagen/P(LLA–CL) fibers; SEM images of RTECs grown on collagen/P(LLA–CL) fibers; H&E images of RTEC morphology and fluorescence images of green fluorescent protein (GFP) expression of RTECs on collagen/P(LLA–CL) fibers; tracheal chondrocyte proliferation on collagen/P(LLA–CL) yarns; H&E images of tracheal chondrocyte penetration and fluorescence images of red fluorescent protein (RFP) expression of tracheal chondrocytes on collagen/P(LLA–CL) yarns); cell penetration into collagen/P(LLA–CL) yarns after being subcutaneously embedded for 2 and 4 weeks; images of the BTLS after cell-seeding and pre-vascularization processing; images of in situ implantation of the BLTS in a rat trachea; H&E staining and immunofluorescence images of bare BLTS after in vivo implantation. See DOI: 10.1039/c6tb02484j |
| ‡ Tong Wu and Hui Zheng contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2017 |