A smart, stretchable resistive heater textile†
Yiming
Li‡
,
Zhitao
Zhang‡
,
Xueyi
Li
,
Jing
Zhang
,
Huiqing
Lou
,
Xiang
Shi
,
Xunliang
Cheng
* and
Huisheng
Peng
 *
*
State Key Laboratory of Molecular Engineering of Polymers, Department of Macromolecular Science, and Laboratory of Advanced Materials, Fudan University, Shanghai 200438, China. E-mail: penghs@fudan.edu.cn
First published on 8th December 2016
Abstract
A new kind of flexible and stretchable strip-shaped thermochromic resistive heater (TRH) has been fabricated by incorporating an aligned carbon nanotube sheet and a thermochromic silicone elastomer. This strip-shaped TRH demonstrates rapid thermal response and high stability even under stretching at a speed of 2 mm s−1. The resulting TRH textiles woven from the strip-shaped TRHs are flexible, stretchable, and breathable, and they can stably work under various deformations such as twisting. The temperatures of TRH textiles during working can be visually evaluated from the designed patterns for both high efficiency and safety. The weaving structure in the TRH textile is available for local heating, which has been demonstrated for thermal therapy.
Resistive heaters as an important kind of temperature adjusters have been applied extensively in fields such as displays, defoggers, sensors and microchannel chips.1–6 Recently, as a new emerging application, resistive heaters have been expected to be combined with the human body for wearable heating and thermal therapy.7–9 For instance, wearable thermal therapy as an effective way for physiotherapy has been widely employed for alleviating pain caused by joint injuries, due to its capability to contribute to the vasodilation effect with moderate increase in temperature.10 Additionally, local heating is envisioned to accelerate the healing of chronic wounds as this process is able to increase collagen deposition in the wound area.11,12 Another potential application exists in thermal-assisted localized drug release from drug coatings that are sensitive to temperature.13
Although wearable resistive heaters are critical and promising for a variety of medical applications,8,9,14 on the one hand, the current rigid planar configuration has remarkably limited their use in the realization of body-conformable resistive heaters.15,16 The human body is soft and curvilinear, while planar resistive heaters are rigid and bulky. This profound mismatch makes them difficult to be effectively combined in an imperceptible way. More importantly, most planar resistive heaters can only work as a whole, which cannot easily realize the local heating on the human body with designed patterns.17–21 Meanwhile, the planar heaters are airtight, which may cause problems for the medical applications where the wounds are required to be breathable.3,17–19,22,23 On the other hand, electronic devices including heaters are expected to be integrated with more functionalities such as intelligence (e.g., they can visually exhibit their working states).24–26
Herein, a new kind of flexible and stretchable strip-shaped thermochromic resistive heater (TRH) has been fabricated by incorporating an aligned carbon nanotube (CNT) sheet and a thermochromic silicone elastomer. This strip-shaped TRH demonstrates rapid thermal response and high stability even under stretching at a speed of 2 mm s−1. The resulting TRH textiles woven from the strip-shaped TRHs are flexible, stretchable, and breathable, and they can stably work under various deformations such as twisting. The temperatures of TRH textiles during working can be visually evaluated from the designed patterns for both high efficiency and safety. The weaving structure in the TRH textile is available for local heating, which has been demonstrated for thermal therapy.
The fabrication of the strip-shaped TRH is schematically exhibited in Fig. 1a. A thermochromic silicone elastomer that has been prepared by spin coating from an admixture of silicone elastomer precursor solution and thermochromic microcapsule ink is first pre-stretched. Aligned CNT sheets are then paved on the stretched thermochromic silicone elastomer substrate. The CNT sheet was drawn from a spinnable CNT array synthesized by chemical vapor deposition (Fig. S1 and S2, ESI†).27 Finally, the substrate is released to the relaxed state to obtain the stretchable strip-shaped TRH. Fig. 1b shows the resulting smart resistive heater textile woven from these as-prepared strip-shaped TRHs, which can be controlled to visually display the real-time temperature with different patterns at a safe voltage for the human body (below 10 V).
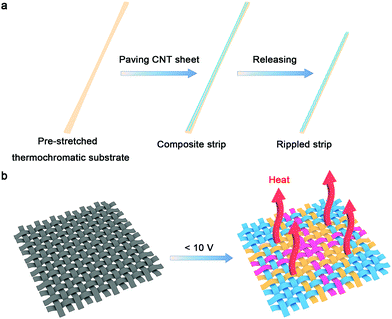 | ||
| Fig. 1 (a) Schematic illustration of the fabrication of stretchable strip-shaped TRH. (b) The resulting smart TRH textile working under an applied voltage below 10 V. | ||
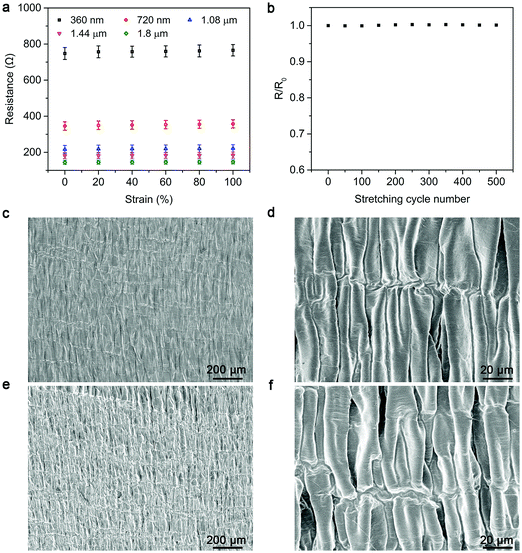
As the heating power of the strip-shaped TRH greatly depends on the used composite electrode, the electrical resistance was first investigated with increasing CNT thickness. The electrical resistances were remarkably decreased from 748 Ω to 145 Ω upon increasing CNT thicknesses from 360 nm to 1.8 μm and then remained almost unchanged at higher thicknesses (Fig. 2a). Therefore, a 1.8 μm-thick CNT layer was mainly employed for the subsequent studies. The resistivity of CNT is calculated to be nearly 6.52 × 10−5 Ω m. The electrical resistance was maintained well under stretching with increasing strains up to 100%, and no obvious fatigue was observed after stretching for 500 cycles even at a strain of 100% (Fig. 2a and b). The high stability in electrical resistance was derived from the designed wrinkle morphology of the CNT layer that could be reversibly switched between the wavy and stretched microstructures (Fig. 2c–f).
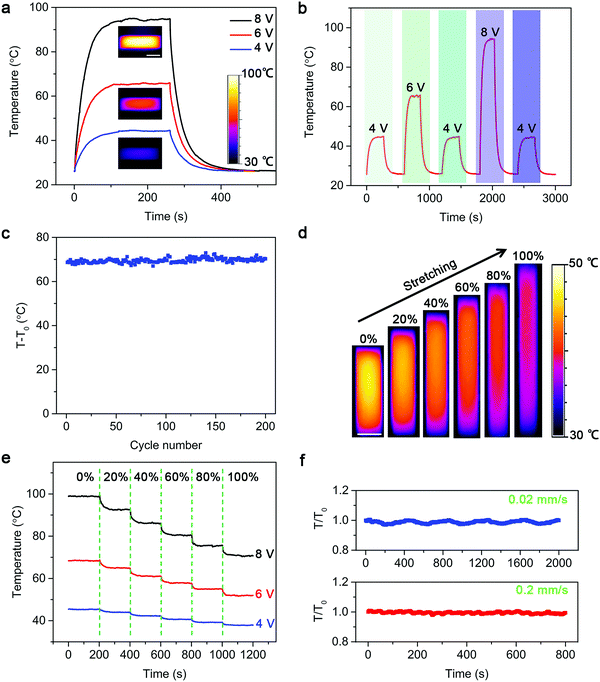
Their Joule heating properties are further carefully studied. The working mechanism of the strip-shaped TRH is shown in Fig. S3 (ESI†). Resistive Joule heating is induced by applying a constant DC voltage at both ends of the CNT electrode. Fig. 3a demonstrates the temperature–time curves of a strip-shaped TRH at different voltages (Fig. S4, ESI†). Here, the average temperature is displayed in the temperature–time curves throughout this work. The temperature was increased with increasing voltage (Fig. S5, ESI†). A rapid temperature response was detected (Fig. S6, ESI†) due to the high electrical and thermal conductivity of aligned CNTs.28–30 The saturated temperature measured by an infrared camera was increased from 45 and 66 to 95 °C with increasing applied voltages from 4 and 6 to 8 V, respectively. Therefore, the temperature of the strip-shaped TRH can be readily controlled by varying the pulse voltage (Fig. 3b and Fig. S7, ESI†). It returned to the original room temperature after removal of the current. The stable Joule heating property was verified in 200 on–off cycles (Fig. 3c).
It is important to maintain the heating efficiency well under deformations during use. Fig. S8 (ESI†) shows the infrared images of a strip-shaped TRH under three-dimensional distortion. The saturated temperature of the strip-shaped TRH remained nearly unchanged after bending at a radius of curvature of 0.5 mm for 500 cycles (Fig. S9, ESI†). Furthermore, the strip-shaped TRH could work normally during stretching, which was attributed to the wrinkle morphology of the CNT layer. The saturated temperatures were gradually decreased from 45, 44, 42, 41 and 39 to 37 °C due to higher heating areas2 under increasing strains from 0, 20%, 40%, 60% and 80% to 100% (Fig. 3d and e), and they were stable after repeated stretching (Fig. S10, ESI†). The dependence of temperature on time was further traced under dynamic stretching and releasing with increasing speeds. The temperatures were maintained by 96% with increasing stretching and releasing speed up to 0.2 mm s−1, indicating a stable thermal performance under dynamic stretching and releasing (Fig. 3f).
Although there existed temperature deviations in the strip-shaped TRHs under distortions, these strips were then woven into flexible and stretchable textiles (Fig. S11 and S12, ESI†), which could effectively reduce the deviations and demonstrate relatively uniform temperature distribution. Each strip-shaped TRH can be separately controlled to heat the target (Fig. S13, ESI†), which is promising for a variety of applications such as local wound healing and thermal-assisted drug delivery that remain challenging for the planar TRHs based on laser ablation patterning and laser cutting.9,11,13,14,31
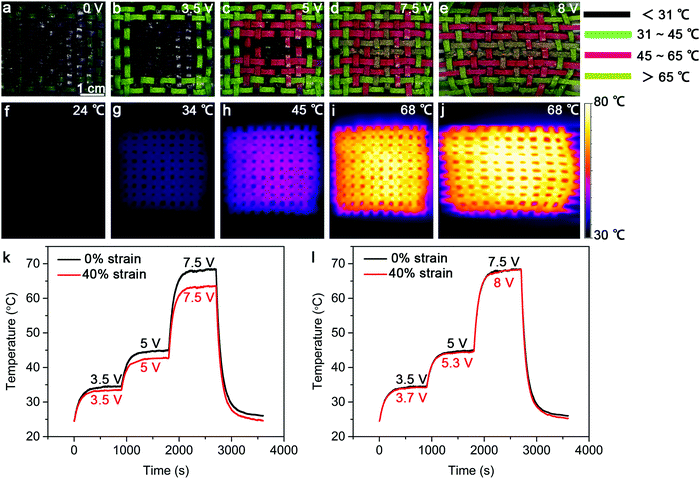
Safety is a key factor for practical applications, and it is highly desired if the heating temperature can be displayed in situ.32 To this end, we constructed strip-shaped TRHs using three kinds of thermochromic microcapsule inks that can change their colors at 31 °C, 45 °C and 65 °C, respectively. These strip-shaped TRHs were then woven into a textile with the designed pattern. As expected, the resulting TRH textile changed its patterns with increasing temperatures (applied voltages) from 24 °C (0 V), 34 °C (3.5 V) and 45 °C (5 V) to 68 °C (7.5 V) (Fig. 4a–d). This smart visual display can help the users to effectively and conveniently judge the temperature range of the TRH textile based on its pattern. Fig. 4f–i further verifies a uniform temperature distribution. Fig. 4e and j further show that the TRH textile can normally work under stretching at a strain of 40%. Note that the saturated temperatures were slightly decreased from 68 °C to 63 °C with increasing strains from 0 to 40% (Fig. 4k), which can be compensated by increasing the applied voltage (Fig. 4l).
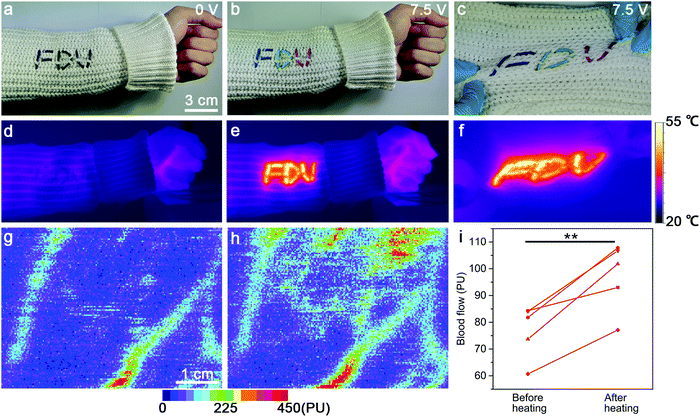
Additionally, these strip-shaped TRHs can also be woven into commercial fabric with different patterns (e.g., “FDU”) for displaying and local heating functions even under stretching (Fig. 5a–f). The resulting TRH textile had a strong potential for thermal therapy due to its local heating function coupled with the above-mentioned body-conformable mechanical property. Generally, the blood flow of the human body can be obviously increased by the effect of thermal expansion of the micro-circulation vascular system.10 Therefore, the blood flows of five healthy adults at the forearm before and after heating at 40 °C for 10 min with the TRH textile were carefully compared by laser Doppler perfusion imaging. The achieved color Doppler images are demonstrated in Fig. 5g and h (see also Fig. S14 in the ESI†). As expected, the mean values of the blood flow flux remarkably increased from 76.92 ± 10.04 to 97.28 ± 12.70 perfusion unit (PU) (n = 5, P < 0.05, Fig. 5i), where n and P correspond to the sample size and probability, respectively. This application might open up a new direction for alleviating the pain and muscle weakness within the human body.33,34
In summary, a novel strip-shaped TRH is fabricated by paving CNT sheets on a pre-stretched thermochromic silicone elastomer to effectively work at a relatively high temperature of 95 °C and a large strain of 100%. These strip-shaped TRHs are successfully woven into a range of smart TRH textiles with patterned heating and visual temperature indication. They have been demonstrated for a variety of applications such as thermal therapy. This work represents a general and effective strategy in the development of high-performance wearable electronic devices.
Experimental section
To prepare the stretchable and thermochromic silicone elastomer, a precursor solution, Ecoflex 00-30 (Smooth-On), was first mixed with different kinds of thermochromic microcapsule inks (Shenzhen Qiansebian Pigment Co., Ltd) at a weight ratio of 5/1. The thermochromic microcapsule inks can change their colors at 31 °C, 45 °C, and 65 °C, respectively. The mixture was then spin-coated on a glass slide at 100 rpm for 60 s, followed by curing at 90 °C for 1 h, and then peeled off from the glass slide. The aligned CNT sheet was directly drawn from a spinnable CNT array and paved on a copper foil that had been treated with ethanol (Sinopharm Chemical Reagent) on the surface. The CNT sheets were transferred to the as-prepared silicone elastomer by attaching the copper foil onto the pre-stretched silicone elastomer and were then peeled off the copper foil. The silicone elastomer was released to the original state to achieve the stretchable strip-shaped TRH. These strip-shaped TRHs were further woven into TRH textiles.Acknowledgements
This work was supported by MOST (2016YFA0203302), NSFC (21634003, 51573027, 51403038, 51673043, 21604012) and STCSM (16JC1400702, 15XD1500400, 15JC1490200).References
- P. Liu, L. Liu, K. Jiang and S. Fan, Small, 2011, 7, 732 CrossRef CAS PubMed.
- J. J. Bae, S. C. Lim, G. H. Han, Y. W. Jo, D. L. Doung, E. S. Kim, S. J. Chae, T. Q. Huy, N. Van Luan and Y. H. Lee, Adv. Funct. Mater., 2012, 22, 4819 CrossRef CAS.
- B. W. An, E.-J. Gwak, K. Kim, Y.-C. Kim, J. Jang, J.-Y. Kim and J.-U. Park, Nano Lett., 2016, 16, 471 CrossRef CAS PubMed.
- D. Kim, L. Zhu, D.-J. Jeong, K. Chun, Y.-Y. Bang, S.-R. Kim, J.-H. Kim and S.-K. Oh, Carbon, 2013, 63, 530 CrossRef CAS.
- Y. Mo, Y. Okawa, M. Tajima, T. Nakai, N. Yoshiike and K. Natukawa, Sens. Actuators, B, 2001, 79, 175 CrossRef CAS.
- K. Sun, A. Yamaguchi, Y. Ishida, S. Matsuo and H. Misawa, Sens. Actuators, B, 2002, 84, 283 CrossRef CAS.
- S. Hong, H. Lee, J. Lee, J. Kwon, S. Han, Y. D. Suh, H. Cho, J. Shin, J. Yeo and S. H. Ko, Adv. Mater., 2015, 27, 4744 CrossRef CAS PubMed.
- S. Choi, J. Park, W. Hyun, J. Kim, J. Kim, Y. B. Lee, C. Song, H. J. Hwang, J. H. Kim, T. Hyeon and D.-H. Kim, ACS Nano, 2015, 9, 6626 CrossRef CAS PubMed.
- D.-H. Kim, S. Wang, H. Keum, R. Ghaffari, Y.-S. Kim, H. Tao, B. Panilaitis, M. Li, Z. Kang, F. Omenetto, Y. Huang and J. A. Rogers, Small, 2012, 8, 3263 CrossRef CAS PubMed.
- L. Brosseau, K. A. Yonge, V. Welch, S. March, M. Judd, G. A. Wells and P. Tugwell, Cochrane Database Syst. Rev., 2003, 89, 694 Search PubMed.
- T. Ikeda, F. Tayefeh, D. I. Sessler, A. Kurz, O. Plattner, B. Petschnigg, H. W. Hopf and D. N. S. J. West, Am. J. Surg., 1998, 175, 33 CrossRef CAS PubMed.
- J. S. Petrofsky, D. Lawson, H. J. Suh, C. Rossi, K. Zapata, E. Broadwell and L. Littleton, Diabetes Technol. Ther., 2007, 9, 535 CrossRef PubMed.
- S.-W. Choi, Y. Zhang and Y. Xia, Angew. Chem., Int. Ed., 2010, 49, 7904 CrossRef CAS PubMed.
- J.-H. Park, J.-W. Lee, Y.-C. Kim and M. R. Prausnitz, Int. J. Pharmacol., 2008, 359, 94 CrossRef CAS PubMed.
- D. R. Cairns, R. P. Witte, D. K. Sparacin, S. M. Sachsman, D. C. Paine, G. P. Crawford and R. R. Newton, Appl. Phys. Lett., 2000, 76, 1425 CrossRef CAS.
- Y. H. Yoon, J. W. Song, D. Kim, J. Kim, J. K. Park, S. K. Oh and C. S. Han, Adv. Mater., 2007, 19, 4284 CrossRef CAS.
- D. Sui, Y. Huang, L. Huang, J. Liang, Y. Ma and Y. Chen, Small, 2011, 7, 3186 CrossRef CAS PubMed.
- S. Ji, W. He, K. Wang, Y. Ran and C. Ye, Small, 2014, 10, 4951 CrossRef CAS PubMed.
- C. Celle, C. Mayousse, E. Moreau, H. Basti, A. Carella and J.-P. Simonato, Nano Res., 2012, 5, 427 CrossRef CAS.
- A. Laforgue, J. Mater. Chem., 2010, 20, 8233 RSC.
- K. Kong, B. K. Deka, M. Kim, A. Oh, H. Kim, Y.-B. Park and H. W. Park, Compos. Sci. Technol., 2014, 100, 83 CrossRef CAS.
- X. Zhang, X. Yan, J. Chen and J. Zhao, Carbon, 2014, 69, 437 CrossRef CAS.
- R. Gupta, K. D. M. Rao, S. Kiruthika and G. U. Kulkarni, ACS Appl. Mater. Interfaces, 2016, 8, 12559 CAS.
- X. Chen, H. Lin, J. Deng, Y. Zhang, X. Sun, P. Chen, X. Fang, Z. Zhang, G. Guan and H. Peng, Adv. Mater., 2014, 26, 8126 CrossRef CAS PubMed.
- X. Chen, H. Lin, P. Chen, G. Guan, J. Deng and H. Peng, Adv. Mater., 2014, 26, 4444 CrossRef CAS PubMed.
- V. K. Thakur, G. Ding, J. Ma, P. S. Lee and X. Lu, Adv. Mater., 2012, 24, 4071 CrossRef CAS PubMed.
- Z. Zhang, K. Guo, Y. Li, X. Li, G. Guan, H. Li, Y. Luo, F. Zhao, Q. Zhang, B. Wei, Q. Pei and H. Peng, Nat. Photonics, 2015, 9, 233 CrossRef CAS.
- Z. Zhang, X. Chen, P. Chen, G. Guan, L. Qiu, H. Lin, Z. Yang, W. Bai, Y. Luo and H. Peng, Adv. Mater., 2014, 26, 466 CrossRef CAS PubMed.
- Z. Zhang, J. Deng, X. Li, Z. Yang, S. He, X. Chen, G. Guan, J. Ren and H. Peng, Adv. Mater., 2015, 27, 356 CrossRef CAS PubMed.
- E. Pop, D. Mann, Q. Wang, K. Goodson and H. Dai, Nano Lett., 2006, 6, 96 CrossRef CAS PubMed.
- R. C. Webb, A. P. Bonifas, A. Behnaz, Y. Zhang, K. J. Yu, H. Cheng, M. Shi, Z. Bian, Z. Liu, Y.-S. Kim, W.-H. Yeo, J. S. Park, J. Song, Y. Li, Y. Huang, A. M. Gorbach and J. A. Rogers, Nat. Mater., 2013, 12, 938 CrossRef CAS PubMed.
- A. Pucci, R. Bizzarri and G. Ruggeri, Soft Matter, 2011, 7, 3689 RSC.
- F. G. J. Oosterveld and J. J. Rasker, Semin. Arthritis Rheum., 1994, 24, 82 CrossRef CAS PubMed.
- D. I. Abramson, Y. Bell, S. Tuck, R. Mtchell and G. Chandrasekharappa, Am. J. Phys. Med., 1961, 40, 5 CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6tc04399b |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2017 |