Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Conformational control of nonplanar free base porphyrins: towards bifunctional catalysts of tunable basicity†
M.
Roucan
 a,
M.
Kielmann
a,
M.
Kielmann
 a,
S. J.
Connon
a,
S. J.
Connon
 *b,
S. S. R.
Bernhard
*b,
S. S. R.
Bernhard
 a and
M. O.
Senge
a and
M. O.
Senge
 *a
*a
aSchool of Chemistry, SFI Tetrapyrrole Laboratory, Trinity College Dublin, The University of Dublin, Trinity Biomedical Sciences Institute, 152-160 Pearse Street, Dublin 2, Ireland. E-mail: sengem@tcd.ie
bSchool of Chemistry, Trinity College Dublin, The University of Dublin, Trinity Biomedical Sciences Institute, 152-160 Pearse Street, Dublin 2, Ireland
First published on 11th December 2017
Abstract
For the first time, free base and N-methylated porphyrins have been utilized as bifunctional organocatalysts in Michael additions and it was found that distortion of the macrocycle is a vital prerequisite for their catalytic activity. Conformational design has been used to tailor the properties of nonplanar porphyrins with regards to availability of the N–H units for hydrogen bonding (distortion-dependent hydrogen bonding) and the basicity of the heterocyclic groups. NMR spectroscopic- and catalyst screening studies provided insight into the likely mode of catalyst action. This unprecedented use of free base and N-substituted porphyrins as organocatalysts opens a new functional role for porphyrins.
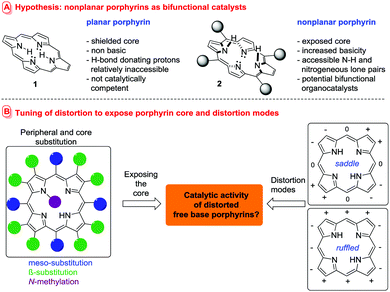
Do porphyrins always require a central metal to be catalytically active? The short answer is ‘No!’ and in the following we detail how conformational design can be used to entice free base porphyrins to act as organocatalysts, revealing a new mode of action for the ubiquitous ‘pigments of life’.1
Porphyrins do not only give color to life but nature utilizes these tetrapyrroles as catalysts and cofactors par excellence for a plethora of essential reactions.2 Some of the most fundamental biological processes involve metalloporphyrin cofactors, e.g., hemes (Fe), (bacterio)chlorophylls (Mg), corphins (Ni) and corrins (Co).1–3 Synthetic porphyrin catalysts aim to reproduce many of these natural processes, notably oxidations, to facilitate synthetic reactions.4 A striking, common feature of all natural and synthetic catalytically active tetrapyrroles is the presence of a central metal ion, with the macrocyclic scaffold serving merely as a fine-tuning molecular frame. Accordingly, the catalytic activity of porphyrins almost exclusively arises from the central metal ion.
Rendering free base porphyrins catalytically active would require participation of the pyrrole N–H and N-lone pairs in chemical reactions and/or hydrogen bonding. In traditional porphyrins these moieties are buried in the macrocycle core and therefore are relatively inaccessible (i.e.1, Fig. 1A). However, any macrocycle distortion resulting in an out-of-plane tilting of the pyrroles will alter this picture and render the core nitrogen atoms spatially accessible (Fig. 1B).5
Ring puckering can be achieved by steric strain from either core or peripheral substitution, as can be the case in highly substituted porphyrins for instance.6 Other classic examples are N-substituted porphyrins7 and (core) porphyrin (di)acids.8,9b The core functionality of these nonplanar9 porphyrins can participate in hydrogen bonding, bind solvent molecules10 or anions,8 and be used as the functional unit of a chiral sensor.11 Dodecasubstituted saddle-distorted porphyrins are metallated several orders of magnitude faster than planar porphyrins, and increased porphyrin distortion can lead to augmented thermodynamic basicity.12 In addition, macrocycle nonplanarity plays a role in the biosynthesis of native porphyrins, where metal chelatases rely on a distortion-mediated mechanism.13 Conformational control of the macrocycle is a key principle to fine-tune the different functional uses of metalloporphyrins in nature.2
Thus, while the general effect of distortion on the availability of the porphyrin lone pairs has been known for decades, the phenomenon has been little studied and completely overlooked as a tool for exploitation in catalysis. We hypothesized that the use of a distortion mechanism5 to facilitate the participation of the metal-free porphyrin core in either hydrogen bonding or base catalysis (or both) could generate powerful and highly tunable organocatalysts (i.e.2, Fig. 1A).
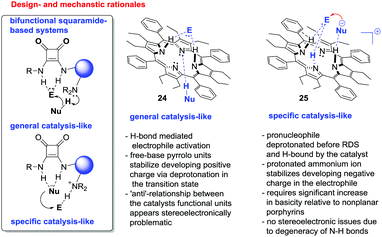
For example, (thio)ureas14 and squaramides15 incorporating basic functionalities have been shown to catalyze a host of reactions through the activation of both the nucleophilic and electrophilic reaction components. Such reactions can occur via either a general catalysis-like mechanism or a specific catalysis-like process (vide infraFig. 2).16,17
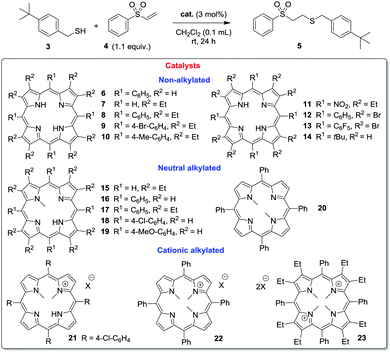
To test this hypothesis, we prepared and evaluated a suite of porphyrins, in which the degree of distortion from planarity, the electronic properties of the macrocycle, and the potential H-bond donating proclivities had been gradually varied as catalysts in the sulfa-Michael addition18 of tert-butyl benzylmercaptan (3) to phenyl vinyl sulfone (4) to afford adduct 5: a reaction likely to be susceptible to bifunctional catalysis. The results of these studies are outlined in Table 1.
| Entry | Catalyst | Δ24a [Å] | λ max [nm] | #NH | Yieldc (%) |
|---|---|---|---|---|---|
| a Δ24 = average deviation of the 24-macrocycle atoms from their least-squares plane as a measure of overall degree of nonplanarity in the solid state. b λ max = soret and long wavelength Q absorption bands in CH2Cl2 (+1% NEt3) as a measure of macrocycle distortion in solution.28 c Determined by 1H NMR spectroscopy using an internal standard, [cat] = 7.1 × 10−2 M. d Performed under dilute conditions: [cat] = 3.6 × 10−3 M. | |||||
| 1 | — | n/a | n/a | n/a | 0 |
| 2 | 6 19a | 0.05, 0.1919a | 417, 515 | 2 | 0 |
| 3 | 7 19b | 0.0219b | 399, 498 | 2 | 0 |
| 4 | 8 20a | 0.5420b | 456, 555 | 2 | >98 (80d) |
| 5 | 9 | — | 459, 515 | 2 | 0 |
| 6 | 10 21 | — | 457, 710 | 2 | >98 (75d) |
| 7 | 11 22 | 0.4022a | 422, 663 | 2 | 0 |
| 8 | 12 12c | 0.6223b | 468, 739 | 2 | 0 |
| 9 | 13 23c | — | 495, 711 | 2 | 0 |
| 10 | 14 9a | — | 446, 692 | 2 | 0 |
| 11 | 15 26a | — | 410, 642 | 1 | <5 |
| 12 | 16 26b | 0.26–0.326c,d | 433, 677 | 1 | 50 |
| 13 | 17 25b | — | 477, 735 | 1 | >98 (<5d) |
| 14 | 18 t.w. | — | 435, 678 | 1 | 3 |
| 15 | 19 t.w. | — | 437, 685 | 1 | 62 |
| 16 | 20 27a | — | 457, 630 | 0 | 5 |
| 17 | 21 t.w. | — | 461, 710 | 1 | >98 (20d) |
| 18 | 22 27b | 0.4427b | 463, 715 | 0 | 0 |
| 19 | 23 27c | 0.6125b | 506, 756 | 0 | 0 |
In the absence of a catalyst, no background reaction was observed at ambient temperature (entry 1). The de facto planar 5,10,25,20-tetraphenyl- and 2,3,7,8,12,13,17,18-octaethyl-porphryins (6 and 7, entries 2 and 3)19 possessed no catalytic activity under the conditions employed at 3 mol% loading. Gratifyingly, a chimera of both these materials – i.e. the highly distorted porphyrin 820 – promoted the reaction to full conversion (entry 4). Under diluted conditions ([cat] = 3.6 × 10−3 M) 80% conversion to 5 was possible. This catalyst system exhibited extraordinary sensitivity to variation of its electronic properties: the tetrakis(4-bromophenyl) analogue 9 was inactive (entry 5) while the tetrakis(4-tolyl) analogue 10 displayed a level of activity on a par with 8 itself (entry 6).21 While a p-bromo substituent is not regarded as a powerful electron withdrawing group (σp = 0.26), we posited that bringing four such substituents to bear on the conjugated macrocycle core brings about the observed modulation of catalyst basicity, leading to inefficient deprotonation of the pronucleophilic substrate.
In line with these observations, the highly sad-distorted9 free base porphyrins 11,2212 and 1312c,23 equipped with multiple electron withdrawing substituents at the meso- and/or β-positions failed to accelerate the reaction (entries 7–9). Ensuing the investigation of sad-distorted porphyrins, an alternative distortion mode, the ruf-distorted 5,10,15,20-tetra(tert-butyl)porphyrin (14, entry 10), was explored. Though highly distorted, no promotion of the reaction was observed due to the N–H being concealed in the porphyrin plane.9a
Next, we examined N-methyl-porphyrins7 as potential catalysts. They are classic inhibitors of ferrochelatase,7,24 where the N-substitution and the consequential macrocycle distortion25 is known to result in increased porphyrin basicity.12a Thus, while these porphyrins contain one less H-bond donating pyrrolo N–H unit than 6–14, they should possess a more accessible and reactive functionality. While N-methylation of 7 (i.e. cat. 15)26a led to only a marginal increase in catalyst efficacy (entry 11), the N-methyl analogue of the similarly inactive catalyst 6 (i.e.16, entry 12)26b–d resulted in a significant improvement to 50% conversion. Disappointingly, the alkylated variant of the efficient catalyst 8 (i.e.17, entry 13)25b was able to catalyze the smooth, quantitative formation of 5 under standard conditions, but not when diluted. Catalysts 18 and 19, which possess N-methyl and meso-aryl groups, but are devoid of octaethyl substitution (entries 14 and 15) did not serve as highly active promoters of the reaction, yet they did exhibit the electronic sensitivity observed in the archetypal free base systems. The N,N′-dimethyl porphyrin 2027a proved a poor catalyst, although it is noteworthy that it is more efficient than 6 (entries 2 and 16). Thus, it is clear that meso-aryl (electron donating) groups, octaethyl substitution, and N-alkylation can be used to improve distortion and catalytic activity. However, the effects of all three modifications together are not additive, and the former two are best utilized in concert from a catalyst design standpoint.
Finally, we were interested in the performance of cationic porphyrins. Compound 21 has a ‘cis’-21,22-dimethylation pattern with a higher degree of distortion than that of the ‘trans’-21,23-dimethylation mode. Despite the presence of the electron-withdrawing aromatic groups, the cationic 21 displayed promising activity under standard conditions, but was a poor promoter of the process under dilute conditions (entry 17). Interestingly, the analogue 22, in which the remaining N–H unit was methylated27b and the electronegative chlorine atom removed could not catalyze the addition (entry 18). A dicationic, permethylated version of the most efficacious catalyst 8 (i.e.23, entry 19)27c was also inactive.
The situation involving cationic porphyrins seems complex. Methylation at both the N21 and N22 positions brings about high levels of distortion25b (which we have shown to be beneficial to activity), however it also generates a positive charge, and we had previously shown (vide supra) that rendering the porphyrin less electron-rich leads to slower catalysis. It is conceivable that delocalization of the charge around the very large aromatic system lessens its impact. In addition, increasing the acidity of the lone pyrrole N–H unit may be relevant. In any case, since 21 is active, despite the presence of electron-withdrawing meso-substituents, it is clear that the contribution from distortion is dominant. Of considerable interest is the failure of 22 as a catalyst, which strongly supports the hypothesis that hydrogen bonding by two porphyrin N–H units – i.e. the original pyrrole N–H and an ammonium ion generated after protonation by the substrate (see 25, Fig. 2) – is also a key facet of catalysis (i.e. a bifunctional mode of operation) in these systems. This correlates well with the fact that 16 (possessing one pyrrole N–H unit) is a mediocre promoter while its analogue with none (i.e.20) is incapable of catalysis under these conditions.29
In order to provide some context regarding the catalytic activity of these distorted porphyrins, we compared the performance of the superior system identified here (8) with a range of amines of disparate basicity under diluted conditions (Table 2). The use of weak amine bases fails to lead to product formation (entries 1 and 2). DMAP promotes the reaction with moderate efficiency (entry 3) while both NEt3 (which is an order of magnitude more basic than DMAP) and 8 can catalyze the reaction to ca. 80% conversion after 24 h (entries 4 and 5).
To confirm that the distorted porphyrins are capable of basicity exceeding some standard amines, we mixed a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio of 8 and DMAP·HCl in CDCl3 (Fig. S2, ESI†). Rapid and quantitative deprotonation of the DMAP conjugate acid was observed using 1H NMR spectroscopic methods, indicating a substantial difference in basicity between the two catalysts.
1 ratio of 8 and DMAP·HCl in CDCl3 (Fig. S2, ESI†). Rapid and quantitative deprotonation of the DMAP conjugate acid was observed using 1H NMR spectroscopic methods, indicating a substantial difference in basicity between the two catalysts.
Given the formation of the bis-protonated analogue of 8 in the experiment outlined above, we investigated if it was possible to form such species under the reaction conditions. Accordingly, we added substrate thiol 3 to 8 and monitored the interaction using 1H NMR spectroscopy (Fig. S9, ESI†). At equimolar levels only traces of porphyrin protonation were detected, however at 10 fold excess of 3 the starting material disappeared and N–H resonances tentatively assigned to both mono- and bis-protonated 8 were observed, with mono-protonation being dominant. Increasing the excess of thiol to those present at the outset of the catalytic process gave rise to significantly more bis-protonated material, which becomes the major product at a substrate![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) catalyst ratio of 100
catalyst ratio of 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and the sole discernible porphyrin species present at a ratio of 200
1 and the sole discernible porphyrin species present at a ratio of 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.
1.
This strongly supports a specific catalysis-like mechanism (see 25, Fig. 2), in which a porphyrin-thiolate ion-pair is catalytically relevant. Given that all porphyrin catalysts evaluated required at least one N–H unit in its free base form to be active, it also seems likely that activation by the electrophile H-bond donation via at least two N–H units (one formed via protonation of the porphyrin by substrate thiol 3) is a feature of the catalysis, i.e. the system is bifunctional. It is unclear at this juncture whether the mono- or bis-protonated cationic porphyrin species (or both) are catalytically competent; however, based on NMR data (see ESI†), it appears likely that the mono-protonated species is the dominant catalyst in solution at both low and high reaction concentration.
In conclusion, we showed that free base and N-substituted porphyrins display catalytic activity in sulfa-Michael reactions and suggest a bifunctional mechanism involving porphyrin amine and imine groups. Distortion and availability of pyrrolic protons appear to be crucial for the catalytic activity. We envisaged that while appropriately designed distorted porphyrins had potential to act via either pathway (i.e.24 or 25, Fig. 2), in view of the ‘saddle’ nature of distorted porphyrins and the experimental data, they appeared to be more amenable to act via the specific-catalysis-type mechanism 25, in which porphyrin protonation allows the catalyst's nucleophile- and electrophile-activating units to reside on the same catalyst hemisphere in an orientation conducive to synergistic cooperation. Studies on the concept of activation, mechanism and structural correlations of an incremental increase of distortion are currently under way.
This work was supported by a grant from Science Foundation Ireland (SFI IvP 13/IA/1894) and the School of Chemistry, TCD.
Conflicts of interest
There are no conflicts to declare.Notes and references
- A. R. Battersby, Nat. Prod. Rep., 2000, 17, 507 RSC
.
- M. O. Senge, S. A. MacGowan and J. M. O’Brien, Chem. Commun., 2015, 51, 17031 RSC
.
- M. O. Senge, A. A. Ryan, K. A. Letchford, S. A. MacGowan and T. Mielke, Symmetry, 2014, 6, 781 CrossRef CAS
.
-
(a) J. T. Groves, T. E. Nemo and R. S. Myers, J. Am. Chem. Soc., 1979, 101, 1032 CrossRef CAS
; (b) B. Meunier, Chem. Rev., 1992, 92, 1411 CrossRef CAS
; (c) J. C. Barona-Castaño, C. C. Carmona-Vargas, T. J. Brocksom and K. T. de Oliveira, Molecules, 2016, 21, 310 CrossRef PubMed
.
- M. O. Senge, ECS Trans., 2015, 66, 1 CrossRef CAS
.
-
(a) C. J. Medforth, M. O. Senge, K. M. Smith, L. D. Sparks and J. A. Shelnutt, J. Am. Chem. Soc., 1992, 114, 9859 CrossRef CAS
; (b) M. O. Senge, Chem. Commun., 2006, 243 RSC
.
-
D. K. Lavallee, The Chemistry and Biochemistry of N-Substituted Porphyrins, VCH Publishers, New York, 1987, 12 Search PubMed
.
-
(a) A. Stone and E. B. Fleischer, J. Am. Chem. Soc., 1968, 90, 2735 CrossRef CAS
; (b) M. O. Senge, T. P. Forsyth, L. T. Nguyen and K. M. Smith, Angew. Chem., Int. Ed. Engl., 1995, 33, 2485 CrossRef
.
-
(a) T. Ema, M. O. Senge, N. Y. Nelson, H. Ogoshi and K. M. Smith, Angew. Chem., Int. Ed. Engl., 1994, 33, 1879 CrossRef
; (b) M. O. Senge, I. Bischoff, N. Y. Nelson and K. M. Smith, J. Porphyrins Phthalocyanines, 1999, 3, 99 CrossRef CAS
; (c) M. O. Senge and W. W. Kalisch, Z. Naturforsch., B: Chem. Sci., 1999, 54, 943 CAS
; (d) M. O. Senge, Z. Naturforsch., B: Chem. Sci., 2000, 55, 336 CrossRef CAS
.
-
(a) W. W. Kalisch and M. O. Senge, Tetrahedron Lett., 1996, 37, 1183 CrossRef CAS
; (b) M. O. Senge and W. W. Kalisch, Inorg. Chem., 1997, 36, 6103 CrossRef CAS PubMed
; (c) M. O. Senge, Z. Naturforsch., B: Chem. Sci., 1999, 54, 821 CAS
.
- Y. Furusho, T. Kimura, Y. Mizuno and T. Aida, J. Am. Chem. Soc., 1997, 119, 5267 CrossRef CAS
.
-
(a) A. Neuberger and J. J. Scott, Proc. R. Soc. London, Ser. A, 1952, 213, 307 CrossRef CAS
; (b) J. Takeda, T. Ohya and M. Sato, Inorg. Chem., 1992, 31, 2877 CrossRef CAS
; (c) P. Bhyrappa, M. Nethaji and V. Krishnan, Chem. Lett., 1993, 869 CrossRef CAS
; (d) O. S. Finikova, A. V. Cheprakov, P. J. Carroll, S. Dalosto and S. A. Vinogradov, Inorg. Chem., 2002, 41, 6944 CrossRef CAS PubMed
.
- S. Al-Karadaghi, R. Franco, M. Hansson, J. A. Shelnutt, G. Isaya and G. C. Ferreira, Trends Biochem. Sci., 2006, 31, 135 CrossRef CAS PubMed
.
- Selected examples:
(a) T. Okino, Y. Hoashi and Y. Takemoto, J. Am. Chem. Soc., 2003, 125, 12672 CrossRef CAS PubMed
; (b) T. Okino, Y. Hoashi, T. Furukawa, X. Xu and Y. Takemoto, J. Am. Chem. Soc., 2005, 127, 119 CrossRef CAS PubMed
; (c) B.-J. Li, L. Jiang, M. Liu, Y.-C. Chen, L.-S. Ding and Y. Wu, Synlett, 2005, 603 CAS
; (d) B. Vakulya, S. Varga, A. Csámpai and T. Soós, Org. Lett., 2005, 7, 1967 CrossRef CAS PubMed
; (e) S. H. McCooey and S. J. Connon, Angew. Chem., Int. Ed., 2005, 44, 6367 ( Angew. Chem. , 2005 , 117 , 6525 ) CrossRef CAS PubMed
; (f) J. Ye, D. J. Dixon and P. S. Hynes, Chem. Commun., 2005, 4481 RSC
.
-
(a) J. P. Malerich, K. Hagihara and V. H. Rawal, J. Am. Chem. Soc., 2008, 130, 14416 CrossRef CAS PubMed
; (b) J. Alemán, A. Parra, H. Jiang and K. A. Jørgensen, Chem. – Eur. J., 2011, 17, 6890 CrossRef PubMed
.
- For mechanistic discussions of bifunctional organocatalysis see:
(a) B. Kótai, G. Kardos, A. Hamza, V. Farkas, I. Pápai and T. Soós, Chem. – Eur. J., 2014, 20, 5631 CrossRef PubMed
; (b) S. Ričko, J. Svete, B. Štefane, A. Perdih, A. Golobič, A. Meden and U. Grošelj, Adv. Synth. Catal., 2016, 358, 3786 CrossRef
; (c) C. Trujillo, I. Rozas, A. Botte and S. J. Connon, Chem. Commun., 2017, 53, 8874 RSC
.
-
(a) A. Hamza, G. Schubert, T. Soós and I. Pápai, J. Am. Chem. Soc., 2006, 128, 13151 CrossRef CAS PubMed
; (b) M. N. Grayson and K. N. Houk, J. Am. Chem. Soc., 2016, 138, 1170 CrossRef CAS PubMed
; (c) M. N. Grayson and K. N. Houk, J. Am. Chem. Soc., 2016, 138, 9041 CrossRef CAS PubMed
.
-
(a) R. Connor and W. M. R. McClellan, J. Org. Chem., 1939, 3, 570 CrossRef CAS
; (b) R. Alleti, W. S. Oh, M. Perambuduru, C. V. Ramana and V. Prakash Reddy, Tetrahedron Lett., 2008, 49, 3466 CrossRef CAS
.
-
(a) M. J. Hamor, T. A. Hamor and J. L. Hoard, J. Am. Chem. Soc., 1964, 86, 1938 CrossRef CAS
; (b) S. J. Silvers and A. Tulinsky, J. Am. Chem. Soc., 1967, 89, 3331 CrossRef CAS PubMed
; (c) J. W. Lauher and J. A. Ibers, J. Am. Chem. Soc., 1973, 95, 5148 CrossRef CAS PubMed
.
-
(a) B. Evans, K. M. Smith and J.-H. Fuhrhop, Tetrahedron Lett., 1977, 5, 443 CrossRef
; (b) A. Regev, T. Galili, C. J. Medforth, K. M. Smith, K. M. Barkigia, J. Fajer and H. Levanon, J. Phys. Chem., 1994, 98, 2520 CrossRef CAS
.
- R.-J. Cheng, Y.-H. Chen, C.-C. Chen, G.-H. Lee, S.-M. Peng and P. P.-Y. Chen, Inorg. Chem., 2014, 53, 8848 CrossRef CAS PubMed
.
-
(a) M. O. Senge, J. Chem. Soc., Dalton Trans., 1993, 3539 RSC
; (b) such porphyrins can also undergo SNAr reactions with thiols: M. Kielmann, K. J. Flanagan, K. Norvaiša, D. Intrieri and M. O. Senge, J. Org. Chem., 2017, 82, 5122 CrossRef CAS PubMed
.
-
(a) G. A. Spyroulias, A. Despotopoulos, C. P. Raptopoulou, A. Terzis and A. G. Coutsolelos, Chem. Commun., 1997, 783 RSC
; (b) K. M. Kadish, M. Lin, E. Van Caemelbecke, G. De Stefano, C. J. Medforth, D. J. Nurco, N. Y. Nelson, B. Krattinger, C. M. Muzzi, L. Jaquinod, Y. Xu, D. C. Shyr, K. M. Smith and J. A. Shelnutt, Inorg. Chem., 2002, 41, 6673 CrossRef CAS PubMed
; (c) D. Mandon, P. Ochsenbein, J. Fischer, R. Weiss, K. Jayaraj, R. N. Austin, A. Gold, P. S. White, O. Brigaud, P. Battioni and D. Mansuy, Inorg. Chem., 1992, 31, 2044 CrossRef CAS
.
- P. R. Ortiz de Montellano, H. S. Beilan and K. L. Kunze, Proc. Natl. Acad. Sci. U. S. A., 1981, 78, 1490 CrossRef CAS
.
-
(a) D. K. Lavallee and O. P. Anderson, J. Am. Chem. Soc., 1982, 104, 4707 CrossRef CAS
; (b) M. O. Senge, W. W. Kalisch and S. Runge, Liebigs Ann./Recl., 1997, 1345 CrossRef CAS
.
-
(a) R. Grigg, A. Sweeney, G. R. Dearden, A. H. Jackson and A. W. Johnson, J. Chem. Soc. D, 1970, 1273 RSC
; (b) H. M. G. Al-Hazimi, A. H. Jackson, A. W. Johnson and M. Winter, J. Chem. Soc., Perkin Trans. 1, 1977, 98 CAS
; (c) unpubl. data; (d) N-methyl-5,10,15,20-tetrakis(p-bromophenyl)porphyrin = 0.266 Å.25a.
-
(a) S. M. S. Chauhan, B. Vijayaraghavan and K. V. Rao, Indian J. Chem., Sect. B: Org. Chem. Incl. Med. Chem., 1987, 26, 122 Search PubMed
; (b) M. O. Senge, J. Porphyrins Phthalocyanines, 1999, 3, 216 CAS
; (c) T. E. Clement, L. T. Nguyen, R. G. Khoury, D. J. Nurco and K. M. Smith, Heterocycles, 1997, 45, 651 CAS
.
- Nonplanar, saddle-distorted porphyrins show a bathochromic shift of the absorption bands.2,6.
- Conformational issues may also impact here, however, 20 is likely to be more distorted than 16.
Footnote |
| † Electronic supplementary information (ESI) available: Catalytic tests, synthesis and characterization of compounds. See DOI: 10.1039/c7cc08099a |
| This journal is © The Royal Society of Chemistry 2018 |