Hierarchical CuCo2S4 nanoarrays for high-efficient and durable water oxidation electrocatalysis†
Lin
Yang
a,
Lisi
Xie
a,
Xiang
Ren
a,
Ziqiang
Wang
a,
Zhiang
Liu
b,
Gu
Du
c,
Abdullah M.
Asiri
 d,
Yadong
Yao
d,
Yadong
Yao
 e and
Xuping
Sun
e and
Xuping
Sun
 *a
*a
aCollege of Chemistry, Sichuan University, Chengdu 610064, Sichuan, China. E-mail: sunxp@scu.edu.cn; sunxp_scu@hotmail.com
bCollege of Chemistry and Chemical Engineering, Qufu Normal University, Qufu 273165, Shandong, China
cChengdu Institute of Geology and Mineral Resources, Chengdu 610081, Sichuan, China
dChemistry Department, Faculty of Science, King Abdulaziz University, Jeddah 21589, Saudi Arabia
eCollege of Materials Science and Engineering, Sichuan University, Chengdu 610064, Sichuan, China
First published on 27th November 2017
Abstract
It is highly attractive to design and develop earth-abundant electrocatalysts toward high-efficiency water oxidation electrocatalysis in alkaline media. In this communication, we report the in situ hydrothermal sulfidization preparation of a hierarchical CuCo2S4 nanoarray on copper foam (CuCo2S4/CF) from its CuCo2-hydroxide nanowire array precursor. When used as a 3D catalyst electrode for water oxidation, the as-prepared CuCo2S4/CF is superior in catalytic activity, demanding overpotentials of only 259 and 295 mV to achieve 60 and 100 mA cm−2 in 1.0 M KOH, respectively. Moreover, it also shows strong electrochemical durability with high turnover frequency values of 0.069 and 0.390 mol O2 s−1 at overpotentials of 300 and 400 mV, respectively.
The drastic depletion of fossil fuels and growing global environmental concerns call for an urgent demand for exploiting eco-friendly and renewable energy carriers.1,2 Clean and sustainable hydrogen is regarded as an ideal candidate to replace fossil fuels.3,4 Water electrolysis provides us an attractive way to produce pure hydrogen on a large scale.5,6 Involving a multi-electron transfer process, anodic water oxidation remains a bottleneck for water electrolysis, thus efficient water oxidation catalysts (WOCs) are required to accelerate the kinetics, for achieving high current densities at minimal overpotentials.7–9 Although Ru- and Ir-based oxides show the highest catalytic activity toward water oxidation, their low abundance and high cost greatly hinder their widespread commercial uses.10
Tremendous efforts have been devoted to developing earth abundant WOCs for more energy-efficient water electrolysis.11–22 Transition metal sulfides (TMSs) have been widely investigated for energy storage and catalysis, such as lithium ion batteries,23–25 supercapacitors,26–28 hydrodesulfurization,29–31 and electrochemical water splitting.32–40 For catalyzing water oxidation, bimetallic TMSs are more competitive than monometallic ones.35,41–43 Among them, big-cell sulphospinels can expose a large number of edge sites, with more rapid electron transfer pathways, smaller optical band gaps, and better redox reactions, offering great benefits to enhance electrochemical performances.38,44–46 Recent works demonstrated that nanoarray catalysts have obvious advantages of exposing more active sites and facilitating diffusion of electrolytes and evolved gas.6,11,13,47,48 Moreover, nanoarray catalysts with hierarchical feature would offer more active sites and have attracted increasing attention.48–51 Therefore, hierarchical structured transition metal sulphospinels hold great promise for electrochemical water oxidation.
Herein, we describe the fabrication of a hierarchical CuCo2S4 nanoarray on copper foam (CuCo2S4/CF) by hydrothermally sulfidizing its bimetallic hydroxide nanowire array precursor (see the ESI† for preparation details). The as-prepared CuCo2S4/CF exhibits superior activity for water oxidation, needing overpotentials of only 259 and 295 mV to achieve 60 and 100 mA cm−2 in 1.0 M KOH, respectively, outperforming monometallic cobalt sulphide on CF (Co–S/CF) and copper sulphide on CF (Cu–S/CF). Notably, this system also demonstrates strong long-term electrochemical durability with high turnover frequency (TOF) values of 0.069 and 0.390 mol O2 s−1 at overpotentials of 300 and 400 mV, respectively.
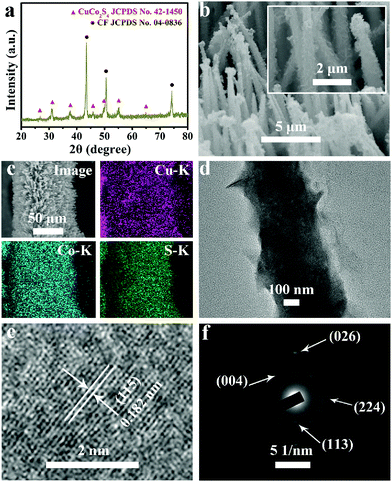
Fig. 1a presents the X-ray diffraction (XRD) patterns for CuCo2S4/CF. The peaks at 26.6°, 31.3°, 38.0°, 47.0°, 50.0°, 55.0°, and 64.4° can be indexed to the (022), (113), (004), (224), (115), (044), and (335) planes of the cubic CuCo2S4 phase (JCPDS No. 42–1450),52–54 respectively, and the three peaks at 43.3°, 50.4°, and 74.1° arise from the CF substrate (JCPDS No. 04–0836). Fig. S1a and S1b show the XRD patterns for Cu–S/CF and Co–S/CF, indexed to the monoclinic (JCPDS No. 83–1462) and hexagonal Cu2S (JCPDS No. 84–0206), and cubic Co3S4 phases (JCPDS No. 47–1738), respectively. The scanning electron microscopy (SEM) images of the hydroxide precursor indicate that it is a nanowire array (Fig. S2, ESI†). After a hydrothermal sulfidization process, the as-prepared CuCo2S4 still preserves the 1D morphology but with many ultrathin nanosheets on the surface, suggesting the formation of the hierarchical structure (Fig. 1b). The low-magnification SEM image in Fig. 1c indicates that the CuCo2S4 is well aligned on the CF substrate, and the corresponding energy-dispersive X-ray (EDX) elemental mapping images verify the uniform distribution of Cu, Co, and S elements. Cu–S/CF, Co–S/CF, and CuCo–S with varied Co2+/Cu2+ ratios were also prepared for comparison (Fig. S2, S3, and S4, ESI†). Fig. S5 (ESI†) shows the EDX spectrum of CuCo2S4/CF, further showing the existence of Cu, Co, and S elements with an atomic ratio close to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4. The high-resolution transmission electron microscopy (HRTEM) analysis (Fig. 1e) taken from CuCo2S4 (Fig. 1d) reveals lattice fringes with an interplanar distance of 0.182 nm indexed to the (115) plane of the cubic CuCo2S4 phase. The selected area electron diffraction (SAED) pattern shows discrete spots of the (113), (004), (224), and (026) planes of crystalline cubic CuCo2S4 (Fig. 1f).
4. The high-resolution transmission electron microscopy (HRTEM) analysis (Fig. 1e) taken from CuCo2S4 (Fig. 1d) reveals lattice fringes with an interplanar distance of 0.182 nm indexed to the (115) plane of the cubic CuCo2S4 phase. The selected area electron diffraction (SAED) pattern shows discrete spots of the (113), (004), (224), and (026) planes of crystalline cubic CuCo2S4 (Fig. 1f).
The X-ray photoelectron spectroscopy (XPS) survey spectrum for CuCo2S4/CF (Fig. 2a) confirms the presence of Cu, Co, and S elements. The binding energies (BEs) at 952.3 and 932.5 eV in the Cu 2p region (Fig. 2b) can be assigned to Cu 2p1/2 and Cu 2p3/2, respectively, indicating the presence of Cu+.53,54 The other two shakeup satellite peaks (identified as Sat.) at 954.0 and 934.5 eV, together with the peak at 942.5 eV, suggest the existence of Cu2+ arising from air exposure.52,55 In the Co 2p region (Fig. 2c), the BEs of Co 2p1/2 and Co 2p3/2 appear at 792.8 and 778.0 eV, respectively, with two shakeup satellites at 796.8 and 780.8 eV, indicating the coexistence of Co2+ and Co3+.53,54 The BE at 162.0 eV in the S 1s region (Fig. 2c) is assigned to the Cu–S and Co–S bonds, and low coordination of S2− at the surface.52,53
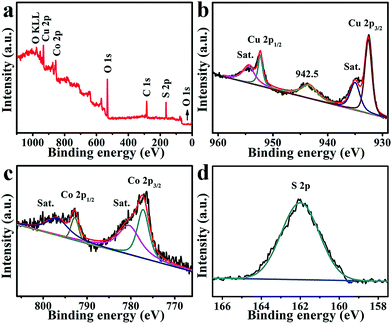 | ||
| Fig. 2 (a) XPS survey spectrum for CuCo2S4. XPS spectra for CuCo2S4 in the (b) Cu 2p, (c) Co 2p, and (d) S 2p regions. | ||
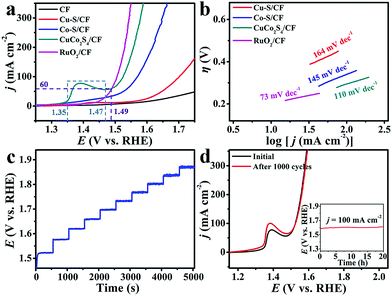
We collected the linear sweep voltammetry (LSV) curves to evaluate the electrocatalytic activity of CuCo2S4/CF (CuCo2S4 loading: 3.6 mg cm−2) toward water oxidation on a typical three-electrode setup with a scan rate of 5 mV s−1 in 1.0 M KOH. For comparison, Cu–S/CF, Co–S/CF, RuO2 (Fig. S6, see ESI† for preparation details) on CF (RuO2/CF, with the same mass loading), and bare CF were also investigated under the same conditions. Owing to the solution resistance, all experimental data were corrected with ohmic potential drop (iR) losses to reflect the intrinsic behavior of the catalysts, and all potentials were reported on a reversible hydrogen electrode (RHE) scale except specifically stated. Fig. 3a shows the LSV curves. As observed, RuO2/CF exhibits high activity for water oxidation with the need of a low overpotential of 276 mV to achieve 100 mA cm−2, whereas the bare CF shows poor catalytic activity. Cu–S/CF is also active for water oxidation, requiring overpotential of 472 mV to afford 100 mA cm−2. Although Co–S/CF shows better catalytic activity for water oxidation, it still needs an overpotential of 340 mV to drive the same current density. Impressively, our CuCo2S4/CF is superior in catalytic activity, demanding an overpotential of only 295 mV to deliver 100 mA cm−2, 177 and 45 mV less than those of monometallic Cu–S/CF and Co–S/CF, respectively. The oxidative feature at 1.35 and 1.47 V (as marked by the blue dash lines) preceding water oxidation is the oxidation of the CuCo2S4/CF catalyst.51 Additionally, it needs a much smaller overpotential of 259 mV to afford the geometric current density of 60 mA cm−2, comparing favorably to the behaviors of most reported non-noble metal WOCs at alkaline pH, like Al–CoP/NF (η50 mA cm−2 = 280 mV),56 MnCo2S4 NA/TM (η50 mA cm−2 = 325 mV),38 NiCo2S4 NA/CC (η100 mA cm−2 = 340 mV),34 NiSe2/Ti (η20 mA cm−2 = 295 mV),57 De-LNiFeP/rGO (η10 mA cm−2 = 258 mV),12etc., and a detailed comparison is listed in Table S1 (ESI†). Fig. 3b presents the Tafel plots of Cu–S/CF, Co–S/CF, CuCo2S4/CF, and RuO2/CF. The Tafel slope of 110 mV dec−1 for CuCo2S4/CF is much smaller than those for Cu–S/CF (164 mV dec−1) and Co–S/CF (145 mV dec−1), implying a more favorable catalytic kinetics on CuCo2S4/CF. We also examined the effect of the Co2+/Cu2+ ratio on the water oxidation activity, and overpotentials of 450, 416, 352, and 311 mV are needed to drive a geometric current density of 100 mA cm−2 for Cu3Co–S/CF, Cu2Co–S/CF, CuCo–S/CF, and CuCo3–S/CF, respectively (Fig. S7, ESI†).
Fig. 3c displays the multi-step chronopotentiometric curve for CuCo2S4/CF. The anodic current density increases from 40 to 400 mA cm−2 with an increment of 40 mA cm−2 per 500 s, and the potential immediately levels off at 1.52 V for the starting current value, remaining constant for the rest 500 s. Similar results are observed for other steps tested up to 400 mA cm−2, demonstrating the excellent mass transport properties, conductivity, and mechanical robustness of the CuCo2S4/CF electrode.13,58 Given that stability is also a critical criterion for the practical applications of catalysts, we thus probed the stability of our CuCo2S4/CF via continuous cyclic voltammetry scanning. The LSV curve shows negligible loss in current density after 1000 cycles compared with the initial one (Fig. 3d), suggesting its superior stability. An electrolysis measurement at a fixed current density of 100 mA cm−2 further demonstrates that this catalyst has strong long-term electrochemical durability, maintaining its catalytic activity for at least 20 h (Fig. 3d, inset). CuCo2S4/CF still retains its hierarchical feature after the stability test (Fig. S8, ESI†). The XRD pattern also shows characteristic peaks of cubic CuCo2S4 but with decreased intensities (Fig. S9, ESI†). A thin amorphous layer about 2–3 nm was formed on the CuCo2S4 surface (Fig. S10, ESI†). We also performed XPS analysis of the sample after oxidation electrolysis. In the Cu 2p region (Fig. S11a, ESI†), the BEs at 934.5 (2p1/2) and 954.6 eV (2p3/2), together with the peaks at 939.5 and 941.7 eV, suggest the existence of CuO.59 The BE at 962.8 eV indicates the presence of Cu(OH)2.60 In the Co 2p region (Fig. S11b, ESI†), the strong peak at 780.4 eV (2p3/2) can be assigned to CoO.61 The peaks at 782.3 (Sat.), 795.4 (2p1/2), and 796.8 eV arise from Co(OH)2.34,62 Another wide peak appearing at 787.0 eV implies the presence of CoOOH.63 The peak for S2− in the S 2p region can hardly be observed (Fig. S11c). In the O 1s region (Fig. S11d, ESI†), the BE at 529.3 eV and a weak peak at 531.3 eV suggest the existence of O2− and OH− species, respectively.64 These observations suggest the formation of an amorphous oxide/(oxy)hydroxide layer on the CuCo2S4 surface as the real active phase.34
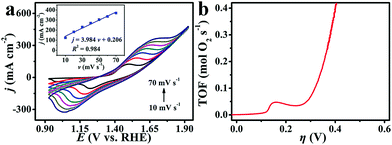
To estimate the electrochemically active surface area, we determined the electrochemical double-layer capacitance (CDL) at the solid/liquid interface for Cu–S/CF, Co–S/CF, CuCo2S4/CF from cyclic voltammograms (CVs).65,66 All CVs were collected in the region of 0.608–0.708 V (Fig. S9a–c, ESI†) to ensure that the current responses are only owing to the charging of the double layer. According to the formula: jc = vCDL (a plot of jc as a function of v yields a straight line with a slope equal to CDL), the CDL values for Cu–S/CF, Co–S/CF, and CuCo2S4/CF are 1.990, 2.390, and 3.300 mF cm−2, respectively (Fig. S9d, ESI†), implying CuCo2S4/CF has a much larger surface area and more exposed active sites.6
To calculate TOF, we applied electrochemistry to quantify the surface concentration of active sites (Fig. 4a).67 We assumed a one-electron oxidation process for the oxidation of Cu and Co metal centers in CuCo2S4.38 There exists a linear dependence between the oxidation peak current density for redox Cu and Co species and scan rates (Fig. 4a, inset). The TOF values for CuCo2S4/CF are calculated to be 0.069 and 0.390 mol O2 s−1 at overpotentials of 300 and 400 mV, respectively (Fig. 4b), much higher than those for reported WOCs like MnCo2S4 NA/TM (∼0.20 mol O2 s−1, η = 400 mV),38 Co3O4/N–PC (0.0015 mol O2 s−1, η = 300 mV),68 and NiCo2O4@Ni–Co–B/CC (0.019 mol O2 s−1, η = 300 mV).69
In summary, a hierarchical CuCo2S4 nanoarray has been developed for durable water oxidation electrolysis with the need of overpotential of 295 mV to afford a geometrical catalytic current density of 100 mA cm−2 in 1.0 M KOH. It also achieves high TOF of 0.069 and 0.390 mol O2 s−1 at overpotentials of 300 and 400 mV, respectively. This nanoarray may hold great promise as an attractive water-oxidizing catalyst material in water-splitting devices toward large-scale production of hydrogen fuels for applications.
This work was supported by the National Natural Science Foundation of China (No. 21575137).
Conflicts of interest
There are no conflicts to declare.References
- J. Chow, R. J. Kopp and P. R. Portney, Science, 2003, 302, 1528–1531 CrossRef PubMed.
- T. R. Cook, D. K. Dogutan, S. Y. Reece, Y. Surendranath, T. S. Teets and D. G. Nocera, Chem. Rev., 2010, 110, 6474–6502 CrossRef CAS PubMed.
- D. G. Nocera, Acc. Chem. Res., 2012, 45, 767–776 CrossRef CAS PubMed.
- M. S. Dresselhaus and I. L. Thomas, Nature, 2001, 414, 332–337 CrossRef CAS PubMed.
- W. Hong, M. Risch, K. A. Stoerzinger, A. Grimaud, J. Suntivich and Y. Shao-Horn, Energy Environ. Sci., 2015, 8, 1404–1427 CAS.
- W. Zhu, R. Zhang, F. Qu, A. M. Asiri and X. Sun, ChemCatChem, 2017, 9, 1721–1743 CrossRef CAS.
- Q. Yin, J. M. Tan, C. Besson, Y. V. Geletii, D. G. Musaev, A. E. Kuznetsov, Z. Luo, K. I. Hardcastle and C. L. Hill, Science, 2010, 328, 342–345 CrossRef CAS PubMed.
- J. Suntivich, K. J. May, H. A. Gasteiger, J. B. Goodenough and Y. Shao-Horn, Science, 2011, 334, 1383–1385 CrossRef CAS PubMed.
- J. Wang, X. Ma, F. Qu, A. M. Asiri and X. Sun, Inorg. Chem., 2017, 56, 1041–1044 CrossRef CAS PubMed.
- Y. Lee, J. Suntivich, K. J. May, E. E. Perry and Y. Shao-Horn, J. Phys. Chem. Lett., 2012, 3, 399–404 CrossRef CAS PubMed.
- X. Ji, S. Hao, F. Qu, J. Liu, G. Du, A. M. Asiri, L. Chen and X. Sun, Nanoscale, 2017, 9, 7714–7718 RSC.
- Y. Liu, H. Wang, D. Lin, C. Liu, P. C. Hsu, W. Liu, W. Chen and Y. Cui, Energy Environ. Sci., 2015, 8, 1719–1724 CAS.
- W. Lu, T. Liu, L. Xie, C. Tang, D. Liu, S. Hao, F. Qu, G. Du, Y. Ma, A. M. Asiri and X. Sun, Small, 2017, 13, 1700805 CrossRef PubMed.
- M. Gao, Y. Xu, J. Jian, Y. Zheng and S. Yu, J. Am. Chem. Soc., 2012, 134, 2930–2933 CrossRef CAS PubMed.
- Z. Zhao, H. Wu, H. He, X. Xu and Y. Jin, Adv. Funct. Mater., 2014, 24, 4698–4705 CrossRef CAS.
- H. Liang, F. Meng, M. Caban–Acevedo, L. Li, A. Forticaux, L. Xiu, Z. Wang and S. Jin, Nano Lett., 2015, 15, 1421–1427 CrossRef CAS PubMed.
- X. Lu, L. Gu, J. Wang, J. Wu, P. Liao and G. Li, Adv. Mater., 2017, 29, 1604437 CrossRef PubMed.
- B. Weng, F. Xu, C. Wang, W. Meng, C. R. Grice and Y. Yan, Energy Environ. Sci., 2017, 10, 121–128 CAS.
- S. Dou, C. Dong, Z. Hu, Y. Huang, J. Chen, L. Tao, D. Yan, D. Chen, S. Shen, S. Chou and S. Wang, Adv. Funct. Mater., 2017, 27, 1702546 CrossRef.
- M. B. Stevens, L. J. Enman, A. S. Batchellor, M. R. Cosby, A. E. Vise, C. D. M. Trang and S. W. Boettcher, Chem. Mater., 2017, 29, 120–140 CrossRef CAS.
- C. Hu, L. Zhang, Z. Zhao, J. Luo, J. Shi, Z. Huang and J. Gong, Adv. Mater., 2017, 29, 1701820 CrossRef PubMed.
- I. A. Moreno-Hernandez, C. A. MacFarland, C. G. Read, B. S. Brunschwig, K. M. Papadantonakis and N. Lewis, Energy Environ. Sci., 2017, 10, 2103–2108 CAS.
- L. Ji, Z. Lin, M. Alcoutlabi and X. Zhang, Energy Environ. Sci., 2011, 4, 2682–2699 CAS.
- X. Xu, W. Liu, Y. Kim and J. Cho, Nano Today, 2014, 9, 604–630 CrossRef CAS.
- Q. Chen, F. Lu, Y. Xia, H. Wang and X. Kuang, J. Mater. Chem. A, 2017, 5, 4075–4083 CAS.
- M. Yan, Y. Yao, J. Wen, L. Long, M. Kong, G. Zhang, X. Liao, G. Yin and Z. Huang, ACS Appl. Mater. Interfaces, 2016, 8, 24525–24535 CAS.
- Z. Xing, Q. Chu, X. Ren, C. Ge, A. H. Qusti, A. M. Asiri, A. O. Al-Youbi and X. Sun, J. Power Sources, 2014, 245, 463–467 CrossRef CAS.
- P. Zhang, B. Y. Guan, L. Yu and X. W. Lou, Angew. Chem., Int. Ed., 2017, 56, 7141–7145 CrossRef CAS PubMed.
- H. Li, J. Liu, J. Li, Y. Hu, W. Wang, D. Yuan, Y. Wang, T. Yang, L. Li, H. Sun, S. Ren, X. Zhu, Q. Guo, X. Wen, Y. Li and B. Shen, ACS Catal., 2017, 7, 4805–4816 CrossRef CAS.
- O. Y. Gutiérrez, S. Singh, E. Schachtl, J. Kim, E. J. Hein and J. A. Lercher, ACS Catal., 2014, 4, 1487–1499 CrossRef.
- W. Lai, Z. Chen, J. Zhu, L. Yang, J. Zheng, X. Yi and W. Fang, Nanoscale, 2016, 8, 3823–3833 RSC.
- X. Ren, W. Wang, R. Ge, S. Hao, F. Qu, G. Du, A. M. Asiri, Q. Wei, L. Chen and X. Sun, Chem. Commun., 2017, 53, 9000–9003 RSC.
- Q. Li, Z. Xing, D. Wang, X. Sun and X. Yang, ACS Catal., 2016, 6, 2797–2801 CrossRef CAS.
- D. Liu, Q. Lu, Y. Luo, X. Sun and A. M. Asiri, Nanoscale, 2015, 7, 15122–15126 RSC.
- N. Yang, C. Tang, K. Wang, G. Du, A. M. Asiri and X. Sun, Nano Res., 2016, 9, 3346–3354 CrossRef CAS.
- Z. Xing, X. Yang, A. M. Asiri and X. Sun, ACS Appl. Mater. Interfaces, 2016, 8, 14521–14526 CAS.
- W. Wang, L. Yang, F. Qu, Z. Liu, G. Du, A. M. Asiri, Y. Yao, L. Chen and X. Sun, J. Mater. Chem. A, 2017, 5, 16585–16589 CAS.
- X. Zhang, C. Si, X. Guo, R. Kong and F. Qu, J. Mater. Chem. A, 2017, 5, 17211–17215 CAS.
- P. Chen, T. Zhou, M. Zhang, Y. Tong, C. Zhong, N. Zhang, L. Zhang, C. Wu and Y. Xie, Adv. Mater., 2017, 29, 1701584 CrossRef PubMed.
- J. Liu, J. Wang, B. Zhang, Y. Ruan, L. Lv, X. Ji, K. Xu, L. Miao and J. Jiang, ACS Appl. Mater. Interfaces, 2017, 9, 15364–15372 CAS.
- W. Fang, D. Liu, Q. Lu, X. Sun and A. M. Asiri, Electrochem. Commun., 2016, 63, 60–64 CrossRef CAS.
- N. Cheng, Q. Liu, A. M. Asiri, W. Xing and X. Sun, J. Mater. Chem. A, 2015, 3, 23207–23212 CAS.
- T. Liu, X. Sun, A. M. Asiri and Y. He, Int. J. Hydrogen Energy, 2016, 41, 7264–7269 CrossRef CAS.
- L. Mei, T. Yang, C. Xu, M. Zhang, L. Chen, Q. Li and T. Wang, Nano Energy, 2014, 3, 36–45 CrossRef CAS.
- L. Shen, J. Wang, G. Xu, H. Li, H. Dou and X. Zhang, Adv. Energy Mater., 2015, 5, 1400977 CrossRef.
- A. M. Elshahawy, X. Li, H. Zhang, Y. Hu, K. H. Ho, C. Guan and J. Wang, J. Mater. Chem. A, 2017, 5, 7494–7506 CAS.
- Z. Pu, Q. Liu, P. Jiang, A. M. Asiri, A. Y. Obaid and X. Sun, Chem. Mater., 2014, 26, 4326–4329 CrossRef CAS.
- L. Xie, C. Tang, K. Wang, G. Du, A. M. Asiri and X. Sun, Small, 2017, 13, 1602755 CrossRef PubMed.
- Z. Li, M. Shao, L. Zhou, R. Zhang, C. Zhang, J. Han, M. Wei, D. G. Evans and X. Duan, Nano Energy, 2016, 20, 294–304 CrossRef CAS.
- Q. Yang, T. Li, Z. Lu, X. Sun and J. Liu, Nanoscale, 2014, 6, 11789–11794 RSC.
- X. Liu, Z. Chang, L. Luo, T. Xu, X. Lei, J. Liu and X. Sun, Chem. Mater., 2014, 26, 1889–1895 CrossRef CAS.
- J. Tang, Y. Ge, J. Shen and M. Ye, Chem. Commun., 2016, 52, 1509–1512 RSC.
- S. E. Moosavifard, S. Fani and M. Rahmanian, Chem. Commun., 2016, 52, 4517–4520 RSC.
- B. Li, F. Yuan, G. He, X. Han, X. Wang, J. Qin, Z. Guo, X. Lu, Q. Wang, I. P. Parkin and C. Wu, Adv. Funct. Mater., 2017, 27, 1606218 CrossRef.
- M. Zhang, K. P. Annamalai, L. Liu, T. Chen, J. Gao and Y. Tao, RSC Adv., 2017, 7, 20724–20731 RSC.
- R. Zhang, C. Tang, R. Kong, G. Du, A. M. Asiri, L. Chen and X. Sun, Nanoscale, 2017, 9, 4793–4800 RSC.
- Z. Pu, Y. Luo, A. M. Asiri and X. Sun, ACS Appl. Mater. Interfaces, 2016, 8, 4718–4723 CAS.
- F. Xie, H. Wu, J. Mou, D. Lin, C. Xu, C. Wu and X. Sun, J. Catal., 2017, 356, 165–172 CrossRef CAS.
- L. Cui, X. Cao, X. Sun, W. Yang and J. Liu, ChemCatChem DOI:10.1002/cctc.201701317.
- J. Yu and J. Ran, Energy Environ. Sci., 2011, 4, 1364–1371 CAS.
- T. J. Chuang, C. R. Bidle and D. W. Rice, Surf. Sci., 1976, 59, 413–429 CrossRef CAS.
- C. Mondal, M. Ganguly, P. K. Manna, S. M. Yusuf and T. Pal, Langmuir, 2013, 29, 9179–9187 CrossRef CAS PubMed.
- J. Yang and T. Sasaki, Chem. Mater., 2008, 20, 2049–2056 CrossRef CAS.
- R. Zhang, Z. Wang, S. Hao, R. Ge, X. Ren, F. Qu, G. Du, A. M. Asiri, B. Zheng and X. Sun, ACS Sustainable Chem. Eng., 2017, 5, 8518–8522 CrossRef CAS.
- C. C. L. McCrory, S. Jung, J. C. Peters and T. F. Jaramillo, J. Am. Chem. Soc., 2013, 135, 16977–16987 CrossRef CAS PubMed.
- J. D. Benck, Z. Chen, L. Y. Kuritzky, A. J. Forman and T. F. Jaramillo, ACS Catal., 2012, 2, 1916–1923 CrossRef CAS.
- M. Xie, L. Yang, Y. Ji, Z. Wang, X. Ren, Z. Liu, A. M. Asiri, X. Xiong and X. Sun, Nanoscale, 2017, 9, 16612–16615 RSC.
- Y. Hou, J. Li, Z. Wen, S. Cui, C. Yuan and J. Chen, Nano Energy, 2015, 12, 1–8 CrossRef CAS.
- X. Ji, X. Ren, S. Hao, F. Xie, F. Qu, G. Du, A. M. Asiri and X. Sun, Inorg. Chem. Front., 2017, 4, 1546–1550 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental section and supplementary figures. See DOI: 10.1039/c7cc07259g |
| This journal is © The Royal Society of Chemistry 2018 |