A chiral scandium-complex-catalyzed asymmetric inverse-electron-demand oxa-Diels–Alder reaction of o-quinone methides with fulvenes†
Jianlin
Zhang
 ,
Lili
Lin
,
Lili
Lin
 *,
Changqiang
He
*,
Changqiang
He
 ,
Qian
Xiong
,
Qian
Xiong
 ,
Xiaohua
Liu
,
Xiaohua
Liu
 and
Xiaoming
Feng
and
Xiaoming
Feng
 *
*
Key Laboratory of Green Chemistry & Technology, Ministry of Education, College of Chemistry, Sichuan University, Chengdu 610064, People's Republic of China. E-mail: lililin@scu.edu.cn; xmfeng@scu.edu.cn; Fax: +86 28 85418249
First published on 6th December 2017
Abstract
A highly efficient asymmetric inverse-electron-demand oxa-Diels–Alder reaction of o-quinone methides with fulvenes has been realized using a chiral N,N′-dioxide/Sc(III) complex as the catalyst. The corresponding optically active chromane derivatives were obtained in high yields with excellent dr and ee values (up to 99% yield, >19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr and 94% ee).
1 dr and 94% ee).
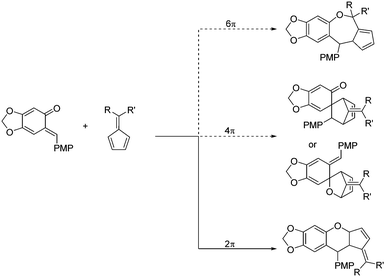
ortho-Quinone methides (o-QMs) are highly reactive intermediates that are used in the synthesis of natural products and bioactive compounds, thus attracting the interest of chemists around the world.1 Because of their inherent highly electrophilic character as highly polarized 1-oxabutadienes, they react readily with a broad range of nucleophiles. Their thrust for aromatization adds to the driving force for such conjugate additions.2 Moreover, the annulation reaction of o-QMs is a hot topic and various reaction partners with o-QMs or other precursors have been discovered, due to the complexity and diversity of the products.3 Among these kinds of reactions, the oxa-Diels–Alder reaction showed significant advantages in providing products containing chromane motifs.4 In 2008, Lectka's group reported the pioneering enantioselective cycloadditions of o-QM with silylketene acetals to give 3,4-disubstituted 3,4-dihydrocoumarins in good yields by using a chiral cinchona alkaloid-derived ammonium fluoride.5 Since then, other kinds of dienophiles, such as ketenes,6 styrenes,7 enamides,8 3-methyl-2-vinylindoles,9 azolactones10 and others,11 were used to realize the enantioselective [4+2] reaction of o-QMs. In spite of these elegant advances, there are no reports on the enantioselective oxa-Diels–Alder reaction between o-QMs and pentafulvenes. The difficulty lies not only in the highly unstable o-QMs, but also in various side reactions on the fulvenes. Fulvenes are a unique type of polar conjugation substance.12 They can be employed as 2π,13 4π,14 or 6π15 reactants, which could participate in different kinds of cycloaddition reactions (Scheme 1). Although this cycloaddition is a challenge, the resulting chromane motifs are present in a large number of natural products and bioactive agents with a wide range of biological activities, including antioxidant, antifungal, antiviral, cytotoxic, and anti-inflammatory activities.16 Therefore, developing an efficient catalytic system to realize the enantioselective oxa-Diels–Alder reaction of o-QMs with fulvenes is meaningful.
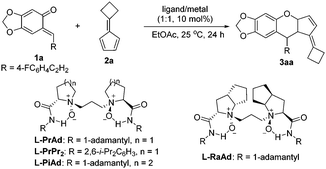
Chiral N,N′-dioxide–metal complexes as privileged catalysts have been successfully applied in a series of asymmetric reactions by our group.17 Accordingly, we assumed that chiral N,N′-dioxide/Lewis acid might realize the reaction. Herein, we documented the asymmetric inverse-electron-demand oxa-Diels–Alder reaction of o-QMs with pentafulvenes using the N,N′-dioxide–metal complex as the catalyst.
Initially, the cycloaddition of o-QM 1a and fulvene 2a was used as the model reaction to optimize the reaction conditions. At first, a series of metal salts coordinating with N,N′-dioxide L-PrAd were tested. Unfortunately, the system was complex in the presence of Cu(OTf)2 (Table 1, entry 1). The complex of Y(OTf)3 could afford modest yield but lower enantioselectivity (65% yield, 11% ee; Table 1, entry 2). When Sc(OTf)3 was selected as the central metal, a higher enantioselectivity was obtained (62% yield, 54% ee, Table 1, entry 3). Then, other chiral N,N′-dioxide ligands were examined. It was found that a ligand with an aromatic amide subunit could sharply improve the yield, but with a decreased enantioselectivity (94% yield, 49% ee; Table 1, entry 4). Further investigating the backbone of the ligands, L-proline-derived L-PrAd was superior to L-pipecolic acid-derived L-PiAd and L-ramipril-derived L-RaAd in enantioselectivity (Table 1, entry 3 vs. entries 5 and 6). When the reaction temperature was decreased to 0 °C, the enantioselectivity was improved significantly (60% yield, 86% ee; Table 1, entry 7). In the process of screening, we found that a slightly higher amount of the ligand was beneficial for not only the enantioselectivity (70% yield, 92% ee; Table 1, entry 8), but also the reproducibility and stability of data. Furthermore, upon changing the proportion of 1a and 2a from 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.1 to 1.5
1.1 to 1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and prolonging the reaction time to 48 h, the yield improved noticeably to 98% with retention of the enantioselectivity (Table 1, entry 9). It is noteworthy that only one diastereomer was observed in these cases. Therefore, the optimal conditions were established: L-PrAd/Sc(OTf)3 as the catalyst in EtOAc at 0 °C for 48 hours.
1 and prolonging the reaction time to 48 h, the yield improved noticeably to 98% with retention of the enantioselectivity (Table 1, entry 9). It is noteworthy that only one diastereomer was observed in these cases. Therefore, the optimal conditions were established: L-PrAd/Sc(OTf)3 as the catalyst in EtOAc at 0 °C for 48 hours.
| Entry | Metal salt | Ligand | Yieldb (%) | eec (%) |
|---|---|---|---|---|
a Unless otherwise noted, all reactions were performed with ligand/metal (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 10 mol%), 1a (0.1 mmol), and 2a (0.11 mmol) in EtOAc (0.5 mL) at 25 °C for 24 h, dr > 19 1, 10 mol%), 1a (0.1 mmol), and 2a (0.11 mmol) in EtOAc (0.5 mL) at 25 °C for 24 h, dr > 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.
b Isolated yield.
c Determined by chiral HPLC analysis.
d Not detected.
e 0 °C for 24 h.
f Ligand/metal (1.05 1.
b Isolated yield.
c Determined by chiral HPLC analysis.
d Not detected.
e 0 °C for 24 h.
f Ligand/metal (1.05![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1).
g
1a (0.15 mmol), 2a (0.1 mmol) at 0 °C for 48 h. 1).
g
1a (0.15 mmol), 2a (0.1 mmol) at 0 °C for 48 h.
|
||||
| 1 | Cu(OTf)2 | L-PrAd | Complex | N.D.d |
| 2 | Y(OTf)3 | L-PrAd | 65 | 11 |
| 3 | Sc(OTf)3 | L-PrAd | 62 | 54 |
| 4 | Sc(OTf)3 | L-PrPr2 | 94 | 49 |
| 5 | Sc(OTf)3 | L-PiAd | 73 | 39 |
| 6 | Sc(OTf)3 | L-RaAd | 77 | 40 |
| 7e | Sc(OTf)3 | L-PrAd | 60 | 86 |
| 8e,f | Sc(OTf)3 | L-PrAd | 70 | 92 |
| 9e,f,g | Sc(OTf)3 | L-PrAd | 98 | 91 |
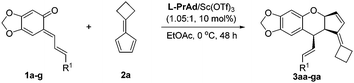
With the optimized reaction conditions in hand, the substrate generality of the reaction was next surveyed. First, a range of o-QMs was examined. As shown in Table 2, o-QMs bearing electron-withdrawing or electron-donating substituents react with 2a in excellent yields, diastereoselectivities and enantioselectivities regardless of the position of the substituent (Table 2, entries 1–6). Moreover, the heteroaryl-substituted o-QM 1g also worked well in this transformation, leading to the corresponding product 3ga in 97% yield and 90% ee (Table 2, entry 7).
| Entry | R1 | Yieldb (%) | eec (%) |
|---|---|---|---|
a All reactions were performed with 1 (0.15 mmol), L-PrAd/Sc(OTf)3 (1.05![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; 10 mol%), and 2a (0.1 mmol) in EtOAc (0.5 mL) at 0 °C for 48 h.
b Isolated yield.
c Determined by chiral HPLC analysis. The dr > 19 1; 10 mol%), and 2a (0.1 mmol) in EtOAc (0.5 mL) at 0 °C for 48 h.
b Isolated yield.
c Determined by chiral HPLC analysis. The dr > 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 was determined by NMR. 1 was determined by NMR.
|
|||
| 1 | 4-FC6H4 (1a) | 98/3aa | 91 |
| 2 | C6H5 (1b) | 97/3ba | 91 |
| 3 | 4-MeOC6H4 (1c) | 95/3ca | 91 |
| 4 | 4-MeC6H4 (1d) | 95/3da | 91 |
| 5 | 3-MeC6H4 (1e) | 98/3ea | 91 |
| 6 | 2-MeC6H4 (1f) | 96/3fa | 95 |
| 7 | 3-Thienyl (1g) | 97/3ga | 90 |
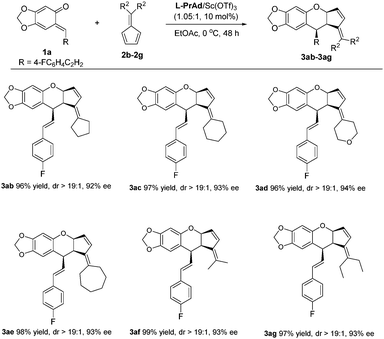
Next, we turned our attention to the scope of fulvenes. First, symmetrical fulvenes were explored. As shown in Scheme 2, fulvenes bearing five to seven-membered rings all transformed smoothly, delivering the corresponding products 3ab–3ae in 96–98% yields with 92–94% ee. It was worth mentioning that fulvene 2d derived from tetrahydro-4H-pyran-4-one was also suitable, affording 3ad in 96% yield and 94% ee. Additionally, the acyclic fulvenes 2f and 2g were also tolerated, giving the products 3af–3ag in excellent yields and ee values (97–99% yields, 93% ee).
Encouraged by the results obtained with symmetrical fulvenes, the scope was extended to unsymmetrical fulvenes. Because of the conjugation effect of the aryl ring with a cyclopentadiene unit, the activities of compounds 5 were not as high as those of compounds 2. Therefore, for the purpose of improving the yield, the temperature of this reaction was increased to 25 °C after stirring at 0 °C for 24 hours. As shown in Table 3, aromatic fulvenes bearing different electronic properties on the aryl rings all worked well and provided the corresponding products in high yields (73–94%), modest ratios of E/Z (1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1–4
1–4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), and good to excellent enantioselectivities (83–96% ee; Table 3, entries 1–6). Remarkably, substrates 5g–5j bearing a heteroaromatic or fused ring were also good reaction partners, affording the corresponding products in 65–92% yields, 1
1), and good to excellent enantioselectivities (83–96% ee; Table 3, entries 1–6). Remarkably, substrates 5g–5j bearing a heteroaromatic or fused ring were also good reaction partners, affording the corresponding products in 65–92% yields, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1–2.3
1–2.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 E/Z ratios and 90–96% ee (Table 3, entries 7–10). The aliphatic cyclohexyl group was also tolerated, leading to 74% yield, 4
1 E/Z ratios and 90–96% ee (Table 3, entries 7–10). The aliphatic cyclohexyl group was also tolerated, leading to 74% yield, 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
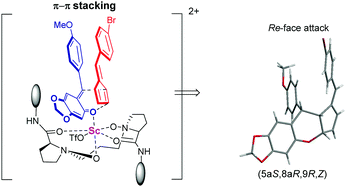
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 E/Z ratio and 92/79% ee (Table 3, entry 11). From Table 3, we could find that the substrates containing flexible aliphatic cyclohexyl groups have better ratios of E/Z than those containing rigid plane aromatic rings. This phenomenon suggested that the π–π interaction between o-QMs and pentafulvenes might account for the low ratio of E/Z in substrates containing aryl rings. The absolute configuration of the product 6d was determined to be (5aS, 8aR, 9R, Z) by X-ray single crystallographic analysis.18
1 E/Z ratio and 92/79% ee (Table 3, entry 11). From Table 3, we could find that the substrates containing flexible aliphatic cyclohexyl groups have better ratios of E/Z than those containing rigid plane aromatic rings. This phenomenon suggested that the π–π interaction between o-QMs and pentafulvenes might account for the low ratio of E/Z in substrates containing aryl rings. The absolute configuration of the product 6d was determined to be (5aS, 8aR, 9R, Z) by X-ray single crystallographic analysis.18
| Entry | R3 | Yieldb (%) | E/Z ratioc | eed (%) |
|---|---|---|---|---|
a All reactions were performed with 4 (0.15 mmol), L-PrAd/Sc(OTf)3 (1.05![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; 10 mol%), and 5 (0.1 mmol) in EtOAc (0.5 mL) at 0 °C for 24 h, then at 25 °C for 24 h.
b Isolated yield.
c Determined by NMR, dr > 19 1; 10 mol%), and 5 (0.1 mmol) in EtOAc (0.5 mL) at 0 °C for 24 h, then at 25 °C for 24 h.
b Isolated yield.
c Determined by NMR, dr > 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.
d Determined by chiral supercritical fluid chromatography (SFC) analysis. PMP = 4-MeOC6H4. 1.
d Determined by chiral supercritical fluid chromatography (SFC) analysis. PMP = 4-MeOC6H4.
|
||||
| 1 | Ph (5a) | 85/6a | 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
90/86 |
| 2 | 4-FC6H4 (5b) | 73/6b | 1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
90/90 |
| 3 | 4-ClC6H4 (5c) | 91/6c | 1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
95/93 |
| 4 | 4-BrC6H4 (5d) | 94/6d | 2.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
96/90 |
| 5 | 4-MeC6H4 (5e) | 94/6e | 2.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
92/89 |
| 6 | 2-MeC6H4 (5f) | 83/6f | 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
92/83 |
| 7 | 1-Nap (5g) | 71/6g | 2.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
96/90 |
| 8 | 2-Nap (5h) | 92/6h | 1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
93/91 |
| 9 | 3-Thienyl (5i) | 55/6i | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
93/93 |
| 10 |
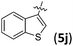
|
90/6j | 1.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
94/92 |
| 11 | Cy (5k) | 74/6k | 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
92/79 |
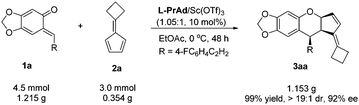
To show the synthetic utility of the current method, a gram-scale synthesis of 3aa was carried out. Under the optimized reaction conditions, o-QM 1a (1.215 g, 4.5 mmol) and fulvene 2a (0.354 g, 3.0 mmol) reacted smoothly, giving 1.153 g (99% yield) of the product 3aa in 92% ee (Scheme 3).
Based on the determination of the absolute configuration of products and our previous work,17 a possible transition-state model was proposed to illustrate the origin of the stereoselectivity (Scheme 4). Firstly, the N-oxides and amide oxygens of L-PrAd coordinated to Sc(III) to form a six-membered chelating ring. The carbonyl group of o-QMs coordinated to the scandium center, then the fulvene prefers to attack it from the Re-face since the Si-face is shielded by the amide moiety, leading to the formation of the product 6d with the (5aS, 8aR, 9R, Z)-configuration. In general, due to the steric hindrance of substituents embedded in unsymmetrical fulvenes, E-products were obtained easily. But in this system, although the E-configuration was the major product, we still got some Z-products. This phenomenon may be caused by the π–π interaction between o-QMs and pentafulvenes. This noncovalent interaction made some difference in stabilizing the transition state, affording Z-products.
In summary, we have developed a chiral N,N′-dioxide L-PrAd-Sc(OTf)3 complex system for the cycloaddition of o-QMs with fulvenes. A series of chromane derivatives were obtained in excellent yields and good to excellent ee values (up to 99% yield and 95% ee). Furthermore, a possible transition state model was proposed to explain the origin of the stereo induction. More endeavours to understand the enantiocontrol of the N,N′-dioxide–metal complex system are in progress.
We appreciate the National Natural Science Foundation of China (No. 21432006 and 21572136).
Conflicts of interest
There are no conflicts to declare.Notes and references
- (a) R. W. Van De Water and T. R. R. Pettus, Tetrahedron, 2002, 58, 5367 CrossRef CAS; (b) H. Amouri and J. Le Bras, Acc. Chem. Res., 2002, 35, 501 CrossRef CAS PubMed; (c) S. B. Ferreira, F. d. C. da Silva, A. C. Pinto, D. T. G. Gonzaga and V. F. Ferreira, J. Heterocycl. Chem., 2009, 46, 1080 CrossRef CAS; (d) Quinone Methides, ed. S. E. Rokita, Wiley, New York, 2009 Search PubMed; (e) T. P. Pathak and M. S. Sigman, J. Org. Chem., 2011, 76, 9210 CrossRef CAS PubMed; (f) N. J. Willis and C. D. Bray, Chem. – Eur. J., 2012, 18, 9160 CrossRef CAS PubMed; (g) W.-J. Bai, J. G. David, Z. G. Feng, M. G. Weaver, K. L. Wu and T. R. R. Pettus, Acc. Chem. Res., 2014, 47, 3655 CrossRef CAS PubMed; (h) Z. Wang and J. Sun, Synthesis, 2015, 3629 Search PubMed.
- (a) Y. Luan and S. E. Schaus, J. Am. Chem. Soc., 2012, 134, 19965 CrossRef CAS PubMed; (b) Y. Huang and T. Hayashi, J. Am. Chem. Soc., 2015, 137, 7556 CrossRef CAS PubMed; (c) J. F. Zheng, L. L. Lin, L. Dai, X. Yuan, X. H. Liu and X. M. Feng, Chem. – Eur. J., 2016, 22, 18254 CrossRef CAS PubMed.
- (a) M.-W. Chen, L.-L. Cao, Z.-S. Ye, G.-F. Jiang and Y.-G. Zhou, Chem. Commun., 2013, 49, 1660 RSC; (b) J. Izquierdo, A. Qrue and K. A. Scheidt, J. Am. Chem. Soc., 2013, 135, 10634 CrossRef CAS PubMed; (c) S. Saha, S. K. Alamsetti and C. Schneider, Chem. Commun., 2015, 51, 1461 RSC; (d) L. Caruana, M. Mondatori, V. Corti, S. Morales, A. Mazzanti, M. Fochi and L. Bernardi, Chem. – Eur. J., 2015, 21, 6037 CrossRef CAS PubMed; (e) Z. Wang, F. Ai, Z. Wang, W. Zhao, G. Zhu, Z. Lin and J. Sun, J. Am. Chem. Soc., 2015, 137, 383 CrossRef CAS PubMed; (f) W. Zhao, Z. Wang, B. Chu and J. Sun, Angew. Chem., Int. Ed., 2015, 54, 1910 CrossRef CAS PubMed; (g) Y. Zhu, L. Zhang and S. Luo, J. Am. Chem. Soc., 2016, 138, 3978 CrossRef CAS PubMed; (h) Z. Lai, Z. Wang and J. Sun, Org. Lett., 2015, 17, 6058 CrossRef CAS PubMed; (i) Q.-Q. Yang and W.-J. Xiao, Eur. J. Org. Chem., 2017, 233 CrossRef CAS; (j) X.-L. Lian, A. Adili, B. Liu, Z.-L. Tao and Z.-Y. Han, Org. Biomol. Chem., 2017, 15, 3670 RSC; (k) X.-L. Jiang, S.-J. Liu, Y.-Q. Gu, G.-J. Mei and F. Shi, Adv. Synth. Catal., 2017, 359, 3341 CrossRef CAS.
- (a) X. Jiang and R. Wang, Chem. Rev., 2013, 113, 5515 CrossRef CAS PubMed; (b) K. A. Jørgensen, Eur. J. Org. Chem., 2004, 2093 CrossRef; (c) H. Pellissier, Tetrahedron, 2009, 65, 2839 CrossRef CAS.
- E. Alden-Danforth, M. T. Scerba and T. Lectka, Org. Lett., 2008, 10, 4951 CrossRef CAS PubMed.
- (a) H. Lv, L. You and S. Ye, Adv. Synth. Catal., 2009, 351, 2822 CrossRef CAS; (b) H. Lv, W.-Q. Jia, L.-H. Sun and S. Ye, Angew. Chem., Int. Ed., 2013, 52, 8607 CrossRef CAS PubMed.
- (a) C.-C. Hsiao, S. Raja, H.-H. Liao, I. Atodiresei and M. Rueping, Angew. Chem., Int. Ed., 2015, 54, 5762 CrossRef CAS PubMed; (b) Y. F. Wong, Z. Wang, W. Hong and J. Sun, Tetrahedron, 2016, 72, 2748 CrossRef CAS.
- (a) S. Saha and C. Schneider, Chem. – Eur. J., 2015, 21, 2348 CrossRef CAS PubMed; (b) S. Saha and C. Schneider, Org. Lett., 2015, 17, 648 CrossRef CAS PubMed.
- J.-J. Zhao, S.-B. Sun, S.-H. He, Q. Wu and F. Shi, Angew. Chem., Int. Ed., 2015, 54, 5460 CrossRef CAS PubMed.
- H. P. Hu, Y. B. Liu, J. Guo, L. L. Lin, Y. L. Xu, X. H. Liu and X. M. Feng, Chem. Commun., 2015, 51, 3835 RSC.
- (a) A. Adili, Z. L. Tao, D. F. Chen and Z. Y. Han, Org. Biomol. Chem., 2015, 13, 2247 RSC; (b) S. K. Alamsetti, M. Spanka and C. Schneider, Angew. Chem., Int. Ed., 2016, 55, 2392 CrossRef CAS PubMed; (c) V. Nair, C. N. Jayan, K. V. Radhakrishnan, G. Anilkumar and N. P. Rath, Tetrahedron, 2001, 57, 5807 CrossRef CAS.
- P. Preethalayam, K. S. Krishnan, S. Thulasi, S. S. Chand, J. Joseph, V. Nair, F. Jaroschik and K. V. Radhakrishnan, Chem. Rev., 2017, 117, 3930 CrossRef CAS PubMed.
- Selected examples: (a) V. Nair, S. Kumar and P. G. Williard, Tetrahedron Lett., 1995, 36, 1605 CrossRef CAS; (b) B.-C. Hong, J.-L. Wu, A. K. Gupta, M. S. Hallur and J.-H. Liao, Org. Lett., 2004, 6, 3453 CrossRef CAS PubMed; (c) H. F. Zheng, C. R. Xu, Y. Wang, T. F. Kang, X. H. Liu, L. L. Lin and X. M. Feng, Chem. Commun., 2017, 53, 6585 RSC.
- Selected examples: (a) D. G. Lonergan and G. Deslongchamps, Tetrahedron, 1998, 54, 14041 CrossRef CAS; (b) G. Nzabamwita, B. Kolani and B. Jousseaume, Tetrahedron Lett., 1989, 30, 2207 CrossRef CAS; (c) S. Muthusamy, S. A. Babu and C. Gunanathan, Tetrahedron Lett., 2002, 43, 5981 CrossRef CAS.
- Selected examples: (a) B.-C. Hong, A. K. Gupta, M.-F. Wu, J.-H. Liao and G.-H. Lee, Org. Lett., 2003, 5, 1689 CrossRef CAS PubMed; (b) M. Potowski, J. O. Bauer, C. Strohmann, A. P. Antonchick and H. Waldmann, Angew. Chem., Int. Ed., 2012, 51, 9512 CrossRef CAS PubMed; (c) Z.-L. He, H.-L. Teng and C.-J. Wang, Angew. Chem., Int. Ed., 2013, 52, 2934 CrossRef CAS PubMed.
- (a) Chemistry of Heterocyclic Compounds: Chromans and Tocopherols, ed. G. P. Ellis and I. M. Lockhart, Wiley-Interscience, New York, 1981, vol. 36 Search PubMed; (b) H. C. Shen, Tetrahedron, 2009, 65, 3931 CrossRef CAS; (c) H. Makabe, Heterocycles, 2013, 87, 2225 CrossRef CAS; (d) S. Khadem and R. Marles, Molecules, 2012, 17, 191 CrossRef CAS PubMed.
- (a) X. H. Liu, L. L. Lin and X. M. Feng, Acc. Chem. Res., 2011, 44, 574 CrossRef CAS PubMed; (b) X. H. Liu, L. L. Lin and X. M. Feng, Org. Chem. Front., 2014, 1, 298 RSC; (c) X. H. Liu, H. F. Zheng, Y. Xia, L. L. Lin and X. M. Feng, Acc. Chem. Res., 2017, 50, 2621 CrossRef CAS PubMed.
- CCDC 1569433 (6d)†.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 1569433. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c7cc08124c |
| This journal is © The Royal Society of Chemistry 2018 |