Correlating morphology and doping effects with the carbon monoxide catalytic activity of Zn doped CeO2 nanocrystals†
Haiwei
Du‡
a,
Yuan
Wang‡
 b,
Hamidreza
Arandiyan
b,
Hamidreza
Arandiyan
 *b,
Jason
Scott
*b,
Jason
Scott
 b,
Tao
Wan
a and
Dewei
Chu
b,
Tao
Wan
a and
Dewei
Chu
 *a
*a
aSchool of Materials Science and Engineering, University of New South Wales, Sydney, NSW 2052, Australia. E-mail: d.chu@unsw.edu.au
bParticles and Catalysis Research Group, School of Chemical Engineering, University of New South Wales, Sydney, NSW 2052, Australia. E-mail: h.arandiyan@unsw.edu.au
First published on 12th December 2017
Abstract
The effects of Zn-doping on CeO2 nanocrystals were investigated for the catalytic oxidation of carbon monoxide (CO). Incorporating Zn2+ into CeO2 nanocubes with an isotropic 3D structure can effectively regulate oxygen vacancy concentration, compared with CeO2 nanorods showing an anisotropic one dimensional 1D structure. The catalytic activity was shown to be governed by a morphology-dependent doping effect.
With many advantages such as easy charge transfer between Ce3+ and Ce4+, a high oxygen storage capacity (OSC) and high thermal stability, cerium oxide (CeO2) has received considerable attention in the catalysis field over the past decades.1a It is well known that the catalytic activity of CeO2 nanocrystals can be modulated by both intrinsic and extrinsic means. On the one hand, several intrinsic factors1b–d including tuning crystal size (particle size of spheres or aspect ratio of nanowires), single particle nanostructuring to control shape/morphology with exposed facets, and architectural design of mesoporous microstructures to achieve a higher specific surface area (SSA), directly play an effective role in catalytic performance. Of these, the facet-selective catalysis is very attractive as different facets can boost selective adsorption properties, and the formation of oxygen vacancies is associated with the terminating facets as well. On the other hand, extrinsic factors mostly originating from the compositional designs also make a great contribution to promoting catalytic efficiency. The main types of compositional modifications can be classified into: (i) metal (such as Au, Ag, Pt and Pd) particles dispersed on CeO2 supports; (ii) metal nanoparticles embedding in CeO2 (ref. 1e) or metal oxides fully covering the CeO2 surface1f to form a core–shell heterostructure; and (iii) metal ions-doped CeO2 nanocrystals. For the former two cases, it has been revealed that more catalytically active sites can be generated by a strong synergistic interaction effect1g,h at the metal–support or metal oxide/CeO2 nano-interfaces, and consequently result in enhanced catalytic performance.
A very promising approach is defect engineering through doping. With different physicochemical properties, the dopants can effectively destabilize the parent oxide host and significantly enhance ionic diffusion or transportation by inducing local structural distortions, stress/strain as well as ionic defects. Doping can not only weaken the Ce–O bonds in the neighbourhood of the dopant by sharing oxygen atoms but also disrupt the lattice structure especially when its own oxide has a different structure compared to the host.2a Typically, the widely used dopants in CeO2 are isovalent (best known is the CeO2–ZrO2 system for example2b) or aliovalent, where the latter case is designed especially to form acceptor-doped CeO2–MxOy solid solutions (M is a di- or tri-valent transition metal or lanthanide2c). Among these dopants, zinc (Zn) with a lower oxidation state is found to effectively improve the reducibility and catalytic performance of CeO2 nanocrystals. Theoretically, simulation work has shown that Zn can significantly decrease the reduction energy of CeO2 by distorting the lattice structure to create under-coordinated oxygen atoms after breaking M–O bonds.2d Experimentally, the enhanced reducibility of Ce1−xZnxO2 has been confirmed by characterizations including H2 temperature-programmed reduction (H2-TPR) measurements and the CO oxidation reaction.2e–g However, the reported Zn-doped CeO2 structures are mainly presented as uniform nanoparticles with little information available on how selectively exposed facets and the morphological effect influence the catalytic performance.
In this work, we show that surface reconstruction and faceting of CeO2 crystals at the nanoscale, when properly controlled Zn doping can provide an important tool to regulate catalytic CO oxidation activity. Ce1−xZnxO2 nanocubes (NCs) and nanorods (NRs) were designed and prepared to investigate the combination of Zn doping and morphological effects on CO oxidation. To prevent the formation of separated ZnO phase in the Ce1−xZnxO2 solid solution, the maximum content of Zn was set to 20 mol%.2f The effects of Zn dopants on the structure and catalytic behaviour of the Ce1−xZnxO2 nanostructures were studied by X-ray diffraction (XRD), transmission electron microscopy (TEM), X-ray photoelectron spectroscopy (XPS), Raman spectra, H2-TPR and electron paramagnetic resonance (EPR) spectroscopy. On incorporating Zn into the ceria lattice, catalytic performance of the CeO2 NCs was significantly improved while the Zn dopant imparted only a slight change in the NRs, highlighting the morphology-dependent doping effect.
TEM images of the as-synthesized Ce1−xZnxO2 NCs and NRs are shown in Fig. 1. From Fig. 1a, the CeO2 NCs exhibit a homogenous cubic shape with a mean grain size of 9.4 nm. An interplanar spacing of 0.273 nm, corresponding to the (200) plane of ceria, can be identified in the high resolution TEM (HRTEM) image (Fig. 1b), indicating that six {100} facets are selectively exposed. Doping with 10 mol% Zn has a slight influence on the morphology (as shown in Fig. S1†). On doping with 20 mol% Zn, the morphology changes to some extent as deformation takes place within the cubic structure, which is due to the local distortion by dopant diffusion into the lattice.2h The mean crystal size increases slightly for the Ce0.8Zn0.2O2 NCs to 11.4 nm (Fig. 1c). Fig. 1d–f show the TEM images of the Ce1−xZnxO2 NRs. The presence of Cl− in the solution synthesis invokes a well-defined rod shape for the CeO2 (Fig. 1d) with a mean rod length of 91.1 nm and a mean width of 6.4 nm (Fig. S2†). Typically, an ideal CeO2 NR should have exposed (100) and (110) planes, while only an interplanar spacing of 0.325 nm (corresponding to the (111) plane) can be seen from the HRTEM image here (Fig. 1e). This is attributed to the presence of numerous {111}-type nanofacets on the (110) planes of ceria NRs,2i and the NRs still have a preferential growth along [110] direction but show dominant (111) planes.2i,j Unlike the Zn-doped CeO2 NCs, doping with 20 mol% Zn has almost no effect on NR morphology (Fig. 1f) while the mean rod length increases significantly to 168.2 nm (Fig. S2c†). The morphology difference between the NCs and NRs derives from the presence of different anions as chloride and nitrate ions can selectively interact with the {111} and {100} facets of the CeO2 nuclei, respectively, controlling the growth rate of different facets by changing the surface free energies.2k,l For these reasons, the presence of Cl− favors the formation of elongated 1D structures like nanowires and NRs, while NO−3 favors the formation of nanoparticles or NCs.
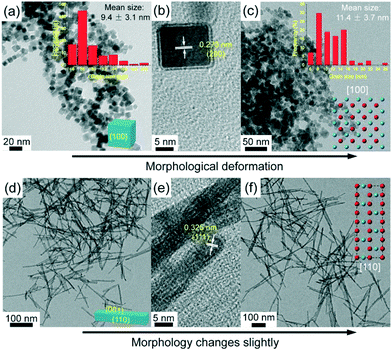 | ||
| Fig. 1 TEM images of Ce1−xZnxO2 samples: CeO2 NCs (a and b), Ce0.8Zn0.2O2 NCs (c), CeO2 NRs (d and e) and Ce0.8Zn0.2O2 NRs (f). | ||
Fig. 2a and b depict the XRD patterns of the as-synthesized Ce1−xZnxO2 NCs. All the diffraction peaks can be indexed to a cubic fluorite phase (JCPDS No. 34-0394) and no peaks corresponding to a ZnO phase were detected, suggesting that Zn has diffused into the CeO2 lattice. In addition the lattice parameter of the Ce1−xZnxO2 NCs decreases gradually (Table S1†) as the (111) diffraction peak with the highest intensity shifts toward a higher degree upon Zn doping. According to the Bragg equation (nλ = 2d![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) sin
sin![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ), a diffraction peak shift toward a higher degree is indicative of a decrease in interplanar spacing. As the ionic radius of Zn2+ (0.90 Å, CN = 8) is smaller than that of Ce4+ (0.97 Å, CN = 8),2m the lattice parameter is expected to be smaller following Zn2+ ion inclusion as the Zn dopant can lead to the lattice shrinkage to some extent. XRD patterns of the Ce1−xZnxO2 NRs (Fig. S3†) displayed a similar cubic fluorite phase with no ZnO phase detected. A slight shift in the (111) diffraction peak toward a higher degree is also observed for the Zn doped NR sample, indicating a similar change in lattice parameter (Table S1†). Additionally, when compared to the NCs, the Ce1−xZnxO2 NRs exhibit broader diffraction peaks which are illustrative of a lower crystallinity.3a,b The nitrogen adsorption–desorption isotherms and pore size distributions of the Ce1−xZnxO2 nanostructures are shown in Fig. S4 and S5,† respectively. All of the Ce1−xZnxO2 samples present similar type IV isotherms and display hysteresis cycles with H3 (p/p0 = 0.7–1.0) hysteresis loops. This type of isotherm normally indicates the present of mesopores3c,d and the corresponding adsorption pore size distribution indicates pores with a diameter range of 3–4 nm. However, the pore sizes are comparable to the particle size distribution of the NCs (Fig. 1) and the width of the NRs (6–9 nm), suggesting that the pore primarily derives from the spaces between nanoparticles. As shown in Table S1,† the SSA increases from 90 m2 g−1 to 105 m2 g−1 upon Zn incorporation into the ceria NCs while there is little change (136 m2 g−1versus 140 m2 g−1) for the ceria NRs.
θ), a diffraction peak shift toward a higher degree is indicative of a decrease in interplanar spacing. As the ionic radius of Zn2+ (0.90 Å, CN = 8) is smaller than that of Ce4+ (0.97 Å, CN = 8),2m the lattice parameter is expected to be smaller following Zn2+ ion inclusion as the Zn dopant can lead to the lattice shrinkage to some extent. XRD patterns of the Ce1−xZnxO2 NRs (Fig. S3†) displayed a similar cubic fluorite phase with no ZnO phase detected. A slight shift in the (111) diffraction peak toward a higher degree is also observed for the Zn doped NR sample, indicating a similar change in lattice parameter (Table S1†). Additionally, when compared to the NCs, the Ce1−xZnxO2 NRs exhibit broader diffraction peaks which are illustrative of a lower crystallinity.3a,b The nitrogen adsorption–desorption isotherms and pore size distributions of the Ce1−xZnxO2 nanostructures are shown in Fig. S4 and S5,† respectively. All of the Ce1−xZnxO2 samples present similar type IV isotherms and display hysteresis cycles with H3 (p/p0 = 0.7–1.0) hysteresis loops. This type of isotherm normally indicates the present of mesopores3c,d and the corresponding adsorption pore size distribution indicates pores with a diameter range of 3–4 nm. However, the pore sizes are comparable to the particle size distribution of the NCs (Fig. 1) and the width of the NRs (6–9 nm), suggesting that the pore primarily derives from the spaces between nanoparticles. As shown in Table S1,† the SSA increases from 90 m2 g−1 to 105 m2 g−1 upon Zn incorporation into the ceria NCs while there is little change (136 m2 g−1versus 140 m2 g−1) for the ceria NRs.
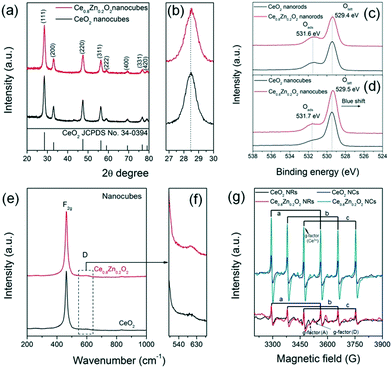 | ||
| Fig. 2 (a and b) XRD patterns, (c and d) O 1s XPS spectra, (e and f) Raman spectra, and (g) EPR spectra of neat CeO2 and Ce0.8Zn0.2O2 nanostructures. | ||
To investigate the chemical states of oxygen on the prepared samples, XPS spectra were compared to obtain detailed information on the surface species with the findings summarized in Table S1.† The Ce 3d spectra from 3d3/2 and 3d5/2 are shown in Fig. S6 and S7† where the contributions are split into ten sub-peaks by curve-fitting. The peaks labelled v, v′′, v′′′, u, u′′ and u′′ are assigned to Ce4+ while v0, u0, v′ and u′ are characteristics of Ce3+. The relative content of Ce3+ is often used to reflect the oxygen defect concentration with the equation defining this given as:
| [Ce3+] = Ce3+(Ce3+ + Ce4+) = A(v0 + v′ + u0 + u′)/A(v0 + v′ + u0 + u′) + A(v + v′′ + v′′′ + u + u′′ + u′′′) |
Raman spectra can be used to identify and clarify the presence of surface defects. From Fig. 2e and f the Raman spectra of the Ce1−xZnxO2 NCs exhibit two obvious peaks; one located at ∼460 cm−1 and corresponding to the Raman-active F2g mode and a symmetrical stretching of the Ce–O vibration in the fluorite structure; the other close to 600 cm−1 and attributed to a defect-induced (D) mode by oxygen vacancies.4b The F2g peak in the Raman spectra of the Ce1−xZnxO2 NRs (Fig. S9†) is broader than that of the NCs (Fig. 2e and f) due to inhomogeneous strain broadening.4c It is known that the surface defect concentration can be evaluated by the area ratio (AD/AF2g) of the Raman spectra. Similar to the XPS results, the area ratio of CeO2 NCs increases upon Zn doping while the increase is less significant for the NRs (Table S1†). The oxygen defect variation induced by doping appears to depend on the morphology whereby the Zn dopant is beneficial for increasing the surface defect sites in the NCs. The surface defects in the Ce1−xZnxO2 NCs are anticipated to arise from an increase in oxygen vacancies following doping with the Zn. Additionally, oxygen defects in metal oxides can be detected by EPR, which is very sensitive to unpaired electrons, for example, Ce3+/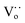 in CeO2. As shown in Fig. 2g, the signal A (g⊥ and g‖) corresponds to Ce3+ stabilized by lattice defects while signal D is ascribed to Ce3+ with easily removable ligands.4d It is apparent that the intensity of g⊥(Ce3+) has increased considerably for the Zn-doped NCs. In the case of the Zn-doped NRs, the intensity change upon Zn doping is negligible. The findings indicate that the Zn dopant concentration in the NCs is higher than for the NRs as the Zn doping is more homogenous in the former one due to its isotropic structure.
in CeO2. As shown in Fig. 2g, the signal A (g⊥ and g‖) corresponds to Ce3+ stabilized by lattice defects while signal D is ascribed to Ce3+ with easily removable ligands.4d It is apparent that the intensity of g⊥(Ce3+) has increased considerably for the Zn-doped NCs. In the case of the Zn-doped NRs, the intensity change upon Zn doping is negligible. The findings indicate that the Zn dopant concentration in the NCs is higher than for the NRs as the Zn doping is more homogenous in the former one due to its isotropic structure.
H2-TPR was conducted to explore the reducibility and the oxygen reactivity of the CeO2 nanostructures and their Zn-doped counterparts (Fig. 3a). The neat CeO2 displayed a two-peak reduction profile; (i) reduction at a lower temperature (328 °C and 388 °C for the NCs and NRs, respectively) which originates from the surface/subsurface reduction of Ce4+ to Ce3+, and (ii) bulk CeO2 reduction at a higher temperature (648 °C and 627 °C for the NCs and NRs, respectively). When compared with the CeO2 NRs, the surface reduction of the CeO2 NCs is less significant. Following Zn doping, the lower temperature reduction peaks (i.e. less than 400 °C) shift to lower temperatures for both the NC and NR samples while the reduction peaks at around 600 °C exhibit no significant temperature change. The shift in the lower temperature reduction peaks indicates that the Zn dopant primarily influences the reducibility of the surface species rather than the bulk material. It is also apparent that the peak area of the low temperature peak for the Zn-doped ceria NCs increases considerably (relative to the neat CeO2 NCs), which is indicative of greater hydrogen consumption by the catalyst surface. The effect may derive from the reduction of Ce4+ and Zn2+ which are in close contact as well as the oxygen vacancies.2e Overall, the Ce0.8Zn0.2O2 NCs exhibit markedly higher redox properties at the lower temperature upon Zn doping. The findings indicate that the promoting effect on CeO2 reducibility with Zn doping is morphology dependent.
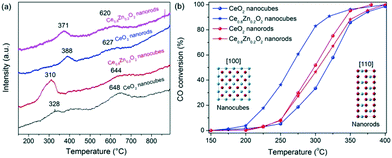 | ||
| Fig. 3 H2-TPR profiles (a) and CO conversion versus temperature (b) for neat CeO2 and Ce0.8Zn0.2O2 nanostructures. | ||
Fig. 3b provides the CO conversion profiles as a function of the temperature for the neat CeO2 and Ce0.8Zn0.2O2 catalysts with NC and NR morphologies. It is apparent that the neat CeO2 NRs performed better than NCs for CO oxidation, possibly as a result of the difference in surface area and/or exposed facets. The same morphology effect has been reported elsewhere.4e However, after incorporating Zn into the CeO2 matrix, the activity of NCs exhibits a significant improvement as is shown by the T10%, T50% and T90% values decreasing from 259 °C, 317 °C and 363 °C for neat CeO2 to 211 °C, 264 °C and 318 °C for the Ce0.8Zn0.2O2 (Table S1†). In contrast to the NCs, the catalytic activity of the Ce0.8Zn0.2O2 NRs remains essentially unchanged at the T10% and T50% values and is slightly increased at the higher temperature (T90%), likely due to the minor doping effect in the NRs. The improvement in catalytic activity for the Ce0.8Zn0.2O2 NC catalysts can be attributed to the increased oxygen vacancy presence as detailed earlier. The oxygen vacancies in Ce0.8Zn0.2O2 appear to be reactive sites which can act as mobile centres for oxygen activation during the CO oxidation reaction. Moreover, in order to study the catalytic stability, Ce0.8Zn0.2O2 NCs were selected to run over repeat cycles. As shown in Fig. S10,† there is very little change in the catalytic activity over five repeat runs, indicating that the Ce–Zn solid solutions are stable for the CO oxidation reaction.
Despite the intrinsic cubic architecture, NCs with six {100} facets can be considered as an isotropic 3-D structure while NRs with {100} and {110} facets are an anisotropic 1D structure, resulting in different atomic arrangements and anisotropic atomic diffusion rates on the different facets.4f In an effort to quantify the influence of morphology on Zn doping concentration, the ratio of Zn 2p/Ce 3d, from the XPS analysis, has been calculated and is provided in Fig. S11.† The assessment indicates that the final Zn/Ce ratio for the particles deviates from the intended composition based on the starting precursors although no peak corresponding to the ZnO phase is observable in the XRD profiles (Fig. 2 and S3†). The partial loss of Zn from the CeO2 nanocrystals is likely attributed to the solution process.4g As shown in Fig. S11,† the ratio of Zn 2p/Ce 3d for the NCs displays a linear trend, indicative of homogeneous diffusion which is due to the isotropic cubic morphology. However, the Zn 2p/Ce 3d ratio for the Ce1−xZnxO2 NRs initially experiences a linear increase after which there is a clear decrease in the slope, indicating inhomogeneous doping induced by different Zn diffusion rates on different facets of the anisotropic 1D NRs. Consequently, doping Zn into the CeO2 structure is governed by the morphological effect where the final ratio of Zn/Ce in the NCs is higher compared to the NRs in all cases. The discrepancy in Zn loading between the NCs and NRs is thought to contribute to the different catalytic activities. The present work demonstrates that the catalytic activity of Zn-doped CeO2 nanostructures is determined by the morphology-dependent doping effect.
Conclusions
In this work, Ce1−xZnxO2 NCs and NRs were synthesized via a hydrothermal method and the morphology-dependent doping effects on lattice structure, microstructure, oxygen defect levels as well as the CO catalytic activity were studied in detail. On comparing with NRs, which possessed an anisotropic 1D structure, NCs containing an isotropic 3D structure were more suited for homogenous doping of Zn into the CeO2 lattice structure. Consequently, the Zn-doped CeO2 NCs displayed an enhanced catalytic efficiency for CO oxidation, originating from the morphology-dependent doping effect and its ensuing contribution to surface oxygen defects formation.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work is funded by the ARC Project (grant no. FT140100032). H. Du thanks Dr. Bill Gong for assistance with XPS measurements and the China Scholarship Council (CSC) for financial support (No. 201406410060).References
- (a) A. Trovarelli, Catal. Rev., 1996, 38, 439–520 CrossRef CAS; (b) E. Grulke, K. Reed, M. Beck, X. Huang, A. Cormack and S. Seal, Environ. Sci.: Nano, 2014, 1, 429–444 RSC; (c) W. Huang and Y. Gao, Catal. Sci. Technol., 2014, 4, 3772–3784 RSC; (d) T. X. Sayle, F. Caddeo, X. Zhang, T. Sakthivel, S. Das, S. Seal, S. Ptasinska and D. C. Sayle, Chem. Mater., 2016, 28, 7287–7295 CrossRef CAS; (e) M. Cargnello, J. D. Jaén, J. H. Garrido, K. Bakhmutsky, T. Montini, J. C. Gámez, R. Gorte and P. Fornasiero, Science, 2012, 337, 713–717 CrossRef CAS PubMed; (f) H. Du, Y. Wang, H. Arandiyan, A. Younis, J. Scott, B. Qu, T. Wan, X. Lin, J. Chen and D. Chu, Mater. Today Commun., 2017, 11, 103–111 CrossRef CAS; (g) Q. Fu, H. Saltsburg and M. Flytzani-Stephanopoulos, Science, 2003, 301, 935–938 CrossRef CAS PubMed; (h) M. Cargnello, V. V. Doan-Nguyen, T. R. Gordon, R. E. Diaz, E. A. Stach, R. J. Gorte, P. Fornasiero and C. B. Murray, Science, 2013, 341, 771–773 CrossRef CAS PubMed.
- (a) V. Shapovalov and H. Metiu, J. Catal., 2007, 245, 205–214 CrossRef CAS; (b) T. Montini, M. Melchionna, M. Monai and P. Fornasiero, Chem. Rev., 2016, 116, 5987–6041 CrossRef CAS PubMed; (c) K. Reed, A. Cormack, A. Kulkarni, M. Mayton, D. Sayle, F. Klaessig and B. Stadler, Environ. Sci.: Nano, 2014, 1, 390–405 RSC; (d) A. B. Kehoe, D. O. Scanlon and G. W. Watson, Chem. Mater., 2011, 23, 4464–4468 CrossRef CAS; (e) O. Laguna, F. R. Sarria, M. Centeno and J. A. Odriozola, J. Catal., 2010, 276, 360–370 CrossRef CAS; (f) O. Laguna, M. Centeno, F. Romero-Sarria and J. A. Odriozola, Catal. Today, 2011, 172, 118–123 CrossRef CAS; (g) S.-L. Zhong, L.-F. Zhang, L. Wang, W.-X. Huang, C.-M. Fan and A.-W. Xu, J. Phys. Chem. C, 2012, 116, 13127–13132 CrossRef CAS; (h) K. Yamamoto, T. Hashishin, M. Matsuda, N. Qiu, Z. Tan and S. Ohara, Nano Energy, 2014, 6, 103–108 CrossRef CAS; (i) C. Yang, X. Yu, S. Heißler, A. Nefedov, S. Colussi, J. Llorca, A. Trovarelli, Y. Wang and C. Wöll, Angew. Chem., Int. Ed., 2017, 56, 375–379 CrossRef CAS PubMed; (j) S. Agarwal, L. Lefferts, B. L. Mojet, D. Ligthart, E. J. Hensen, D. R. Mitchell, W. J. Erasmus, B. G. Anderson, E. J. Olivier and J. H. Neethling, ChemSusChem, 2013, 6, 1898–1906 CrossRef CAS PubMed; (k) Q. Wu, F. Zhang, P. Xiao, H. Tao, X. Wang, Z. Hu and Y. Lu, J. Phys. Chem. C, 2008, 112, 17076–17080 CrossRef CAS; (l) A. Trovarelli and J. Llorca, ACS Catal., 2017, 7, 4716–4735 CrossRef CAS; (m) R. Shannon, Acta Crystallogr., Sect. A, 1976, 32, 751–767 CrossRef.
- (a) S. Wang, L. Zhao, W. Wang, Y. Zhao, G. Zhang, X. Ma and J. Gong, Nanoscale, 2013, 5, 5582–5588 RSC; (b) Q. Dai, H. Huang, Y. Zhu, W. Deng, S. Bai, X. Wang and G. Lu, Appl. Catal., B, 2012, 117, 360–368 CrossRef; (c) H. Arandiyan, J. Scott, Y. Wang, H. Dai, H. Sun and R. Amal, ACS Appl. Mater. Interfaces, 2016, 8, 2457–2463 CrossRef CAS PubMed; (d) B. Gao, J. Deng, Y. Liu, Z. Zhao, X. Li, Y. Wang and H. Dai, Chin. J. Catal., 2013, 34, 2223–2229 CrossRef CAS.
- (a) K. Kuntaiah, P. Sudarsanam, B. M. Reddy and A. Vinu, RSC Adv., 2013, 3, 7953–7962 RSC; (b) J. McBride, K. Hass, B. Poindexter and W. Weber, J. Appl. Phys., 1994, 76, 2435–2441 CrossRef CAS; (c) Z. Wu, M. Li, J. Howe, H. M. Meyer III and S. H. Overbury, Langmuir, 2010, 26, 16595–16606 CrossRef CAS PubMed; (d) E. Abi-aad, R. Bechara, J. Grimblot and A. Aboukais, Chem. Mater., 1993, 5, 793–797 CrossRef CAS; (e) Z. Wu, M. Li and S. H. Overbury, J. Catal., 2012, 285, 61–73 CrossRef CAS; (f) M. Melchionna and P. Fornasiero, Mater. Today, 2014, 17, 349–357 CrossRef CAS; (g) Y. Yang, X. Liu, Y. Yang, W. Xiao, Z. Li, D. Xue, F. Li and J. Ding, J. Mater. Chem. C, 2013, 1, 2875–2885 RSC.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7cy01999h |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2018 |
