Home food preparation techniques impacted the availability of natural antioxidants and bioactivities in kale and broccoli†
Lu
Yu
 a,
Boyan
Gao
a,
Boyan
Gao
 a,
Yanfang
Li
a,
Thomas T. Y.
Wang
b,
Yinghua
Luo
a,
Jing
Wang
a,
Yanfang
Li
a,
Thomas T. Y.
Wang
b,
Yinghua
Luo
a,
Jing
Wang
 *c and
Liangli (Lucy)
Yu
*c and
Liangli (Lucy)
Yu
 *a
*a
aDepartment of Nutrition and Food Science, University of Maryland, College Park, MD 20742, USA. E-mail: lyu5@umd.edu
bDiet, Genomics and Immunology Laboratory, USDA-ARS, Beltsville, MD 20705, USA
cBeijing Advanced Innovation Center for Food Nutrition and Human Health and Beijing Higher Institution Engineering Research Center of Food Additives and Ingredients, Beijing Technology & Business University (BTBU), Beijing 100048, China. E-mail: wangjing@th.btbu.edu.cn
First published on 1st December 2017
Abstract
This study evaluated the effects of grinding and chopping with/without microwaving on the health-beneficial components, and antioxidant, anti-inflammation and anti-proliferation capacities of commercial kale and broccoli samples. The availability of indole-3-carbinol (I3C) was evaluated using high-performance liquid chromatography. The total phenolic contents, the scavenging activities against DPPH, oxygen, hydroxyl and ABTS cation radicals, and cell-based antioxidant activities were determined for the antioxidant capacities. The results indicated that chopping released the least nutraceutical components and antioxidant compounds. Microwaving had no effect on the I3C release from kale, but resulted in an elevated (more than 2-fold) release of I3C from broccoli. In addition, the choice of a blender affected the availability of the anti-proliferative compounds from the vegetables, while it had no effect on the availability of their anti-inflammatory compounds. In summary, different food preparation methods could strongly impact the availability of bioactive factors in the selected vegetables. These findings suggest that choosing an appropriate food processing method for each vegetable might be critical to obtain desirable health-beneficial effects.
1. Introduction
Dark green leafy vegetables from the Cruciferae family are excellent sources of protein, carbohydrates, dietary fiber, minerals and antioxidants.1–3 Cruciferous vegetables such as broccoli and kale contain a large amount of phytochemicals with potential antioxidant, antimutagenic, antifungal and antiviral activities.4–6 Cruciferous vegetables differ from other vegetables as they are rich in phytochemical glucosinolates. The metabolites of glucosinolates, such as indole-3-carbinol, are well known for a broad spectrum of anti-cancer properties with low toxicity. Cruciferous vegetables may reduce the risk of prostate, breast, liver, colon, cervix and lung cancers.7–11Among all dark green vegetable subgroups, broccoli is the most consumed (37.7% of the subgroups),3 and kale contains the overall highest content of vitamin C (0.82 mg g−1 of fresh weight) as well as β-carotenes (0.09 mg g−1 of fresh weight).12 Compared to the accumulating research findings on chemical profiles,13 antioxidant activities14 and health-promoting properties of green leafy vegetables including kale and broccoli,15 how home food preparation may alter the desirable availability of their natural antioxidants and beneficial effects is less known. A recent study investigated the effects of food preparation methods on the availability of the beneficial properties of carrots and blueberries.16 The results from this study indicated that home food preparation methods may alter the availability of health components from carrots and blueberries, and the impact might also depend on the fruit types.16 Therefore, it is interesting to know whether and how home food preparation may impact the availability of the health-beneficial properties of commonly consumed vegetables.
Recently, fresh vegetable (e.g. salads) consumption has been considered as a good way to retain essential nutrients and dietary fiber. In this study, the potential effects of grinding and chopping with/without microwaving on the availability of antioxidant components, antioxidant activities, anti-inflammatory properties and anti-proliferation effects were evaluated using kale and broccoli as the probe vegetables. Two vegetables were selected to be able to draw a general conclusion. The results from this study are important for advancing the knowledge on how home food preparation may impact the overall availability of the nutritional value and health properties of vegetables.
2. Materials and methods
2.1. Materials
All of the vegetables were purchased from local supermarkets in College Park. Five home-used blenders, including Nutribullet 600, Nutribullet Pro 900, Nutribullet RX, Oster Versa 1400 and Vitamix 5200 were gifted from Capital Brans, LLC (Los Angeles, California). A Luna 5u C18 reversed-phase column (4.6 × 250 mm, 5 μm) was purchased from Phenomenex (Torrance, CA). 2,2′-Azinobis(2-amidinopropane) dihydrochloride (AAPH) was purchased from Wako Chemicals, USA (Richmond, VA). Thirty percent ACS-grade H2O2 was purchased from Fisher Scientific (Fair Lawn, NJ). The human hepatocarcinoma cell line HepG2/C3A, human prostate cancer cell line LNCaP, and human leukemic cell line THP-1 were purchased from the American Type Culture Collection. Lipopolysaccharides (LPS) from Escherichia coli 0111:B4, iron(III) chloride, fluorescein (FL), 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylicacid (Trolox), 2,2-diphenyl-1-picrylhydrazyl radical (DPPH), HPLC grade water, acetic acid, sodium acetate, HEPES, hydrocortisone, penicillin, streptomycin, gentamicin, insulin, and dimethyl sulfoxide were purchased from Sigma-Aldrich (St Louis, MO, USA). 2′,7′-Dichlorofluorescin diacetate (DCFHDA), Williams’ medium E without phenol red, RPMI medium with phenol red, and Hanks’ balanced salt solution (HBSS) were purchased from Gibco Life Technologies (Grand Island, NY, USA). 2,2′-Azobis(2-amidinopropane) dihydrochloride (ABAP) was obtained from Wako Chemicals (Richmond, VA, USA). An ATPlite Luminescence Assay System Kit was obtained from PerkinElmer Life and Analytical Sciences (Boston, MA. USA). DMEM, antibiotic–antimycotic, fetal bovine serum, and TRIzol reagent were purchased from Invitrogen (Carlsbad, CA, USA). All other chemicals were of analytical reagent grade and used without any further purification.2.2. Sample preparation
50 g and 100 g of commercial fresh kale and broccoli (including stem and leaves) samples were ground for 15 s with 200 mL of water in 5 different commercially available blenders. The ground vegetables were divided into two parts, and one part was microwaved at 700 watts for 30 s. An equal volume of 100% acetone was added to the un-microwaved or microwaved samples to obtain the final 50% acetone extraction at ambient temperature. After being kept at ambient temperature for 6 h, the mixtures were centrifuged and the supernatants were kept at −20 °C for further analyses.2.3. HPLC
I3C in kale and broccoli were quantified using a Shimadzu LC-20AD with an auto-sampler plus a C18, 4.6 × 250 mm, 5 μm column. A binary gradient system was used with mobile phase A consisting of 0.1% formic acid in acetonitrile and mobile phase B consisting of 0.1% formic acid in water. The flow rate was kept at 1 mL min−1. HPLC conditions for I3C analysis: 0–15 min, 20% A to 50% A; 15–16 min, 50% A to 100% A; 16–20 min, 100% A; 20–20.1 min, 100% A to 20% A. The absorbance at a wavelength of 254 nm was detected at ambient temperature. Indole-3-carbonal was used as an external standard. The limitation of quantification (LOQ) for I3C is 1.36 μM.2.4. Total phenolic content (TPC)
The TPC of each 50% acetone kale and broccoli extract was measured according to a laboratory procedure described previously.17 Briefly, 100 μL of the extract, 500 μL of the Folin–Ciocalteu reagent, 1.5 mL of 20% sodium carbonate, and 1.5 mL of ultrapure water comprised the reaction mixture. Gallic acid was used as the standard and the reactions were conducted in triplicate and results were reported as gallic acid equivalents (GAE) per gram of fresh weight of vegetables. Absorbance was read at 765 nm on a Genesys 20 spectrophotometer (Thermo Scientific, Waltham, MA) after 2 h of reaction at ambient temperature.2.5. Relative DPPH˙ scavenging capacity
A laboratory protocol was used for the DPPH˙ scavenging capacity estimation that was performed using a Victor3 multi-label plate reader (PerkinElmer, Turku, Finland).18 Briefly, each reaction mixture contained 100 μL of 50% acetone extracts at different concentrations and 100 μL of 0.2 mM DPPH˙ solution. Briefly, 100 μL of DPPH˙ solution (0.2 mM) was added to 100 μL of the blank, the standard or 50% acetone extracts to start the radical-antioxidant reaction. The absorbance at 517 nm was measured against a blank of 50% acetone at the time points and used to estimate the remaining radical levels according to a standard curve. The percentage of the radical remaining at 40 min is determined and Trolox was used as the standard and the reactions were conducted in triplicate and results were represented as Trolox equivalents (TE) per gram of fresh weight of vegetables.2.6. Oxygen radical absorbance capacity (ORAC)
The ORAC assay was conducted according to a previously reported laboratory protocol.19 Fluorescein (FL) was used as the fluorescent probe and a Victor3 multilabel plate reader (PerkinElmer, Turku, Finland) was used to measure the fluorescence. In the initial reaction mixtures, 225 μL of freshly made 8.16 × 10−8 M FL and 30 μL of the 50% acetone extract of the samples, and the Trolox standard or blank were combined and placed in the pre-heated 96-well plate in the plate reader at 37 °C. After 25 μL of freshly made 0.36 M AAPH was added to each well, the fluorescence of the assay mixture was recorded once every two minutes for three hours at 37 °C, with excitation and emission wavelengths of 485 nm and 535 nm, respectively. Trolox equivalents (TE) were calculated for the samples based on the area under the curve (AUC) calculations. The results are expressed as micromoles of TE per gram of fresh samples.2.7. Hydroxyl radical scavenging capacity (HOSC)
The HOSC assay was conducted using FL as the fluorescent probe on a Victor3 multi-label plate reader (PerkinElmer, Turku, Finland) according to a previously reported laboratory protocol.20 The reaction mixture contained 170 μL of 9.28 × 10−8 M FL, 30 μL of the samples, 40 μL of 0.1990 M H2O2 and 60 μL of 3.43 M FeCl3. The fluorescence of the reaction mixture was recorded once every minute for 2 h without a temperature control. Excitation and emission wavelengths were 485 nm and 535 nm, respectively. 9.28 × 10−8 M FL was prepared freshly using 75 mM sodium phosphate buffer (pH 7.4). Trolox equivalents (TE) were calculated for the samples using the same AUC calculations.22 The results are expressed as micromoles of TE per gram of vegetables.2.8. ABTS cation radical scavenging capacity
The radical-scavenging capacities of sample extracts were evaluated against ABTS˙+ generated by the chemical method according to a previously reported protocol.21 Briefly, ABTS˙+ was prepared by oxidizing 5 mM aqueous solution of ABTS, 2,2′-azinobis-3-ethylbenzothiazoline-6-sulfonic acid diammonium salt, with manganese dioxide at ambient temperature for 30 min. The ABTS˙+ antioxidant reaction mixture contained 1.0 mL of ABTS˙+ with an absorbance of 0.8 at 734 nm, and 100 μL of sample extracts or 50% acetone as the blank. The absorbance at 734 nm was measured at 1 min of the reaction, and results are expressed as micromoles of Trolox equivalents (TE) per gram of fresh vegetables.2.9. Cell-based antioxidant capacity
Cell-based antioxidant capacity assays were performed according to a previously published protocol.22 Briefly, HepG2/C3A, 6 × 104 cells per well, was seeded in 96-well plates and kept in a 37 °C incubator under 5% CO2. After 24 h, the culture medium was removed and replaced with the fresh medium containing the test extracts/compounds. After 24 h of pretreatment, the medium was removed and the cells were washed with pre-warmed HBSS. The HepG2/C3A cells were then treated with the testing extracts and 25 μM DCFHDA for 1 h. Next, 100 μL of 600 μM ABAP at 37 °C was added to the cells. Fluorescence was read at 5 min intervals for 1 h with an emission at 538 nm and an excitation at 485 nm at 37 °C. Cell-based antioxidant activities of the samples were expressed as milligrams of gallic acid per gram of fresh vegetables.2.10. Anti-inflammatory capacity
To determine the anti-inflammatory activities of the extracts, human THP-1 monocytes were cultured in 6-well plates overnight to reach an 80% confluence. The cells were incubated at 37 °C under 5% CO2 for 24 h. Extracts (1 mg mL−1) were added to the cells 24 h prior to induction, respectively. After 24 h incubation, LPS was added into the media at the initial concentration of 10 ng mL−1. After induction, the culture media were discarded and the cells were collected to perform total RNA isolation and real-time PCR.23,24RNA isolation and real-time PCR were performed according to a previously published protocol.25 After LPS induction, the cells were washed with 1 × PBS, and the TRIzol reagent was added for total RNA isolation. The StrataScript First Strand complementary DNA Synthesis kit was used to reverse transcribe complementary DNA. Real-time PCR was performed on an ABI Prism 7000 Sequence Detection System using TaqMan Universal PCR Master Mix. IL-6, COX-2 and TNF-α mRNAs were determined. The mRNA amounts were normalized to an internal control Tbp mRNA. The following amplification parameters were used for PCR: 50 °C for 2 min, 95 °C for 10 min, and 46 cycles of amplification at 95 °C for 15 s and 60 °C for 1 min.
2.11. Anti-proliferative capacity
Vegetable extracts were also examined for their potential anti-proliferative activities using androgen responsive prostate LNCaP cells. The cells were grown at 1 × 104 cells per mL at 37 °C under 5% carbon dioxide in RPMI medium supplemented with 10% fetal bovine serum and 1% antibiotic/antimycotic. The treatment was performed at a final concentration of 1% DMSO in triplicate. An ATP-Lite 1 step kit (PerkinElmer Life and Analytical Sciences, Shelton, CT) was used to determine cell proliferation.26 The emitted luminescence was determined using a Victor3 plate reader (PerkinElmer, Turku, Finland) immediately prior to treatment and at 0, 24, 48, 72 and 96 h after the initial treatment. The treatment media were replaced every 24 h.2.12. Statistics
Tests were conducted in triplicate, with the data reported as the mean ± standard deviation. The significance level of differences in the means was detected using one-way ANOVA and Tukey's test. The statistics were analyzed using SPSS for Windows (version rel. 10.0.1, 1999, SPSS Inc., Somers, NY). The statistical significance was defined at P ≤ 0.05.3. Results and discussion
3.1. Effects of food preparation methods on releasable health component I3C in kale and broccoli
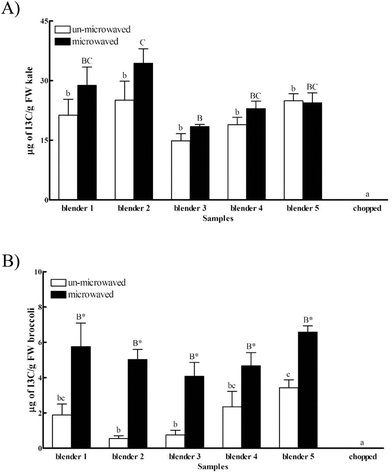
Indole-3-carbinol (I3C) is a health-promoting component derived from the hydrolysis of glucosinolates, and is present at a relatively high level in Cruciferae vegetables.27 I3C may reduce the risk of hormone-related cancers, such as prostate, breast and cervical cancers.8,28,29 The inhibitory effects of I3C and its metabolites have been investigated through cancer cell-cycle/survival regulation such as Akt-NF-kB signaling involved pathways, caspase activation, estrogen metabolism, and cyclin-dependent kinase activities.30The availability of I3C was significantly enhanced with grinding (14.86–25.08 μg g−1 of fresh kale) as compared to chopping (below the limit of detection), suggesting the effect of particle size on food factor release (Fig. 1A). The particle sizes of the ground kale and broccoli were 10 times smaller than their chopped counterparts (data shown in the ESI†). No significant difference was observed between kale samples ground with different blenders without microwaving. A 1.9-fold increase of I3C availability was found in kale samples ground using blender 2 as compared to blender 3 with microwaving. Together, these data suggested a possible combined effect of the particle size and intrinsic enzyme activity on the releasable level of I3C from kale.
A similar trend was observed for broccoli. The availability of I3C in the chopped broccoli was below the limit of detection (LOD) (Fig. 1B), confirming the potential influence of particle size on component availability from food products. Grinding not only resulted in the difference of particle size, but might also break the plant cell wall and release enzymes such as myrosinase, which might hydrolyze glucobrassicin and cause a higher releasable I3C level.11
No difference was found in the releasable amount of I3C from the broccoli samples with or without microwaving treatment (Fig. 1B), whereas a significant difference was observed in I3C availability with or without microwaving. Interestingly, microwaving significantly elevated the extractable amount of I3C from broccoli regardless of the blenders used. For instance, the availability of I3C in broccoli was increased by 3.1, 9.1 and 1.9 fold, respectively, using the blenders 1, 2 and 5 with microwaving as compared to their un-microwaved counterparts. The observation might be explained by the interactions between I3C and the vegetable matrix and thermal effects during microwaving. Microwaving might change the physical properties of foods,31 and the possibly elevated hydrolysis32 of chemical bonding between I3C and the vegetable matrix might contribute to the overall changes of I3C availability. The thermal effect was supported by the enhanced release of natural antioxidants from whole wheat pizza crust by thermal treatment,33 and the increased antioxidant activities of whole rye bread fractions after baking.34 In brief, these data support the possible impact of intrinsic enzymes and heat on the beneficial component release from a selected vegetable.
3.2. Effects of food preparation approaches on the releasable total phenolic content (TPC)
Grinding significantly increased the availability of the TPC as compared to chopping (Table 1), suggesting the impact of particle size on TPC release. The TPC values of kale extracts ranged from 1.59 to 2.33 mg of gallic acid per g fresh kale. This range is lower than the TPC values of 20.06–42.83 mg gallic acid per g fresh kale reported by Fiol's group.35 This difference might be explained by the different kale cultivars and extraction methods in the two studies. Without microwaving, kale extracts prepared using different blenders exhibited significant differences in the extractable TPC, indicating the effect of blenders on TPC release. The releasable TPC of kale extracts prepared using blenders 1 and 5 significantly differed from that of blender 4 (by 1.3-fold). No effect on the TPC release could be observed from kale using different blenders with microwaving. But the TPC release of kale extracts prepared using blenders 1 and 5 was significantly decreased as compared to that without microwaving. The data suggested a possible effect of multiple enzymes on TPC availability.36| TPCa (mg GA FW−1) | ORACb (μmole Trolox FW−1) | HOSCc (μmole Trolox FW−1) | |||||
|---|---|---|---|---|---|---|---|
| Kale | Broccoli | Kale | Broccoli | Kale | Broccoli | ||
| a TPC: total phenolic content. b ORAC: oxygen radical absorbance capacity. c HOSC: hydroxyl radical scavenging capacity. | |||||||
| Un-microwaved | Blender 1 | 2.14c ± 0.24 | 0.47b ± 0.09 | 28.97cd± 1.75 | 11.14b ± 0.37 | 1184.75b ± 68.19 | 508.66b ± 136.10 |
| Blender 2 | 2.03bc ± 0.08 | 0.39b ± 0.20 | 35.46d ± 4.65 | 12.60b ± 1.65 | 1163.52b ± 101.54 | 389.01b ± 35.18 | |
| Blender 3 | 1.79bc ± 0.15 | 0.36b ± 0.13 | 30.03cd ± 2.21 | 14.44b ± 1.94 | 1225.6b ± 51.59 | 457.26b ± 15.63 | |
| Blender 4 | 1.59b ± 0.10 | 0.35b ± 0.12 | 22.53bc ± 4.22 | 14.63b ± 1.36 | 1210.64b ± 115.69 | 519.31b ± 25.20 | |
| Blender 5 | 2.12c ± 0.17 | 0.28b ± 0.10 | 13.63b ± 1.28 | 14.76b ± 0.85 | 1262.57b ± 172.53 | 471.13b ± 84.14 | |
| Chopped | N/A | N/A | N/A | N/A | N/A | N/A | |
| Microwaved | Blender 1 | 2.33B ± 0.24 | 0.32B* ± 0.06 | 24.47BC ± 1.57 | 16.48B* ± 2.37 | 1254.06B ± 62.52 | 446.06B ± 88.06 |
| Blender 2 | 2.29B ± 0.51 | 0.30B ± 0.05 | 27.84BC ± 4.54 | 17.39B* ± 3.65 | 1299.17B ± 64.66 | 521.26B* ± 109.99 | |
| Blender 3 | 1.87B ± 0.09 | 0.29B ± 0.02 | 26.98BC ± 6.17 | 16.10B ± 1.65 | 1062.97B ± 151.60 | 446.89B ± 86.38 | |
| Blender 4 | 1.69B ± 0.12 | 0.28B ± 0.01 | 21.74B ± 6.15 | 15.82B ± 1.02 | 1099.41B ± 88.14 | 482.49B ± 81.64 | |
| Blender 5 | 2.09B ± 0.32 | 0.27B ± 0.03 | 36.65C* ± 5.45 | 15.32B ± 0.62 | 1136.10B ± 201.09 | 416.59B ± 56.89 | |
Similar to kale, the releasable TPC levels of broccoli samples prepared using blenders are significantly higher than those in chopped broccoli extracts. The availability of the TPC from blended broccoli samples ranged from 0.28 to 0.47 mg gallic acid per g of fresh weight. This range was lower than a reported mean of 0.99 mg gallic acid per g of fresh broccoli,37 suggesting that different cultivars, locations (USA versus France) and extraction methods (water extraction versus 70% acetone extraction) might affect the releasable TPC from broccoli. Together, the particle size difference between ground and chopped kale/broccoli samples had a pivotal effect on the food component release. Agreeing with a previous observation, the data from this study showed that enzymes, such as glucosinolate hydrolase, could alter I3C availability.11
3.3. Effects of home food preparation methods on available DPPH radical scavenging capacity
The DPPH˙ scavenging capacities of kale prepared using different blenders and chopping are presented in Table 2S.† Grinding resulted in significantly greater DPPH˙ scavenging capacities of kale extracts as compared to chopping, indicating a strong impact of particle size on the release of DPPH˙ scavenging capacities from kale. DPPH˙ scavenging capacities in kale extracts prepared using blenders ranged from 0.85 to 1.34 μmol Trolox per g fresh kale without microwaving. The DPPH˙ scavenging capacity of dried kale ranges from 44.4 to 59.0 μmol Trolox per g, which is approximately equal to 3.62 to 4.73 μmol Trolox per g fresh kale.38 The DPPH˙ scavenging capacity range observed is lower than but comparable to that reported by Ilyasoğlu and Burnaz.38 The difference in the DPPH˙ scavenging capacities might be due to the different kale cultivars, growing conditions, and extraction methods. There was no difference between the availability of DPPH˙ scavenging capacities of kale extracts prepared using different blenders without microwaving. With microwaving, kale extracts prepared using blenders 1 and 5 significantly differed in their DPPH radical scavenging capacities. Also notable was that kale extracts prepared using blender 1 differed in their DPPH˙ scavenging capacities with and without microwaving. These data indicated a possible enzyme effect (such as the inactivation of glucosinolate hydrolase) on the DPPH˙ scavenging capacity release from kale.The DPPH˙ scavenging capacities of broccoli extracts prepared using blenders were significantly greater than those of the chopped ones. No difference between the blenders in releasing DPPH˙ scavenging capacities from broccoli was observed with or without microwaving. Furthermore, no difference was detected between the microwaved broccoli extract and its un-microwaved counterpart using the same blender. The result indicated that particle size is a crucial factor for the availability of DPPH˙ scavenging capacities from broccoli, and less of an enzyme effect was involved. Together, reducing the particle size and intrinsic enzymes might have a different impact on the DPPH˙ scavenging capacity release from different vegetables.
3.4. Effects of home food preparation methods on available oxygen radical absorbance capacity (ORAC)
The ORAC value ranged from 13.63 to 35.46 μmol Trolox per g of fresh kale (Table 1). This range is comparable with the reported ORAC value of kale (17.7 μmol Trolox per g on the basis of wet weight).4 The availability of the ORAC was significantly greater in kale extracts prepared using blenders as compared to those prepared with chopping. Interestingly, different blenders exhibited significant differences in releasing the ORAC from kale with and without microwaving. For instance, the kale extract prepared using blender 2 exhibited a 2.6-fold greater ORAC as compared to that using blender 5. In addition, the ORAC value of the kale extract prepared using blender 5 was significantly increased (by 2.7-fold) by microwaving immediately after grinding.The ORAC value of broccoli extracts ranged from 15.32 to 17.39 μmol of Trolox per g of fresh broccoli, which is greater than the reported level of 0.50 to 0.55 μmol Trolox per g for fresh broccoli39 depending on the different cultivars and extraction methods. Similar to kale, the ORAC values of broccoli extracts prepared using blenders were significantly greater than those of the chopped broccoli. No difference could be found in the availability of the ORAC value of broccoli extracts prepared using different blenders with or without microwaving. However, microwaving after grinding was able to significantly increase the available level of ORAC values in the broccoli extracts prepared using blender 1 and blender 2, by 1.5-fold and 1.4-fold, respectively. Together, these data indicated that a smaller particle size was associated with a greater available ORAC value from both kale and broccoli, and intrinsic enzymes also might impact the available ORAC from kale and broccoli.
3.5. Effects of home food preparation methods on available hydroxyl radical scavenging capacities (HOSC)
The hydroxyl radical is one of the most reactive species generated in biological systems that could be associated with oxidative damage at the cellular level and numerous chronic diseases.40 The HOSC of kale extracts prepared using different blenders ranged from 1163.52 to 1262.57 μmol Trolox per g fresh kale without microwaving treatment (Table 1). Neither different blenders nor microwaving treatment had any significant effect on the HOSC release from kale.Grinding also resulted in a significant increase of HOSC release in broccoli extracts as compared to chopping. The HOSC value ranged from 389.01 to 508.66 μmol Trolox per g fresh broccoli. Different blenders had the same capability in releasing HOSC from broccoli with or without microwaving. Interestingly, microwaving treatment increased the HOSC release from broccoli when blender 2 was used, suggesting a significant role of enzymes in HOSC release. Together, these data indicated that particle size played an important role in releasing the hydroxyl radical scavenging capacities from both kale and broccoli, while the inactivation of enzymes might impact HOSC release differently for individual vegetable types.
3.6. Effects of home food preparation methods on ABTS cation radical scavenging capacities
Grinding resulted in a higher potential to scavenge ABTS cation radicals as compared to chopping for both kale and broccoli extracts (Table 2S†). The ABTS˙+ scavenging capacities of kale extracts prepared with blenders ranged from 1.00 to 1.42 μmol Trolox per g fresh kale. This range was lower than but comparable to the reported ABTS˙+ scavenging capacity of kale at 6.21 to 6.33 mmoles Trolox per 100 g dried kale,38 which is approximately equal to 5.84 to 5.95 μmol Trolox per g fresh kale. No difference was found among the five blenders in releasing the ABTS˙+ scavenging components from kale with or without microwaving. Microwaving after grinding had no effect on releasing the ABTS˙+ scavenging components from kale.The ABTS˙+ scavenging capacities of the broccoli extracts ranged from 0.91 to 1.24 μmol Trolox per g fresh broccoli, which was lower than the reported range (0.42–2.8 μmol Trolox per g fresh broccoli).41 The ABTS˙+ scavenging capacities of broccoli extracts prepared using blenders 3 and 4 without microwaving were significantly different from each other, suggesting a possible effect of particle size on antioxidant release. In addition, microwaving was able to enhance the ABTS˙+ scavenging component release from broccoli. The data suggested the possible effects of particle size, multiple enzymes and thermal treatment on the ABTS˙+ scavenging component release from vegetables, and these effects may also depend on the vegetable type.
3.7. Effects of home food preparation methods on cell-based antioxidant capacities
The effects of grinding using different blenders on the cell-based antioxidant capacities of kale and broccoli extracts were examined in HepG2/C3A mammalian liver cells. No significant difference between the blenders could be found in releasing cell-based antioxidants from kale and broccoli extracts at an initial concentration of 1 mg fresh vegetable equivalent per mL (data not shown).3.8. Effects of home food preparation methods on available anti-inflammatory capacities
Immune cells such as monocytes and macrophages provide primary defense in response to pathogens.42 In response to the stimulation from the bacterial outer membrane components, such as lipopolysaccharides (LPS), the pattern recognition receptors (PRRs) of macrophages recognize pathogen-associated molecular patterns and stimulate inflammation responses such as the increase of interleukins and COX-2 production.42 The anti-inflammatory effects of compounds from kale and broccoli were reported. I3C attenuated the expression of IL-6 in both Raw 264.7 and THP-1 cells.43 In this study, grinding was not able to release more inhibitors for IL-6 and COX-2 mRNA expressions in the cultured monocytes at an initial concentration of 1 mg fresh vegetable equivalent per mL (data not shown).3.9. Effects of home food preparation methods on available anti-proliferative activities
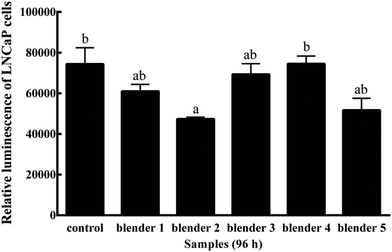
Kale and broccoli have been reported to contain phytochemicals with the potential to reduce the risk of cancer through stimulating cell apoptosis and protecting DNA from damage.44,45 I3C is known as an anti-cancer agent for prostate cells. PC-3 prostate cancer cells were significantly inhibited by I3C at 0.2 mmol L−1,46 which is much higher than the I3C content detected in kale (0.05 mmol L−1) and broccoli (0.02 mmol L−1). In this study, the effects of blenders on prostate cancer cell growth had been investigated. Kale extracts prepared using blender 2 showed a significant inhibition of LNCaP cell growth when compared to the control 96 h after treatment (P ≤ 0.01) (Fig. 2) at the initial treatment concentration of 1 mg fresh vegetable equivalent per mL. Broccoli extracts prepared using different blenders did not show significant anti-proliferative potential in 96 h (data not shown). The data suggested a possible effect of blender selection on releasing anti-proliferative components from vegetables, but a more expensive blender was not necessary to release a greater amount of beneficial properties.It needs to be pointed out that any research has its limitations and will not be able to answer all questions. In this study, I3C was measured as a probe compound to investigate the effects of different food preparation methods on bioactive compound release from kale and broccoli, whereas the extracts were used to determine the processing effects on available bioactivities. It is known that other components potentially in the kale and broccoli extracts, including but not being limited to sulforaphane,47 luteolin,48 endogenous metabolites of vitamin C49,50 and phenolic acids,51 were also reported to have anti-inflammatory or anticancer activities. These compounds could also contribute to the health-beneficial properties of kale and broccoli extracts prepared from the kale and broccoli samples from different preparation methods. Additional research is required to further investigate how food preparation techniques may alter the availability of each of the bioactive compounds and their beneficial activities.
4. Conclusions
The study examined the potential effects of home food preparation approaches on the availability of health-beneficial components using kale and broccoli. The results suggested that home food preparation approaches may alter the release of food bioactives from the vegetable matrix. The type of food matrix (type of vegetables) also may alter the effectiveness of a processing approach on a selected bioactive release. These observations suggest the importance of home food preparation methods on the overall nutritional value and health-beneficial properties of a food product. The observations also suggest the importance of consumer education and communication on the overall values from diets.Conflicts of interest
None declared.Acknowledgements
A gift from Capital Brands, LLC (Los Angeles, CA) and a funding from Beijing Advanced Innovation Center for Food Nutrition and Human Health, the Beijing Excellent Talents Funding for the Youth Scientist Innovation Team.References
- H. R. Vasanthi, S. Mukherjee and D. K. Das, Potential health benefits of broccoli- a chemico-biological overview, Mini-Rev. Med. Chem., 2009, 9, 749–759 CrossRef CAS PubMed.
- I. Herr and M. W. Büchler, Dietary constituents of broccoli and other cruciferous vegetables: Implications for prevention and therapy of cancer, Cancer Treat. Rev., 2010, 36, 377–383 CrossRef CAS PubMed.
- A. D. T. Fabbri and G. A. Crosby, A review of the impact of preparation and cooking on the nutritional quality of vegetables and legumes, Int. J. Gastron. Food Sci., 2016, 3, 2–11 CrossRef.
- G. Cao, E. Sofic and R. L. Prior, Antioxidant capacity of tea and common vegetables, J. Agric. Food Chem., 1996, 44, 3426–3431 CrossRef CAS.
- G. Goldberg, Plants: Diet and Health, Blackwell Science, Oxford, U.K., 2003 Search PubMed.
- N. Hounsome, B. Hounsome, D. Tomos and G. Edwards-Jones, Plant metabolites and nutritional quality of vegetables, J. Food Sci., 2008, 73, R48–R65 CrossRef CAS PubMed.
- S. Graham, J. Marshall, C. Mettlin, T. Rzepka, T. Nemoto and T. Byers, Diet in the epidemiology of breast cancer, Am. J. Epidemiol., 1982, 116, 68–75 CrossRef CAS PubMed.
- S. R. Chinni, Y. W. Li, S. Upadhyay, P. K. Koppolu and F. H. Sarkar, Indole-3-carbinol (I3C) induced cell growth inhibition, G1 cell cycle arrest and apoptosis in prostate cancer cells, Oncogene, 2001, 20, 2927–2936 CrossRef CAS PubMed.
- J. R. Weng, C. H. Tsai, S. K. Kulp and C. S. Chen, Indole-3-carbinol as a chemopreventive and anti-cancer agent, Cancer Lett., 2008, 262, 153–163 CrossRef CAS PubMed.
- T. T. Y. Wang, N. W. Schoene, J. A. Milner and Y. S. Kim, Broccoli-derived phytochemicals indole-3-carbinol and 3,3′-diindolylmethane exerts concentration-dependent pleiotropic effects on prostate cancer cells: Comparison with other cancer preventive phytochemicals, Mol. Carcinog., 2012, 51, 244–256 CrossRef CAS PubMed.
- N. Fujioka, V. Fritz, P. Upadhyaya, F. Kassie and S. S. Hecht, Research on Cruciferous vegetables, Indole-3-Carbinol, and cancer prevention: a tribute to Lee W. Wattenberg, Mol. Nutr. Food Res., 2016, 60, 1228–1238 CAS.
- J. Singh, A. K. Upadhyay, A. Bahadur and K. P. Singh, Dietary antioxidants and minerals in Crucifers, J. Veg. Crop Prod., 2004, 10, 33–41 Search PubMed.
- H. M. Shi, Y. Zhao, J. H. Sun, L. L. Yu and P. Chen, Chemical profiling of glucosinolates in cruciferous vegetables-based dietary supplements using ultra-high performance liquid chromatography coupled to tandem high resolution mass spectrometry, J. Food Compos. Anal., 2017, 61, 67–72 CrossRef CAS.
- A. Podsedek, Natural antioxidants and antioxidant capacity of Brassica vegetables: A review, LWT–Food Sci. Technol., 2007, 40, 1–11 CrossRef CAS.
- J. H. Pan, B. Abernathy, Y. J. Kim, J. H. Lee, J. H. Kim, E. C. Shin and J. K. Kim, Cruciferous vegetables and colorectal cancer prevention through microRNA regulation: A review, Crit. Rev. Food Sci. Nutr., 2017, 1–13 CrossRef PubMed.
- B. Y. Gao, L. Yu, J. Liu, T. T. Y. Wang, Y. H. Luo, L. L. Yu, H. J. Zhang, L. X. Gong and J. Wang, Home-based preparation approaches altered the availability of health beneficial components from carrot and blueberry, Food Sci. Nutr., 2017, 1–12 CrossRef CAS PubMed.
- L. L. Yu, S. Haley, J. Perret and M. Harris, Antioxidant properties of hard winter wheat extracts, Food Chem., 2002, 78, 457–461 CrossRef CAS.
- Z. H. Cheng, J. Moore and L. L. Yu, High-throughput relative DPPH radical scavenging capacity assay, J. Agric. Food Chem., 2006, 54, 7429–7436 CrossRef CAS PubMed.
- J. Moore, J. G. Liu, K. Zhou and L. L. Yu, Effects of genotype and environment on the antioxidant properties of hard winter wheat bran, J. Agric. Food Chem., 2006, 54, 5313–5322 CrossRef CAS PubMed.
- J. Moore, J. J. Yin and L. L. Yu, Novel fluorometric assay for hydroxyl radical scavenging capacity (HOSC) estimation, J. Agric. Food Chem., 2006, 54, 617–626 CrossRef CAS PubMed.
- K. Q. Zhou and L. L. Yu, Antioxidant properties of bran extracts from Trego wheat grown at different locations, J. Agric. Food Chem., 2004, 52, 1112–1117 CrossRef CAS PubMed.
- L. Yu, H. Q. Huang, L. L. Yu and T. T. Y. Wang, Utility of Hesperidinase for food function research: enzymatic digestion of botanical extracts alters cellular antioxidant capacities and anti-inflammatory properties, J. Agric. Food Chem., 2014, 62, 8640–8647 CrossRef CAS PubMed.
- H. Q. Huang, Z. H. Cheng, H. M. Shi, W. B. Xin, T. T. Y. Wang and L. L. Yu, Isolation and characterization of two flavonoids, engeletin and astilbin, from the leaves of Engelhardia roxburghiana and their potential anti-inflammatory properties, J. Agric. Food Chem., 2011, 59, 4562–4569 CrossRef CAS PubMed.
- J. Liu, Y. F. Li, H. M. Shi, T. T. Y. Wang, X. L. Wu, X. J. Sun and L. L. Yu, Components characterization of total tetraploid jiaogulan (Gynostemma pentaphyllum) saponin and its cholesterol-lowering properties, J. Funct. Foods, 2016, 23, 542–555 CrossRef CAS.
- S. E. Trasino, Y. S. Kim and T. T. Y. Wang, Ligand, receptor, and cell type-dependent regulation of ABCA1 and ABCG1 mRNA in prostate cancer epithelial cells, Mol. Cancer Ther., 2009, 8, 1934–1945 CrossRef CAS PubMed.
- M. Slavin, W. Kenworthy and L. L. Yu, Antioxidant properties, phytochemical composition, and antiproliferative activity of Maryland-grown soybeans with colored seed coats, J. Agric. Food Chem., 2009, 57, 11174–11185 CrossRef CAS PubMed.
- Y. C. Chang, J. Riby, G. H. Chang, B. C. Peng, G. Firestone and L. F. Bjeldanes, Cytostatic and antiestrogenic effects of 2-(indol-3-ylmethyl)-3, 3′-diindolylmethane, a major in vivo, product of dietary indole-3-carbinol, Biochem. Pharmacol., 1999, 58, 825–834 CrossRef CAS PubMed.
- G. Y. C. Wong, L. Bradlow, D. Sepkovic, S. Mehl, J. Mailman and M. Osborne, Dose-ranging study of Indole-3-Carbinol for breast cancer prevention, J. Cell. Biochem., 1997, 67, 111–116 CrossRef.
- F. Yuan, D. Z. Chen, K. Liu, D. W. Sepkovic, H. L. Bradlow and K. Auborn, Anti-estrogenic activities of indole-3-carbinol in cervical cells: implication for prevention of cervical cancer, Anticancer Res., 1999, 19, 1673–1680 CAS.
- L. R. Ferguson and R. C. Schlothauer, The potential role of nutritional genomics tools in validating high health foods for cancer control: Broccoli as example, Mol. Nutr. Food Res., 2011, 56, 126–146 Search PubMed.
- M. S. Venkatesh and G. S. V. Raghavan, An overview of microwave processing and dielectric properties of agri-food materials, Biosyst. Eng., 2004, 88, 1–18 CrossRef.
- S. T. Chen, S. H. Chiou and K. T. Wang, Enhancement of chemical reactions by microwave irradiation, J. Chin. Chem. Soc., 1991, 38, 85–91 CrossRef CAS.
- J. Moore, M. Luther, Z. H. Cheng and L. L. Yu, Effects of baking conditions, dough fermentation, and bran particle size on antioxidant properties of whole-wheat pizza crusts, J. Funct. Foods, 2009, 57, 832–839 CAS.
- Y. J. Lu, D. Luthria, E. P. Fuerst, A. M. Kiszonas, L. L. Yu and C. F. Morris, Effect of processing on phenolic composition of dough and bread fractions made from refined and whole wheat flour of three wheat varieties, J. Agric. Food Chem., 2014, 62, 10431–10436 CrossRef CAS PubMed.
- M. Fiol, A. Weckmüller, S. Neugart, M. Schreiner, S. Rohn, A. Krumbein and L. W. Kroh, Thermal-induced changes of kale's antioxidant activity analyzed by HPLC–UV/Vis-online-TEAC detection, Food Chem., 2013, 138, 857–865 CrossRef CAS PubMed.
- A. Korus, Level of Vitamin C, Polyphenols, and Antioxidant and Enzymatic Activity in Three Varieties of Kale (Brassica Oleracea L. Var. Acephala) at Different Stages of Maturity, Int. J. Food Prop., 2011, 14, 1069–1080 CrossRef CAS.
- P. Brat, S. Georgé, A. Bellamy, L. D. Chaffaut, A. Scalbert, L. Mennen, N. Arnault and M. J. Amiot, Daily polyphenol intake in France from fruit and vegetables, J. Nutr., 2006, 136, 2368–2373 CAS.
- H. Ilyasoğlu and N. A. Burnaz, Effect of domestic cooking methods on antioxidant capacity of fresh and frozen kale, Int. J. Food Sci. Technol., 2014, 18, 1298–1305 Search PubMed.
- Q. Liu, C. S. C. Tan, H. Yang and S. F. Wang, Treatment with low-concentration acidic electrolysed water combined with mild heat to sanitise fresh organic broccoli (Brassica oleracea), Food Sci. Technol., 2017, 79, 594–600 CAS.
- Free Radicals in Biology and Medicine, ed. B. Halliwell and J. M. C. Gutteridge, Oxford University Press, New York, 3rd edn, 1989 Search PubMed.
- H. T. Aldrich, P. Kendall, M. Bunning, F. Stonaker, O. Kulen and C. Stushnoff, Environmental temperatures influence antioxidant properties and mineral content in broccoli cultivars grown organically and conventionally, J. AcroCrop Sci., 2011, 2, 1–10 Search PubMed.
- H. Q. Huang, A. Fletcher, Y. G. Niu and T. T. Y. Wang, Characterization of lipopolysaccharide-stimulated cytokine expression in macrophages and monocytes, Inflammation Res., 2012, 61, 1329–1338 CrossRef CAS PubMed.
- M. L. Wang, C. K. Shih, H. P. Chang and Y. H. Chen, Antiangiogenic activity of indole-3-carbinol in endothelial cells stimulated with activated macrophages, Food Chem., 2012, 134, 811–820 CrossRef CAS PubMed.
- C. Bonnesen, I. M. Eggleston and J. D. Hayes, Dietary indoles and isothiocyanates that are generated from cruciferous vegetables can both stimulate apoptosis and confer protection against DNA damage in human colon cell lines, Epidemiol. Prev., 2001, 61, 6120–6130 CAS.
- A. R. Kristal and J. W. Lampe, Brassica vegetables and prostate cancer risk: a review of the epidemiological evidence, Nutr. Cancer, 2002, 42, 1–9 CrossRef PubMed.
- H. R. Frydoonfar, D. R. McGrath and A. D. Spigelman, The effect of indole-3-carbinol and sulforaphane on a prostate cancer cell line, Aust. N. Z. J. Surg., 2003, 73, 154–156 CrossRef.
- K. J. Woo and T. K. Kwon, Sulforaphane suppresses lipopolysaccharide-induced cyclooxygenase-2 (COX-2) expression through the modulation of multiple targets in COX-2 gene promoter, Int. Immunopharmacol., 2007, 7, 1776–1783 CrossRef CAS PubMed.
- K. Shimoi, H. Okada, M. Furugori, T. Goda, S. Takase, M. Suzuki, Y. Hara, H. Yamamoto and N. Kinae, Intestinal absorption of luteolin and luteolin 7-O-beta-glucoside in rats and humans, FEBS Lett., 1998, 438, 220–224 CrossRef CAS PubMed.
- D. Zhang and Y. Hamauzu, Phenolics, ascorbic acid, carotenoids and antioxidant activity of broccoli and their changes during conventional and microwave cooking, Food Chem., 2004, 88, 503–509 CrossRef CAS.
- S. J. Padayatty, A. Katz, Y. H. Wang, P. Eck, O. Kwon, J. Lee, S. L. Chen, C. Corpe, A. Dutta, S. K. Dutta and M. Levine, Vitamin C as an antioxidant: evaluation of its role in disease prevention, J. Am. Coll. Nutr., 2003, 22, 18–35 CrossRef CAS PubMed.
- U. Gawlik-Dziki, M. Jeżyna, M. Świeca, D. Dziki, B. Baraniak and J. Czyż, Effect of bioaccessibility of phenolic compounds on in vitro anticancer activity of broccoli sprouts, Food Res. Int., 2012, 49, 469–476 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7fo00948h |
| This journal is © The Royal Society of Chemistry 2018 |