Evaluation of protective effect of different dietary fibers on polyphenolic profile stability of maqui berry (Aristotelia chilensis (Molina) Stuntz) during in vitro gastrointestinal digestion
Manuel
Viuda-Martos
 *a,
Raquel
Lucas-Gonzalez
a,
Carmen
Ballester-Costa
a,
José A.
Pérez-Álvarez
a,
Loreto A.
Muñoz
*a,
Raquel
Lucas-Gonzalez
a,
Carmen
Ballester-Costa
a,
José A.
Pérez-Álvarez
a,
Loreto A.
Muñoz
 b and
Juana
Fernández-López
a
b and
Juana
Fernández-López
a
aIPOA Research Group. Agro-Food Technology Department. Escuela Politécnica Superior de Orihuela. Miguel Hernández University, Orihuela, Alicante, Spain. E-mail: mviuda@umh.es; Fax: +34966749677; Tel: +34 966749661
bUniversidad Central de Chile, Facultad de Ingeniería, Santiago de Chile, Chile
First published on 18th December 2017
Abstract
The aim of this work was to determine the protective effect of different dietary fibers on (i) the recovery and bioaccessibility indexes, and (ii) the stability of polyphenolic compounds (phenolic acids, flavonoids and anthocyanins) of maqui berry powder subjected to in vitro gastrointestinal digestion (GID). The extracts obtained in each phase (oral, gastric and intestinal) of GID were used to analyze the stability of polyphenolic compounds by HPLC, and the bioaccessibility of these compounds was also determined. At the end of the GID process, the mixture of maqui berry with the different fibers increased the bioaccessibility index of the phenolic and flavonoid compounds in all cases. The results obtained suggest that the anthocyanins and phenolic acids and flavonoid compounds present in maqui are stabilized through dietary fiber interactions, which might provide sufficient levels for absorption during gastrointestinal digestion. The gums sodium carboxymethyl cellulose, xanthan gum and guar gum provided the best protective effect.
Introduction
Some Chilean native berries are underexplored but could probably be used as food matrices to obtain new powdered products for use as ingredients in the development of functional foods. For example, after suitable technological treatment, such powders could be used as natural antioxidative agents due to their high content of bioactive compounds, mainly polyphenolic compounds, vitamins and minerals.One of these Chilean berries is commonly named Chilean wineberry or “maqui berry” (Aristotelia chilensis (Molina) Stuntz). It is a wild, edible berry from central and southern Chile. The berries, which are about 6 mm in diameter, are extremely rich in bioactive compounds, mainly phenolic acids, flavonoids, anthocyanins and vitamins.1 The scientific literature contains numerous reports that mention the health benefits related with maqui evaluation in in vitro or animal models, including the inhibition of inflammation processes, its anti-diabetic and cardio protective effects, the inhibition of adipogenesis and the prevention of low-density lipoprotein oxidation.2–4 As mentioned above, the biological effects of maqui berry could be attributed to its rich source of bioactive compounds, mainly anthocyanins, interest in which has increased after the correlation made between their consumption and a lower risk to develop several chronic diseases.5 The role of anthocyanins as health promoters includes antioxidant, anti-inflammatory and anti-cancer activities and even protective effects against various metabolic, degenerative and cardiovascular diseases.6 However, anthocyanins are very unstable compounds, being very sensitive to temperature, light and changes in pH conditions.7 Thus, as mentioned Manach et al.8 the bioavailability of these substances has been accepted as being very low and, consequently, their study is very complex.
To avoid this problem, the consumption of anthocyanin-rich products along with other food matrices could help to protect these compounds from the degradation produced by the pH variations that occur in the different stages of the digestive process. Therefore, the structure and composition of the food matrix in which anthocyanins are included are factors that can either enhance or prevent the release and stability of these compounds during digestion, affecting their effectiveness.9 In this way, McDougall et al.10 reported that when raspberries a known source of anthocyanins, are consumed with other foods such as bread, cereals, ice-cream or cooked meat, the anthocyanin content is not affected after gastric digestion.
One food component that could protect bioactive compounds during gastric digestion is dietary fiber (DF), which, when in solution, can impact the digestion process. The dominant factors involved in the influence of DF on bioactive compounds digestion are: (i) the physical trapping of the bioactive compounds within structured assemblies, as occurs in fruit tissue, and (ii) the enhanced viscosity of gastric fluids, which restricts the peristaltic mixing process that promotes transport of enzymes to their substrates, bile salts to unmicellized fat, and soluble bioactive compounds to the gut wall.11
In vitro gastrointestinal digestion (GID) models have been used to mimic the events occurring during digestion and offer the opportunity to analyze the effect of physical and chemical parameters and their role in the bioaccessibility of bioactive compounds.12 In addition, GID models provide an alternative to animal and human models for the screening of food ingredients; indeed, in vitro techniques are ethically superior, faster and less expensive than the corresponding in vivo techniques.13 Thus, the aim of this work was to determine the protective effect of different dietary fibers on (i) the recovery and bioaccessibility indexes and (ii) the stability of polyphenolic compounds (phenolic acids, flavonoids and anthocyanins) of maqui berry powder subjected to in vitro gastrointestinal digestion.
Materials and methods
Plant material and dietary fibers
Maqui berry (Aristotelia chilensis (Molina) Stuntz) samples were provided by South-Am Freeze Dry S.A. The following dietary fibers were used: high molecular weight 75–85% deacetylated chitosan (CHF); xanthan gum (XG) from Xanthomonas campestris, G1253, molecular weight ≈2000 kDa and guar gum (GG), all purchased from Sigma-Aldrich Chemical Co. (Steinheim, Germany). Long-chain inulin from chicory (IF) (chain length: 2 to 60, mean Degree of polymerisation documented as ≥10; Orafti GR) provided by BENEO (Morris Plaines, NJ, USA); β-glucan (BG) (Glucagel, 75.6% purity from barley) supplied by PolyCell Technologies LLC, (Crookston, MN, USA); Sodium carboxymethyl cellulose (CMC) (Blanose™ 7H4XF) provided by Ashland Speciality Ingredients, (Covington, USA); Pectin (PF) (GENU pectin type LM-106 AS-YA), from citrus peel with degree of methylation of 18–22% and pKa ∼ 4, supplied by CP Kelco (San Diego, USA) and Cellulose Microcrystalline NF, (CF) purchased from Fagron, Inc. (St Paul, MN, USA).Sample preparation
The model systems were composed by 1 g of maqui berry powder which was mixed in a glass beaker with 4 g of the different dietary fibers analyzed and 40 mL of distilled water then, the blend was shaken for 5 min until a homogenized mixture was obtained. After that, the samples were stored, in the darkness, at 4 °C overnight. The stability of the system was visually analyzed, observing if the presence of syneresis or phase separation occurred. The different samples obtained were: maqui + chitosan (M-CHF); maqui + xanthan gum (M-XG); maqui + guar gum (M-GG); maqui + inulin (M-IF); maqui + β-glucan (M-BG); maqui + sodium carboxymethyl cellulose (M-CMC); maqui + Pectin (M-PF) and maqui + cellulose (M-CF).Simulated in vitro gastrointestinal digestion
In vitro gastrointestinal digestion of samples was performed according to the method described by Gullón et al.14 (Fig. 1). The samples were subjected to simulated oral, gastric and intestinal digestion. Instead of withdrawing aliquots from the reaction vessel at the end of the digestion process, individual digestions were carried out for each phase of digestion. The oral phase was simulated by adding 9 mL of water and 1 mL of 100 U mL−1 α-amylase diluted in 1 mM CaCl2 (adjusted to pH 6.9 with 1 M NaHCO3) to 2.4 g of sample. The mixture was vortexed and incubated at 37 °C for 5 min. For the gastric phase, the pH was adjusted to 2.0 ± 0.05 with HCl (6 M), and 1 mL of pepsin (10.8 mg mL−1 of 0.1 M HCl) was added. The mixture was incubated for 2 h in a shaking water bath at 37 °C and 70 rpm. After each of these phases, the mixtures obtained were centrifuged for 12 min at 8000g at 4 °C, yielding the chyme-soluble fraction (CSF) and the pellet fraction (PF). For the intestinal phase, the pH was adjusted to 7.0 ± 0.05 with NaOH (6 M), and 2.5 mL pancreatin (8 mg mL−1 of 0.5 M NaHCO3) and 2.5 mL bile salt mixture (50 mg mL−1 of 0.5 M NaHCO3) were added. The mixtures were incubated for 2 h in a shaking water bath at 37 °C and 70 rpm. After the incubation, the resulting mixtures were transferred to dialysis membranes with a cut-off weight of 3 kDa and dialyzed overnight against 0.5 M NaHCO3 in a shaking water bath at 37 °C and 70 rpm. At the end of the incubation, the fractions contained in the membranes were collected as unabsorbed fractions (OUT) and the dialysates as the absorbed fractions (IN). All the obtained fractions were lyophilized and stored at −20 °C until analysis.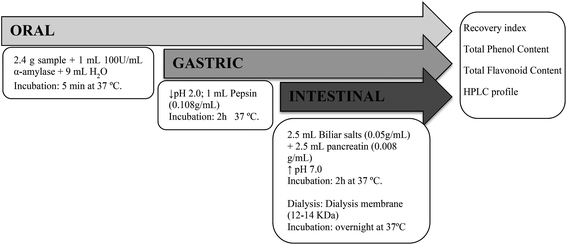 | ||
| Fig. 1 Graphic representation of the static in vitro gastrointestinal digestion procedure carried out with samples. | ||
Recovery index and bioaccessibility index
To determine the effect of the different fibers analyzed on the digestion process of the phenolic and flavonoid compounds two different indexes were studied: the recovery index and the bioaccessibility index. For the recovery index, the indications of Pineda-Vadillo et al.9 were used. Thus, for each phenolic group analyzed, the amount released from the food matrix into the digestive fluids (soluble fraction) and the proportion that remained insoluble after the oral, gastric and intestinal digestions was calculated as follows:| Soluble (%) = (PCS/PCD) × 100 | (i) |
| Insoluble (%) = (PCI/PCD) × 100 | (ii) |
| Total recovery = Soluble (%) + Insoluble (%). | (iii) |
The bioaccessibility percentage was calculated following the indications of Ortega et al.15 For phenolic or flavonoids compounds, the bioaccessibility was defined as the percentage of each group of compounds that was solubilized in IN sample after intestinal digestion phase. Thus, this index defines the proportion of the polyphenolic compounds that could become available for absorption into the systemic circulation:
| Bioaccessibility index (%) = (PCS/PCD) × 100 | (iv) |
Total phenol and total flavonoid content
The total phenol content (TPC) of lyophilized samples obtained during the different phases of in vitro gastrointestinal digestion was determined using Folin–Ciocalteu's reagent,16 while the method based on that described by Blasa et al.17 was used for the total flavonoid content (TFC). Methanolic solutions of lyophilized samples, with concentrations of between 20 and 60 mg mL−1, were used for both analysis. In TPC, gallic acid (GA) was the reference standard and the results were expressed as mg GA equivalents per g sample. In TFC, different concentrations of rutin were used for calibration. The results were expressed in mg rutin equivalents (RE) per g of sample.Determination of polyphenolic compounds
To determine the polyphenolic profile by High Performance Liquid Chromatography (HPLC) of the samples obtained in each phase of in vitro gastrointestinal digestion, the methodology described by Genskowsky et al.1 was followed. The samples were injected into a Hewlett-Packard HPLC series 1200 instrument equipped with C18 column, Mediterranea Sea18, 5 μm particle size (25 × 0.4 cm) (Teknokroma, Barcelona, Spain). Phenolic compounds were analyzed, in standard and sample solutions, using a gradient elution at 1 mL min−1. The mobile phases were composed of a mixture of two solvents. Solvent A contained formic acid in water (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 99, v/v) and solvent B was composed of acetonitrile (100%). The detection was made by UV absorption at 280, 360 and 520 nm. Polyphenolic compound identification was carried out by comparing UV absorption spectra and retention times of each compound with those of pure standards injected in the same conditions. When standards were unavailable, the compounds were tentatively identified by comparing their UV/Vis spectra with previously published data.4,18,19 Anthocyanins were quantified based on the linear curves of authentic standards. All standards used were supplied by Extrasyntehse (Extrasyntehse, Genay, France). Delphinidin-3-glucoside calibration was used for the quantification of delphinidin derivatives, while the cyanidin-3-glucoside calibration was used for cyanidin-derivatives. The estimated concentrations were subsequently multiplied by the respective molecular-weight-correction factor.20
99, v/v) and solvent B was composed of acetonitrile (100%). The detection was made by UV absorption at 280, 360 and 520 nm. Polyphenolic compound identification was carried out by comparing UV absorption spectra and retention times of each compound with those of pure standards injected in the same conditions. When standards were unavailable, the compounds were tentatively identified by comparing their UV/Vis spectra with previously published data.4,18,19 Anthocyanins were quantified based on the linear curves of authentic standards. All standards used were supplied by Extrasyntehse (Extrasyntehse, Genay, France). Delphinidin-3-glucoside calibration was used for the quantification of delphinidin derivatives, while the cyanidin-3-glucoside calibration was used for cyanidin-derivatives. The estimated concentrations were subsequently multiplied by the respective molecular-weight-correction factor.20
Statistical assay
Statistical analysis and comparisons among means were carried out using the statistical package SPSS 19.0 (SPSS Inc., Chicago, IL). All experiments were carried out in triplicate and data are reported as mean ± standard deviation. The differences in mean values between the concentration of bioactive compounds obtained in the different phases of the in vitro gastrointestinal digestion were analyzed by one-way analysis of variance (ANOVA). Tukey's post hoc test was applied for comparison of means, while differences were considered significant at p < 0.05.Results and discussion
Recovery index and bioaccessibility index
Polyphenolic compounds (phenolic acids, flavonoids and anthocyanins) are plant food constituents which are associated to many health benefits. These substances have been demonstrated to reduce the risk of developing several chronic diseases such as cancer, diabetes and cardiovascular disorders.21 Thus, the presence of polyphenolic compounds in foods, especially in fruits, could be particularly important for consumers due to their beneficial health properties.22Table 1 shows the total phenolic content (TPC) and total flavonoid content (TFC) of maqui berry and maqui berry mixed with different dietary fibers. Maqui berry showed the highest TPC (p < 0.05), while the addition of fibers to maqui berry lowered these levels (p < 0.05). However, no differences were found (p > 0.05) between different samples containing the different fibers, except for M-CMC and M-CHF, which showed the lowest (p < 0.05) values. As regard TFC, the behavior varied in the mixture of maqui berry with the different fibers. Thus, M-XG and M-GG showed the highest (p < 0.05) values with no statistical differences (p > 0.05) with maqui berry alone. On the other hand the samples M-CHF and M-CMC again showed the lowest (p < 0.05) TFC values with no statistical differences between them (p > 0.05). In previous works, several authors reported the interactions between different groups of polyphenolic compounds (phenolic acids, flavonoids and anthocyanins) and components such as polysaccharides of dietary fiber,23,24 which may have an important impact on the bioaccessibility and bioavailability of these compounds.
| Maqui | M-CMC | M-PF | M-IF | M-XG | M-GG | M-BG | M-CHF | M-C | |
|---|---|---|---|---|---|---|---|---|---|
| TPC: Total phenolic content, values expressed as mg GAE per g maqui; TFC: total flavonoid content, values expressed as mg RE per g maqui. M-CMC: Maqui + sodium carboxymethylcellulose; M-PF: maqui + Pectin; M-IF: maqui + inulin; M-XG: maqui + xanthan gum; M-GG: maqui + guar gum; M-BG: maqui + β-glucan; M-CHF: maqui + Chitosan and M-CF: maqui + cellulose. Values followed by the same lower case letter within the same row are not significantly different (p > 0.05) according to Tukey's Multiple Range Test. | |||||||||
| TPC | 51.48 ± 0.36a | 39.54 ± 0.14c | 49.72 ± 0.32b | 49.88 ± 0.21b | 50.06 ± 0.18b | 49.99 ± 0.35b | 49.78 ± 0.17b | 33.30 ± 0.44d | 49.50 ± 0.27b |
| TFC | 88.82 ± 0.47a | 53.60 ± 0.41e | 82.80 ± 0.474d | 85.90 ± 0.68b | 88.03 ± 0.24a | 88.58 ± 0.21a | 85.47 ± 0.61b | 54.67 ± 0.39e | 84.55 ± 0.21c |
For this reason the samples were submitted to an in vitro gastrointestinal digestion process. The total phenolic (TP) recovery index obtained after each phase (oral, gastric and intestinal) of in vitro gastrointestinal digestion of maqui berry fruits and maqui berry mixed with different fibers is shown in Fig. 2. After oral digestion, the M-IF sample showed the highest (p < 0.05) recovery index (89.21%) followed by M-CF and M-GG (68.87 and 69.36%, respectively) with no statistically differences (p > 0.05) between them. M-CMC, M-XG and M-CHF had the lowest (p < 0.05) recovery index with no statistical differences between them (p > 0.05). The total recovery index of phenolic compounds of maqui berry after the oral phase was 78.26%; this value was higher than all the maqui samples mixed with different fibers except M-IF. It is important to notice that, in this phase, all samples analyzed showed a higher recovery index (p < 0.05) in the chyme soluble fraction than in the pellet fraction. To understand the low recovery of compounds studied in the digestive media after the oral incubation phase, the composition of the food matrix submitted to digestion should be considered. Some studies have suggested that the presence of fibers could interact with bioactive compounds and affect their release into the digestive media due to their gelation properties.25 Gastric phase had a strong effect on the TP recovery index since the percentage of phenolic compounds recuperated was in all cases higher than 100%, except in the case of M-GG. Thus, M-PF showed the highest (p < 0.05) recovery index (183.8%) followed by M-CMC (176.39%). Again, in all samples analyzed, except M-XG and M-CHF, the recovery index in this phase was higher than that obtained from maqui berry (109.25%). It is important to note that in all samples analyzed, except the M-CMC, the recovery index was higher (p < 0.05) in the chyme soluble fraction than in the pellet fraction. The phenolic compounds released from the test matrix after gastric digestion could be due to the bond of these compounds to fiber being broken. This could be attributed to the acidic pH and enzymatic activity which increases the extractability of polyphenolic compounds (phenolic acids) from the food matrix.26
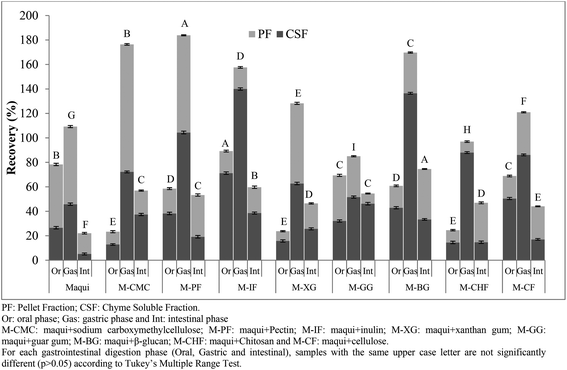 | ||
| Fig. 2 Recovery index of total phenolic content (TPC) obtained after each phase (oral, gastric and intestinal) of in vitro gastrointestinal digestion of maqui berry added to different dietary fibers. | ||
At the end of intestinal phase, the total phenolic recovery was very different from that associated with the gastric phase. M-BG showed the highest (p < 0.05) values followed by M-CMC, M-IF, M-PF and M-GG with no statistical differences between them. In all cases, the recovery index of phenolic compounds was higher than that obtained for maqui berry (22.10%), which indicates the protective effect of dietary fibers on the stability of phenolic compounds during the different phases of gastrointestinal digestion.
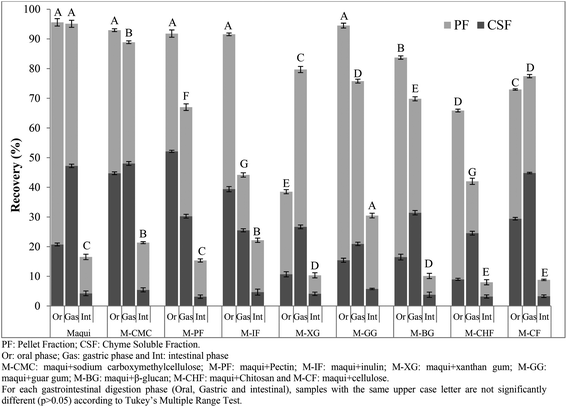
The total flavonoid (TF) recovery index obtained after each phase (oral, gastric and intestinal) of in vitro gastrointestinal digestion of maqui berry fruits mixed with different fibers were shown in Fig. 3. After the oral phase, contrasting results were obtained for the recovery index. On the one hand the maqui berry and M-CMC, M-PF, M-IF and MGG samples showed values close to 100% with no statistically significant differences (p > 0.05) between these samples. On the other hand the samples M-CF, M-CHF and, particularly, M-XG were strongly affected (p < 0.05) with recovery index values of 72.96, 65.86 and 38.52%, respectively. In all the samples analyzed except M-PF, the total flavonoid recovery indexes were higher (p < 0.05) in the pellet fractions than in the chyme soluble fractions. The gastric phase of digestion also produced a variable effect on the recovery index. Thus, the TF recovery index for M-CF and M-XG were greater than the values obtained in the oral phase. On the other hand, the TF recovery index for M-CMC was only slightly affected, while the TF recovery index for M-PF; M-IF, M-GG, M-BG and M-CHF were strongly affected with values of between 42.01 and 79.09%. These results agree with those of Pineda-Vadillo et al.9 who described how the recovery of polyphenolic compounds of grape extracts added to different food matrices decreased after the gastric phase. Nevertheless, the values obtained for all maqui berry samples mixed with different fibers were lower than that those obtained for maqui berry alone (95.10%). It is possible that flavonoid compounds are strongly bound to the fiber matrix and may not be released by the action of digestive enzymes or by the effect of pH, leading to a significant decrease in their concentrations after gastric digestion.
In the last phase of gastrointestinal digestion, the intestinal phase, the total flavonoid recovery index of maqui berry was 16.54%. A decrease in the amount of polyphenols after intestinal digestion has been widely reported by the scientific community.15,26,27 Losses of polyphenolic compounds during intestinal phase of digestion were thought to be due to an increase in pH values as mentioned Sengul et al.28 However, in the samples in which maqui was mixed with sodium carboxymethyl cellulose, inulin or guar gum, the flavonoid recovery indexes were higher (p < 0.05) than those obtained for the maqui berry alone, with values of 21.35, 22.16 and 30.47%, respectively. Thus, the behavior of polyphenolic compounds (phenolic acids and flavonoids) during in vitro gastrointestinal digestion depends on the composition of the matrix in which these compounds are found or added, the resistance and susceptibility of this matrix to digestive enzymes, and the conditions in the gastrointestinal tract, such as pH.29 Additionally, other factors such as (i) chemical reactions, especially oxidation and polymerization, may lead to the formation of other phenolic derivatives (ii) interactions with bile salts, (iii) changes in molecular structures due to enzymatic action and, consequently, solubility could produce drastic losses in the bioactive compounds.30–32 Furthermore, the increase in viscosity due to the ability of the soluble dietary fiber to retain water leads to a reduced diffusion rate of bioactive compounds, which can not be absorbed.33
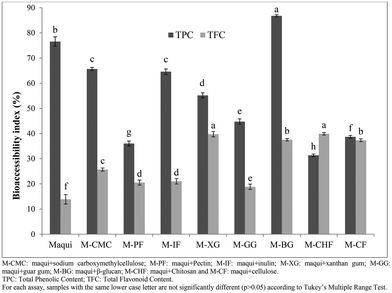
Bioaccessibility and bioavailability are terms related to the release and absorption of nutrients, vitamins, bioactive compounds, and other components from food matrices.34 In this respect, bioaccessibility refers to the correct release of several nutrients and specific bioactive compounds present in the food matrix due to the different processes that occur during gastrointestinal digestion. Fig. 4 shows the bioaccessibility index of the total phenolic content and total flavonoid content obtained after the intestinal phase of in vitro gastrointestinal digestion of maqui berry mixed with different dietary fibers. As regards to total phenolic content, M-BG had the highest (p < 0.05) bioaccessibility index (86.77%) followed by the M-CMC and M-IF (bioaccessibility index of 65.66 and 64.60%, respectively) with no statistical differences (p > 0.05) between them. The M-CHF showed the lowest (p < 0.05) bioaccessibility index (31.37%). With reference to the bioaccessibility index of the total flavonoid content (Fig. 4), M-CHF, M-XG and M-BG showed the highest values (p < 0.05) with no statistical differences between them (p > 0.05), while the M-PF and M-GG had the lowest (p < 0.05) bioaccessibility indexes (20.52 and 18.84% respectively) with no statistical differences (p > 0.05) between them.
The bioaccessibility index of phenolic and flavonoid compounds present in maqui berry after the last phase of gastrointestinal digestion was 76.55 and 13.87%, respectively, values similar to those reported by Lucas-Gonzalez et al.22 who mentioned that the bioaccessibility index of phenolic and flavonoid compounds of maqui berry were 78.19 and 14.10% respectively. In all cases, the mixture of maqui berry with different fibers increased the bioaccessibility index of the flavonoid compounds, which might be explained by a protective effect afforded by the different fibers to the flavonoid compounds in the upper phases of gastrointestinal digestion.
On the other hand, the bioaccessibility index of phenolic compounds was reduced when the maqui berry was mixed with the different fibers, except in the case of β-glucan. This phenomenon could be explained by interaction of phenolic compounds with the fiber matrix during the development of the gastrointestinal digestion of maqui berry, which influenced their bioaccessibility. These results agree with Sengul et al.,28 who mentioned that several carbohydrates, such as starch, gelatinized starch, cellulose or pectin, had adverse effects on the total phenolic content in the last phase of gastrointestinal digestion.
Nevertheless, as mentioned by Cummings et al.35 the release of bioactive compounds from the fiber matrix into the surrounding intestinal fluids is inversely proportional to particle size and directly proportional to the solute gradient, in this case all samples had a particle size lower than 40 mesh. Palafox-Carlos et al.36 mentioned that it is also affected by the following factors: the physical state of the solute (for example, whether it is present in solid form or is already dissolved in water trapped within the particle); the physical structure of the particle (i.e. whether it is readily deformed, like a sponge, so that dissolved solids can be squeezed out by peristaltic contractions, or rigid, so that solutes must diffuse out); and the surface properties of the particle (i.e. surface-tension effects).37
Stability of polyphenolic compounds during simulated in vitro gastrointestinal digestion
Table 2 showed the polyphenolic profile of maqui berry and maqui berry extracts mixed with different fibers. A total of nineteen polyphenolic compounds were found in maqui berry, identified as anthocyanins (eight compounds), flavonols (ten compounds) and ellagitannins (one compound). In maqui berry samples mixed with different fibers fourteen compounds were identified, comprising anthocyanins (eight compounds); flavonols (five compounds), and ellagitannins (one compound). As regards the anthocyanins content, only delphinidin and cyanidin derivatives were identified in all the samples analyzed, which agrees with the findings of several authors.1,4 In M-IF, M-PF, M-CHF and MCF, delphinidin-3-sambubioside was the major (p < 0.05) anthocyanin with values between 0.97 and 7.12 mg g−1 maqui, while in M-GG and M-BG the predominant anthocyanin (p < 0.05) was delphinidin-3-glucoside. In M-CMC and M-XG no differences were found between delphinidin-3-sambubioside and delphinidin-3-glucoside. In all the samples in which the maqui was mixed with the different fibers, the values obtained for all anthocyanins identified were lower (p < 0.05) than those obtained in maqui berry alone. This phenomenon could be explained by binding interactions with the fiber. Thus, Padayachee et al.25 found that several dietary fibers such as cellulose and pectin are able to bind anthocyanins. As regards non-anthocyanin compounds, ellagic acid was found in the highest (p < 0.05) concentration in all samples except M-CHF. Quercetin and quercetin-derivatives (three compounds) and myricetin and myricetin-derivatives (two compounds) were also identified in all the samples analyzed. Again, all the non-anthocyanin compounds identified in the samples in which the maqui was mixed with fibers had lower values (p < 0.05) than in the samples of maqui berry. This might be attributed to physicochemical interactions that probably occur between these compounds and the components of the different fibers assayed, causing the polyphenolic compounds to be physically trapped, thus affecting their quantification.36| Compound | Maqui | M-IF | M-PF | M-CMC | M-XG | M-GG | M-CHF | M-CF | M-BG |
|---|---|---|---|---|---|---|---|---|---|
| Values expressed as mg g−1 maqui. M-CMC: Maqui + sodium carboxymethylcellulose; M-PF: maqui + Pectin; M-IF: maqui + inulin; M-XG: maqui + xanthan gum; M-GG: maqui + guar gum; M-BG: maqui + β-glucan; M-CHF: maqui + Chitosan and M-CF: maqui + cellulose. Values followed by the same lower case letter within the same row are not significantly different (p > 0.05) according to Tukey's Multiple Range Test. Values followed by the same upper case letter within the same column are not significantly different (p > 0.05) according to Tukey's Multiple Range Test. n.d.: Non detected. | |||||||||
| Delphinidin-3-sambubioside-5-glucoside | 2.99 ± 0.04Fa | 0.37 ± 0.02Fd | 0.44 ± 0.03Gc | 0.31 ± 0.02Ed | 0.57 ± 0.03Cb | 0.62 ± 0.06Eb | 0.44 ± 0.04Dc | 0.65 ± 0.08Eb | 0.40 ± 0.02Fc |
| Delphinidin-3,5-diglucoside | 4.35 ± 0.06Ca | 0.84 ± 0.03Ee | 0.61 ± 0.06Ff | 0.44 ± 0.04Dg | 0.40 ± 0.03Dg | 1.36 ± 0.07Cb | 0.66 ± 0.03Cf | 1.09 ± 0.06Cc | 0.91 ± 0.05Cd |
| Cyanidin-3-sambubioside-5-glucoside | 2.79 ± 0.12Fa | 1.28 ± 0.07Db | 1.20 ± 0.03Db | 0.84 ± 0.02Bc | 0.41 ± 0.03Dd | 0.35 ± 0.02Fd | 0.41 ± 0.04Dd | 0.84 ± 0.05Dc | 0.48 ± 0.02Fd |
| Cyanidin-3,5-diglucoside | 4.05 ± 0.10Da | 2.05 ± 0.13Cb | 2.24 ± 0.10Cb | 0.71 ± 0.05Cc | 0.81 ± 0.04Bc | 0.56 ± 0.02Ed | 0.76 ± 0.03Bc | 0.40 ± 0.02Fe | 0.46 ± 0.03Fe |
| Delphinidin-3-sambubioside | 7.25 ± 0.14Ba | 7.12 ± 0.15Aa | 3.94 ± 0.11Ab | 1.30 ± 0.06Af | 1.42 ± 0.08Af | 2.05 ± 0.11Bd | 0.97 ± 0.03Ag | 3.53 ± 0.05Ac | 1.75 ± 0.08Be |
| Delphinidin-3-glucoside | 7.89 ± 0.14Aa | 4.31 ± 0.10Bb | 2.88 ± 0.12Bc | 1.34 ± 0.08Ad | 1.28 ± 0.11Ad | 2.83 ± 0.14Ac | 0.78 ± 0.06Be | 2.85 ± 0.12Bc | 3.04 ± 0.14Ac |
| Cyanidin-3-sambubioside | 3.48 ± 0.06Ea | 2.30 ± 0.08Cb | 1.29 ± 0.06Dc | 0.44 ± 0.06Df | 0.34 ± 0.04Df | 0.51 ± 0.04Ee | 0.36 ± 0.03DEf | 0.98 ± 0.06Cd | 0.62 ± 0.06Ee |
| Cyanidin-3-glucoside | 1.99 ± 0.05Ga | 1.12 ± 0.04Db | 0.80 ± 0.03Ec | 0.52 ± 0.02De | 0.39 ± 0.03Df | 0.80 ± 0.03Dc | 0.32 ± 0.03Ef | 0.88 ± 0.03Dc | 0.71 ± 0.04Dd |
| Myricetin-3-galactoside | 0.05 ± 0.01Ma | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Myricetin-3-glucoside | 0.17 ± 0.02Ka | 0.11 ± 0.03Gab | 0.13 ± 0.02Iab | 0.08 ± 0.02Gb | 0.14 ± 0.02Eab | 0.13 ± 0.01Gab | 0.13 ± 0.02Gab | 0.09 ± 0.03Gb | 0.09 ± 0.02Hb |
| Quercetin-galloyl-hexoside | 0.16 ± 0.01K | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Quercetin-galloyl-hexoside | 0.12 ± 0.01L | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Rutin | 0.45 ± 0.06Ja | 0.36 ± 0.02Fab | 0.28 ± 0.04Hb | 0.13 ± 0.02Fc | 0.15 ± 0.01Ec | 0.16 ± 0.03Gc | 0.19 ± 0.03Fc | 0.11 ± 0.03Gc | 0.15 ± 0.02Gc |
| Ellagic acid | 0.94 ± 0.04Ha | 0.76 ± 0.08Eb | 0.83 ± 0.06Eb | 0.61 ± 0.06Cc | 0.38 ± 0.04Dd | 0.50 ± 0.04Ec | 0.22 ± 0.06Fe | 0.56 ± 0.04Ec | 0.60 ± 0.05Ec |
| Quer-3-glucoside | 0.10 ± 0.02L | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Quer-3-xyloside | 0.03 ± 0.01M | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Dimethoxy-quercetin | 0.51 ± 0.01Ia | 0.36 ± 0.02Fb | 0.25 ± 0.07Hb | 0.30 ± 0.03Eb | 0.29 ± 0.04Dbc | 0.36 ± 0.03Fb | 0.23 ± 0.03Fc | 0.41 ± 0.05Fb | 0.39 ± 0.05Fb |
| Myricetin | 0.11 ± 0.03La | 0.07 ± 0.01Gab | 0.05 ± 0.01Jb | 0.07 ± 0.01Gab | 0.07 ± 0.03Fab | 0.05 ± 0.01Hb | 0.06 ± 0.01Hb | 0.07 ± 0.02Gab | 0.07 ± 0.03Hab |
| Quercetin | 0.09 ± 0.02La | 0.04 ± 0.01Hb | 0.04 ± 0.01Jb | 0.03 ± 0.01Hb | 0.07 ± 0.01Fab | 0.06 ± 0.02Hab | 0.05 ± 0.01Hb | 0.04 ± 0.01Gb | 0.06 ± 0.01Hab |
To ascertain the stability of polyphenolic compounds of maqui berry and maqui berry extracts mixed with different fibers, the physical and chemical processes of the human digestion were simulated. After the oral phase (Table 3), the same compounds identified in the undigested sample were identified in the digested sample, although at lower concentrations (p < 0.05) with respect to the undigested sample which showed the slight release of phenolic compounds present in the matrix. Again, delphinidin-3-sambubioside and delphinidin-3-glucoside were found in the highest (p > 0.05) concentrations. In the maqui berry mixed with different fibers (Table 3) only eleven compounds were identified as polyphenolic compounds. These corresponded to seven anthocyanins, except in samples M-GG, M-CHF and M-BG (six anthocyanins) and four non-anthocyanins except in samples M-GG, M-CF and M-BG (three compounds). Of the anthocyanin compounds, delphinidin-3-sambubioside was the main component (p < 0.05) in all the samples analyzed with values ranging between 0.63 and 5.57 mg g−1 maqui except in M-BG, M-CMC and M-GG, where the predominant anthocyanin was delphinidin-3-glucoside (p < 0.05). In general term, all the anthocyanins identified in the maqui mixed with the fibers showed lower concentrations (p < 0.05) than the corresponding undigested samples. The decrease in concentration of main components, delphinidin-3-sambuboside, ranged between 15.92 and 39.42%, the M-CMC and M-CHF samples showing the greatest decrease (39.42 and 34.00%, respectively). In the case of delphinidin-3-glucoside, a decrease of between 7.93 and 43.46% was found, M-CMC and M-CHF again showing the greatest decrease (41.28 and 43.46%, respectively). As regard the non-anthocyanins, all showed lower concentrations (p < 0.05) with respect to the undigested sample. Thus, ellagic acid, the main component, decreased between 8.14 and 53.58%, while dimethoxy-quercetin decreased between 7.25 and 31.08% compared with the undigested sample (p < 0.05). These values confirm the effect that fiber has on the retention of these compounds in their matrix by means of different mechanisms, such as hydrogen bonding, covalent bonding or hydrophobic interactions, which render them unavailable for release in this phase. As mentioned by Quirós-Sauceda et al.38 the composition, functional group substitution and physical properties of the fibers are key factors in their interaction with polyphenolic compounds.
| Compound | Maqui | M-IF | M-PF | M-CMC | M-XG | M-GG | M-CHF | M-CF | M-BG |
|---|---|---|---|---|---|---|---|---|---|
| Values expressed as mg g−1 maqui. M-CMC: Maqui + sodium carboxymethylcellulose; M-PF: maqui + Pectin; M-IF: maqui + inulin; M-XG: maqui + xanthan gum; M-GG: maqui + guar gum; M-BG: maqui + β-glucan; M-CHF: maqui + Chitosan and M-CF: maqui + cellulose. Values followed by the same upper case letter within the same column are not significantly different (p > 0.05) according to Tukey's Multiple Range Test. Values followed by the same lower case letter within the same row are not significantly different (p > 0.05) according to Tukey's Multiple Range Test. n.d.: Non detected. | |||||||||
| Delphinidin-3-sambubioside-5-glucoside | 1.73 ± 0.01D | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Delphinidin-3,5-diglucoside | 2.24 ± 0.04Ba | 0.46 ± 0.08F | 0.42 ± 0.06Fe | 0.31 ± 0.03Cf | 0.53 ± 0.04Ce | 1.09 ± 0.04Cb | 0.47 ± 0.05Be | 0.80 ± 0.05Cc | 0.66 ± 0.08Cd |
| Cyanidin-3-sambubioside-5-glucoside | 1.47 ± 0.06Ea | 1.08 ± 0.17Db | 0.58 ± 0.03Ec | 0.35 ± 0.02Cd | 0.12 ± 0.03Ef | 0.35 ± 0.03Ed | 0.35 ± 0.03Cd | 0.57 ± 0.04Dc | 0.25 ± 0.03Fe |
| Cyanidin-3,5-diglucoside | 2.05 ± 0.05Ca | 1.69 ± 0.13Cb | 1.63 ± 0.10Cb | 0.30 ± 0.03Cc | 0.41 ± 0.07Dc | n.d. | n.d. | n.d. | n.d. |
| Delphinidin-3-sambubioside | 3.16 ± 0.15Ab | 5.57 ± 0.25Aa | 3.02 ± 0.13Ac | 0.79 ± 0.04Bg | 1.09 ± 0.08Af | 1.73 ± 0.07Bd | 0.63 ± 0.05Ah | 2.96 ± 0.05Ac | 1.36 ± 0.08Be |
| Delphinidin-3-glucoside | 3.26 ± 0.25Aa | 3.60 ± 0.19Ba | 2.65 ± 0.14Bb | 0.84 ± 0.03Ad | 0.97 ± 0.08Bd | 2.24 ± 0.11Ac | 0.57 ± 0.06Ae | 2.45 ± 0.07Bb | 2.50 ± 0.19Ab |
| Cyanidin-3-sambubioside | 0.65 ± 0.11Fc | 1.59 ± 0.24Ca | 0.94 ± 0.08Db | 0.22 ± 0.06De | 0.17 ± 0.02Ee | 0.36 ± 0.02Ed | 0.22 ± 0.03De | 0.73 ± 0.03Cc | 0.35 ± 0.04Ed |
| Cyanidin-3-glucoside | 0.61 ± 0.02Fb | 0.73 ± 0.09Ea | 0.61 ± 0.07Eb | 0.17 ± 0.02Dd | 0.17 ± 0.01Ed | 0.43 ± 0.03Dc | 0.17 ± 0.02D | 0.53 ± 0.05Db | 0.50 ± 0.04Dbc |
| Myricetin-3-galactoside | 0.02 ± 0.01H | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Myricetin-3-glucoside | 0.08 ± 0.01D | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Quercetin-galloyl-hexoside | 0.05 ± 0.01F | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Quercetin-galloyl-hexoside | 0.05 ± 0.01F | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Rutin | 0.24 ± 0.03Ba | 0.22 ± 0.04Ha | 0.20 ± 0.02Ia | 0.02 ± 0.00Ec | 0.03 ± 0.01Gc | n.d. | 0.07 ± 0.02Fb | n.d. | n.d. |
| Ellagic acid | 0.36 ± 0.02Aa | 0.34 ± 0.08Ga | 0.32 ± 0.06Ga | 0.18 ± 0.05Db | 0.06 ± 0.01Fc | 0.22 ± 0.01Fb | 0.04 ± 0.01Fc | 0.31 ± 0.04Ea | 0.24 ± 0.04Fb |
| Quer-3-glucoside | 0.05 ± 0.01G | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Quer-3-xyloside | 0.02 ± 0.00I | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Dimethoxy-quercetin | 0.27 ± 0.04Ca | 0.25 ± 0.03Hab | 0.25 ± 0.04Hab | 0.21 ± 0.02Db | 0.06 ± 0.01Fc | 0.19 ± 0.03Fb | 0.12 ± 0.02Ec | 0.19 ± 0.03Fb | 0.21 ± 0.01Fb |
| Myricetin | 0.04 ± 0.01G | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Quercetin | 0.08 ± 0.01Ea | 0.07 ± 0.02Ia | 0.07 ± 0.01Ja | 0.03 ± 0.01Eb | 0.07 ± 0.01Fa | 0.04 ± 0.01Gb | 0.05 ± 0.02Fab | 0.06 ± 0.01Gab | 0.07 ± 0.01Ga |
In the gastric phase (Table 4) again, the same compounds identified in the undigested sample were identified in the digested maqui sample. Although concentrations were much lower than in the undigested sample, all the anthocyanins identified showed an increase (p < 0.05) in concentration compared with the oral phase values. This behavior is the same as that shown by the maqui samples mixed with different fibers. The compounds identified in the oral phase were again identified after gastric digestion but with higher concentrations. As mentioned by Pineda-Vadillo et al.9 the high stability of polyphenols against degradation in acidic gastric media maintained these compounds practically unaltered during the gastric phase. In this phase, delphinidin-3-glucoside was the main component (p < 0.05) quantified in all the samples analyzed except in M-PF and M-CF, where the predominant anthocyanin was delphinidin-3-sambubioside. Of note is the higher concentration (p < 0.05) of delphinidin-3-sambubioside and delphinidin-3-gluoside in M-CMC and M-XG than in the undigested maqui. This fact could be explained by the enzymatic activity and/or pH conditions which would help break down the high molecular weight phenols that may initially be bound to fiber.
| Compound | Maqui | M-IF | M-PF | M-CMC | M-XG | M-GG | M-CHF | M-CF | M-BG |
|---|---|---|---|---|---|---|---|---|---|
| Values expressed as mg g−1 maqui. M-CMC: Maqui + sodium carboxymethylcellulose; M-PF: maqui + Pectin; M-IF: maqui + inulin; M-XG: maqui + xanthan gum; M-GG: maqui + guar gum; M-BG: maqui + β-glucan; M-CHF: maqui + Chitosan and M-CF: maqui + cellulose. Values followed by the same upper case letter within the same column are not significantly different (p > 0.05) according to Tukey's Multiple Range Test. Values followed by the same lower case letter within the same row are not significantly different (p > 0.05) according to Tukey's Multiple Range Test. n.d.: Non detected. | |||||||||
| Delphinidin-3-sambubioside-5-glucoside | 2.18 ± 0.01E | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Delphinidin-3,5-diglucoside | 3.87 ± 0.00Ca | 0.60 ± 0.02Ee | 0.58 ± 0.09Ce | 0.49 ± 0.08Ee | 1.05 ± 0.11Ccd | 0.86 ± 0.09Cd | 1.01 ± 0.12Dc | 1.05 ± 0.12Dcd | 1.22 ± 0.17Cb |
| Cyanidin-3-sambubioside-5-glucoside | 1.96 ± 0.06Fa | 1.46 ± 0.11Cb | n.d. | 1.74 ± 0.15Da | 0.78 ± 0.07Dd | 0.47 ± 0.07Dd | 0.77 ± 0.11DEd | 0.55 ± 0.08Ed | 1.29 ± 0.14Cc |
| Cyanidin-3,5-diglucoside | 2.79 ± 0.01Da | 1.75 ± 0.14Bb | n.d. | 2.47 ± 0.27Ca | 1.21 ± 0.14Cc | n.d. | 1.37 ± 0.14Cc | 1.37 ± 0.17Cc | n.d. |
| Delphinidin-3-sambubioside | 4.87 ± 0.04Bc | 4.58 ± 0.20Ac | 2.31 ± 0.17Ad | 5.69 ± 0.23Bb | 4.75 ± 0.18Bc | 2.29 ± 0.24Ba | 2.53 ± 0.21Bd | 4.72 ± 0.19Ac | 4.28 ± 0.13Bc |
| Delphinidin-3-glucoside | 5.26 ± 0.03Ab | 4.70 ± 0.13Ac | 0.67 ± 0.09Bf | 7.40 ± 0.03Aa | 5.31 ± 0.12Ab | 4.25 ± 0.21Ac | 3.15 ± 0.11Ad | 2.71 ± 0.14Be | 4.78 ± 0.14Ab |
| Cyanidin-3-sambubioside | 1.53 ± 0.01Ha | 1.00 ± 0.09Db | 0.53 ± 0.04Cd | 1.63 ± 0.12Da | 0.91 ± 0.09Cc | 0.92 ± 0.11Cc | 0.81 ± 0.09Dc | 0.44 ± 0.07Fd | 1.12 ± 0.08Cb |
| Cyanidin-3-glucoside | 1.89 ± 0.00Ga | 0.93 ± 0.06Dc | 0.16 ± 0.02Ee | 1.52 ± 0.19Db | 1.03 ± 0.14Cc | 0.93 ± 0.11Cc | 0.63 ± 0.05Ed | 0.95 ± 0.11Dc | 1.05 ± 0.21Cc |
| Myricetin-3-galactoside | 0.04 ± 0.01M | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Myricetin-3-glucoside | 0.11 ± 0.00L | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Quercetin-galloyl-hexoside | 0.08 ± 0.00L | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Quercetin-galloyl-hexoside | 0.08 ± 0.01L | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Rutin | 0.22 ± 0.02Ka | n.d | 0.10 ± 0.02Eb | 0.17 ± 0.03Fa | 0.11 ± 0.02Gb | 0.06 ± 0.01Fc | 0.14 ± 0.03Gb | n.d. | n.d. |
| Ellagic acid | 0.47 ± 0.04Ia | 0.35 ± 0.04Fb | 0.49 ± 0.07Ca | 0.42 ± 0.08Ea | 0.35 ± 0.03Eb | 0.36 ± 0.07Db | 0.30 ± 0.06F | 0.50 ± 0.02Ea | 0.39 ± 0.04Db |
| Quer-3-glucoside | 0.02 ± 0.00K | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Quer-3-xyloside | 0.08 ± 0.01L | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Dimethoxy-quercetin | 0.23 ± 0.00Ma | 0.21 ± 0.06Ga | 0.25 ± 0.03Da | 0.28 ± 0.09Fa | 0.24 ± 0.06Fa | 0.25 ± 0.03Ea | 0.19 ± 0.04Ga | 0.28 ± 0.02Ga | 0.27 ± 0.02Ea |
| Myricetin | 0.32 ± 0.03J | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Quercetin | 0.06 ± 0.01Lc | 0.07 ± 0.01Hc | 0.10 ± 0.02Ec | 0.21 ± 0.03Fa | 0.10 ± 0.04Gc | 0.18 ± 0.04Eab | n.d. | 0.16 ± 0.03Hb | 0.25 ± 0.03Ea |
As regards non-anthocyanins compounds, there was a slight increase (p < 0.05) in their concentrations over the values obtained in oral digestion phase, as occurred with anthocyanins. In any case, there was a decrease (p < 0.05) in concentrations with respect to the values of test matrix. The decrease in the concentration of polyphenolic compounds, seen in all the assayed samples, compared with undigested samples could be due to the fact that these compounds can form insoluble complexes. They bind to components of the pancreatin or bile salts present in the medium, which causes changes in their molecular weight, solubility and chemical structure.10,39
In the last phase of gastrointestinal digestion (Table 5), a drastic reduction in polyphenolic compounds was observed. In maqui berry and M-PF, M-CMC, M-XG, M-GG and M-BG, only six compounds were identified (delphinidin-3,5-diglucoside, cyanidin-3,5-diglucoside, delphinidin-3-sambubioside, delphinidin-3-glucoside, ellagic acid and dimethoxy-quercetin). As regards the anthocyanins, delphinidin-3-glucoside showed the highest (p < 0.05) concentration in all the analyzed samples, followed by delphinidin-3-sambubioside. Note that in the IN samples (serum-available) no anthocyanins were found, which agrees with the findings of Cebeci and Şahin-Yeşilçubuk,40 who reported that anthocyanins were not detected in the IN fractions of blueberry and blueberry added to milk or oat. This could be explained by the instability of anthocyanins at basic pH values as mentioned by several authors.41,42 The fact that anthocyanins were not detected in the IN fraction of intestinal digestion does not imply the complete loss of these compounds. Thus, in the OUT samples (colon-available) the three gums (CMC, XG and GG) showed the highest values for the anthocyanins identified, perhaps because the structure formed by these gums protects the anthocyanins from alkaline pH values, thus preventing their deterioration. This could be regarded as being beneficial because these compounds are transported to the large intestine, where fibrous material is fermented by gut bacteria.24,25
| Compound | Maqui | M-IF | M-PF | M-CMC | M-XG | M-GG | M-CHF | M-CF | M-BG | |
|---|---|---|---|---|---|---|---|---|---|---|
| Values expressed as μg g−1 maqui. M-CMC: Maqui + sodium carboxymethylcellulose; M-PF: maqui + Pectin; M-IF: maqui + inulin; M-XG: maqui + xanthan gum; M-GG: maqui + guar gum; M-BG: maqui + β-glucan; M-CHF: maqui + Chitosan and M-CF: maqui + cellulose. Values followed by the same upper case letter within the same column are not significantly different (p > 0.05) according to Tukey's Multiple Range Test. Values followed by the same lower case letter within the same row are not significantly different (p > 0.05) according to Tukey's Multiple Range Test. n.d.: Non detected. | ||||||||||
| Delphinidin-3,5-diglucoside | IN | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| OUT | 5.23 ± 0.06 | n.d. | 4.52 ± 0.03 | 5.99 ± 0.07 | 5.78 ± 0.06 | 5.63 ± 0.06 | n.d. | n.d. | 4.62 ± 0.02 | |
| Total | 5.23 ± 0.06dE | — | 4.52 ± 0.03eE | 5.99 ± 0.07aD | 5.78 ± 0.06bF | 5.63 ± 0.06cE | — | — | 4.62 ± 0.02eE | |
| Cyanidin-3,5-diglucoside | IN | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| OUT | 3.69 ± 0.05 | n.d. | 4.56 ± 0.08 | 5.69 ± 0.07 | 5.52 ± 0.05 | 5.63 ± 0.02 | n.d. | n.d. | 3.85 ± 0.07 | |
| Total | 3.69 ± 0.05cF | — | 4.56 ± 0.08bE | 5.69 ± 0.07aE | 5.52 ± 0.05aE | 5.63 ± 0.02aE | — | — | 3.85 ± 0.07cF | |
| Delphinidin-3-sambubioside | IN | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| OUT | 15.23 ± 0.12 | 11.85 ± 0.12 | 11.67 ± 0.06 | 19.88 ± 0.09 | 20.15 ± 0.07 | 20.47 ± 0.05 | n.d. | n.d. | 16.98 ± 0.06 | |
| Total | 15.23 ± 0.12eD | 11.85 ± 0.12fD | 11.67 ± 0.06dD | 19.88 ± 0.09cD | 20.15 ± 0.07bD | 20.47 ± 0.05aD | — | — | 16.98 ± 0.06dD | |
| Delphinidin-3-glucoside | IN | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| OUT | 18.24 ± 0.12 | 16.23 ± 0.12 | 22.36 ± 0.12 | 25.39 ± 0.12 | 24.89 ± 0.12 | 27.66 ± 0.12 | n.d. | 19.89 ± 0.12 | 21.22 ± 0.12 | |
| Total | 18.24 ± 0.12eC | 16.23 ± 0.12fC | 22.36 ± 0.12cB | 25.39 ± 0.12bB | 24.89 ± 0.12bB | 27.66 ± 0.12aB | — | 19.89 ± 0.12dB | 21.22 ± 0.12cB | |
| Ellagic acid | IN | 12.02 ± 0.06 | n.d. | 16.31 ± 0.02 | 15.07 ± 0.06 | 14.15 ± 0.06 | 7.89 ± 0.11 | n.d. | n.d. | 11.35 ± 0.04 |
| OUT | 28.21 ± 0.09 | 45.23 ± 0.09 | 30.28 ± 0.07 | 33.59 ± 0.07 | 31.29 ± 0.06 | 27.69 ± 0.09 | 30.28 ± 0.05 | 32.25 ± 0.07 | 27.98 ± 0.09 | |
| Total | 40.23 ± 1.11dA | 45.23 ± 0.09cA | 46.59 ± 0.06bA | 48.66 ± 0.12aA | 45.44 ± 0.09cA | 35.58 ± 0.08eA | 30.28 ± 0.05gA | 32.25 ± 0.07fA | 39.33 ± 0.08dA | |
| Dimethoxy-quercetin | IN | 8.86 ± 0.03 | n.d. | 6.69 ± 0.02 | 10.85 ± 0.04 | 8.69 ± 0.04 | 9.35 ± 0.05 | n.d. | n.d. | 7.54 ± 0.04 |
| OUT | 11.03 ± 0.04 | 17.22 ± 0.05 | 7.87 ± 0.07 | 13.78 ± 0.09 | 14.56 ± 0.02 | 13.21 ± 0.04 | 17.56 ± 0.05 | 15.32 ± 0.05 | 12.09 ± 0.03 | |
| Total | 19.89 ± 0.05dB | 17.22 ± 0.05eB | 14.56 ± 0.04gC | 24.63 ± 0.07aB | 23.25 ± 0.04bC | 22.56 ± 0.04cC | 17.56 ± 0.05eB | 15.32 ± 0.05fC | 19.63 ± 0.02dC | |
As regard non-anthocyanins (Table 5) only two compounds were identified (ellagic acid and dimethoxy-quercetin) in all the samples of maqui or maqui mixed with different fibers. In both the IN and OUT fractions, dimethoxy-quercetin was the main (p < 0.05) component followed by ellagic acid. As occurs with anthocyanins, CMC, XG and GG had the highest values (p < 0.05) for the non-anthocyanin compounds identified.
It is important to notice that in all the samples analyzed, expect M-IF, M-CHF and M-CF, both ellagic acid and dimethoxy-quercetin compounds were detected in the IN fraction (serum-available) samples. However, the concentrations were higher (p < 0.05) in the OUT fraction than in the IN fraction. The low concentration of polyphenolic compounds in the last phase of gastrointestinal digestion may be due to the low stability of these compounds at basic pH values. For this reason, Fernández and Labra43 reported that polyphenolic compounds are not stable under neural and/or basic pH conditions, whilst Chen et al.44 stated that this instability, under alkaline conditions, may be attributed to the fact that polyphenolic compounds undergo changes, such as oxidation, polymerization and transformation.
Conclusion
The results obtained in the present study provide further evidence about the in vitro digestion stability of polyphenolic compounds of maqui berry mixed with different dietary fibers. The results obtained demonstrate that the stabilization of anthocyanins and phenolic acids and flavonoid compounds present in maqui as a result of interaction with different dietary fibers might help provide sufficient levels for absorption during gastrointestinal digestion, especially in the early phases of gastrointestinal digestion. In future work the lyophilized maqui can be used as an ingredient to develop functional foods. One way to protect the bioactive compounds (polyphenolic compounds) present in its composition would be by adding it together with dietary fibers and mainly with gums. xanthan gum, guar gum and sodium carboxymethyl cellulose provide the best results in this context.Conflicts of interest
There are no conflicts to declare.References
- E. Genskowsky, L. A. Puente, J. A. Pérez-Álvarez, J. Fernández-López, L. A. Muñoz and M. Viuda-Martos, Determination of polyphenolic profile, antioxidant activity and antibacterial properties of maqui [Aristotelia chilensis (Molina) Stuntz] a Chilean blackberry, J. Sci. Food Agric., 2016, 96, 4235–4242 CrossRef CAS PubMed.
- M. E. Schreckinger, J. Lotton, M. A. Lila and E. G. de Mejia, Berries from South America: A comprehensive review on chemistry, health potential, and commercialization, J. Med. Food, 2010, 13, 233–246 CrossRef CAS PubMed.
- L. E. Rojo, D. Ribnicky, S. Logendra, A. Poulev, P. Rojas-Silva, P. Kuhn, R. Dorn, M. H. Grace, M. A. Lila and I. Raskin, In vitro and in vivo anti-diabetic effects of anthocyanins from Maqui berry (Aristotelia chilensis), Food Chem., 2012, 131, 387–396 CrossRef CAS PubMed.
- J. E. Brauch, M. Buchweitz, R. M. Schweiggert and R. Carle, Detailed analyses of fresh and dried maqui (Aristotelia chilensis (Mol.) Stuntz) berries and juice, Food Chem., 2016, 190, 308–316 CrossRef CAS PubMed.
- F. Marques Peixoto, I. Fernandes, A. C. Gouvêa, M. C. Santiago, R. G. Borguini, N. Mateus, V. Freitas, R. L. O. Godoy and I. M. Ferreira, Simulation of in vitro digestion coupled to gastric and intestinal transport models to estimate absorption of anthocyanins from peel powder of jabuticaba, jamelão and jambo fruits, J. Funct. Foods, 2016, 24, 373–381 CrossRef CAS.
- M. Riaz, M. Zia-Ul-Haq and B. Saad, The role of anthocyanins in health as antioxidant, in bone health and as heart protecting agents, in Anthocyanins and Human Health: Biomolecular and therapeutic aspects Cham, Springer International Publishing, 2016, pp. 87–107 Search PubMed.
- C. R. Welch, Q. Wu and J. E. Simon, Recent advances in anthocyanin analysis and characterization, Curr. Anal. Chem., 2008, 4(2), 75–101 CrossRef CAS PubMed.
- C. Manach, A. Scalbert, C. Morand, C. Rémésy and L. Jimenez, Polyphenols: food sources and bioavailability, Am. J. Clin. Nutr., 2004, 79, 727–747 CAS.
- C. Pineda-Vadillo, F. Nau, C. Guerin Dubiard and V. Cheynier, et al., In vitro digestion of dairy and egg products enriched with grape extracts: Effect of the food matrix on polyphenol bioaccessibility and antioxidant activity, Food Res. Int., 2016, 88, 284–292 CrossRef CAS.
- G. J. McDougall, P. Dobson, P. Smith, A. Blake and D. Stewart, Assessing potential bioavailability of raspberry anthocyanins using an in vitro digestion system, J. Agric. Food Chem., 2005, 53(15), 5896–5904 CrossRef CAS PubMed.
- L. Montagne, J. R. Pluske and D. J. Hampson, A review of interactions between dietary fibre and the intestinal mucosa, and their consequences on digestive health in young monogastric animals, Anim. Feed Sci. Technol., 2003, 108, 95–117 CrossRef.
- Y. Ting, Q. Zhao, C. Xia and Q. Huang, Using in vitro and in vivo models to evaluate the oral bioavailability of nutraceuticals, J. Agric. Food Chem., 2015, 63(5), 1332–1338 CrossRef CAS PubMed.
- M. Minekus, M. Alminger, P. Alvito, S. Balance, T. Bohn and C. Bourlieu, et al., A standardized static in vitro digestion method suitable for food- An international consensus, Food Funct., 2014, 5, 1113–1124 CAS.
- B. Gullón, M. E. Pintado, X. Barber, J. Fernández-López, J. A. Pérez-Álvarez and M. Viuda-Martos, In vitro gastrointestinal digestion of pomegranate peel (Punica granatum) flour obtained from co-products: Changes in the antioxidant potential and bioactive compounds stability, J. Funct. Foods, 2015, 19, 617–628 CrossRef.
- N. Ortega, A. Macia, M. P. Romero, J. Reguant and M. J. Motilva, Matrix composition effect on the digestibility of carob flour phenols by an in vitro digestion model, Food Chem., 2011, 124, 65–71 CrossRef CAS.
- V. L. Singleton and J. A. Rossi, Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents, Am. J. Enol. Vitic., 1965, 16, 144–158 CAS.
- M. Blasa, M. Candiracci, A. Accorsi, P. M. Piacentini, M. C. Albertini and E. Piatti, Raw Millefiori honey is packed full of antioxidants, Food Chem., 2005, 97, 217–222 CrossRef.
- U. A. Fischer, R. Carle and D. R. Kammerer, Identification and quantification of phenolic compounds from pomegranate (Punica granatum L.) peel, mesocarp, aril and differently produced juices by HPLC-DAD-ESI/MSn, Food Chem., 2011, 127, 807–821 CrossRef CAS PubMed.
- C. Fredes, G. G. Yousef, P. Robert, M. H. Grace, M. A. Lila, M. Gómez, M. Gebauer and G. Montenegro, Anthocyanin profiling of wild maqui berries (Aristotelia chilensis [Mol.] Stuntz) from different geographical regions in Chile, J. Sci. Food Agric., 2014, 94, 2639–2648 CrossRef CAS PubMed.
- A. Chandra, J. Rana and Y. Li, Separation, identification, quantification, and method validation of anthocyanins in botanical supplement raw materials by HPLC and HPLC-MS, J. Agric. Food Chem., 2001, 49, 3515–3521 CrossRef CAS PubMed.
- L. Hooper and A. Cassidy, A review of the health care potential of bioactive compounds, J. Sci. Food Agric., 2006, 86, 1805–1813 CrossRef CAS.
- R. Lucas-Gonzalez, S. Navarro-Coves, J. A. Pérez-Álvarez, J. Fernández-López, L. A. Muñoz and M. Viuda-Martos, Assessment of polyphenolic profile stability and changes in the antioxidant potential of maqui berry (Aristotelia chilensis (Molina) Stuntz) during in vitro gastrointestinal digestion, Ind. Crops Prod., 2016, 94, 774–782 CrossRef CAS.
- D. Sun-Waterhouse, B. G. Smith, C. J. O′connor and L. D. Melton, Effect of raw and cooked onion dietary fibre on the antioxidant activity of ascorbic acid and quercetin, Food Chem., 2008, 111(3), 580–585 CrossRef CAS.
- A. Padayachee, G. Netzel, M. Netzel, L. Day, D. Zabaras, D. Mikkelsenand and M. J. Gidley, Binding of polyphenols to plant cell wall analogues–Part 2: Phenolic acids, Food Chem., 2012, 135, 2287–2292 CrossRef CAS PubMed.
- A. Padayachee, G. Netzel, M. Netzel, L. Day, D. Zabaras, D. Mikkelsenand and M. J. Gidley, Binding of polyphenols to plant cell wall analogues–Part 1: Anthocyanins, Food Chem., 2012, 134, 155–161 CrossRef CAS.
- M. J. Rodríguez-Roque, M. A. Rojas-Graü, P. Elez-Martínez and O. Martín-Belloso, Soymilk phenolic compounds, isoflavones and antioxidant activity as affected by in vitro gastrointestinal digestion, Food Chem., 2013, 136, 206–212 CrossRef PubMed.
- R. Martínez-Las Heras, A. Pinazo, A. Heredia and A. Andrés, Evaluation studies of persimmon plant (Diospyros kaki) for physiological benefits and bioaccessibility of antioxidants by in vitro simulated gastrointestinal digestion, Food Chem., 2017, 214, 478–485 CrossRef PubMed.
- H. Sengul, E. Surek and D. Nilufer-Erdil, Investigating the effects of food matrix and food components on bioaccessibility of pomegranate (Punica granatum) phenolics and anthocyanins using an in-vitro gastrointestinal digestion model, Food Res. Int., 2014, 62, 1069–1079 CrossRef CAS.
- L. Siracusa, T. Kulisic-Bilusic, O. Politeo, I. Krause, B. Dejanovic and G. Ruberto, Phenolic composition and antioxidant activity of aqueous infusions from Capparis spinosa L. and Crithmum maritimum L. before and after submission to a two-step in vitro digestion model, J. Agric. Food Chem., 2011, 59, 12453–12459 CrossRef CAS PubMed.
- K. Kida, M. Suzuki, N. Matsumoto, F. Nanjo and Y. Hara, Identification of biliary metabolites of (-)-epigallocatechin gallate in rats, J. Agric. Food Chem., 2000, 48, 4151–4155 CrossRef CAS PubMed.
- A. Gil-Izquierdo, M. I. Gil, F. Ferreres and F. A. Tomás-Barberán, In vitro availability of flavonoids and other phenolics in orange juice, J. Agric. Food Chem., 2001, 49, 1035–1041 CrossRef CAS PubMed.
- J. Kroll, H. M. Rawel and S. Rohn, Reactions of plant phenolics with food proteins and enzymes under special consideration of covalent bonds, Food Sci. Technol. Res., 2003, 9, 205–218 CrossRef CAS.
- P. J. Wood, Relationships between solution properties of cereal β-glucans and physiological effects – A review, Trends Food Sci. Technol., 2004, 15, 313–320 CrossRef CAS.
- M. Porrini and P. Riso, Factors influencing the bioavailability of antioxidants in foods: a critical appraisal, Nutr., Metab. Cardiovasc. Dis., 2008, 18(10), 647–650 CrossRef PubMed.
- J. H. Cummings, L. M. Edmond and E. A. Magee, Dietary carbohydrates and health: do we still need the fibre concept?, Clin. Nutr. Suppl., 2004, 1(2), 5–17 CrossRef.
- H. Palafox-Carlos, J. F. Ayala-Zavala and G. A. González-Aguilar, The role of dietary fiber in the bioaccessibility and bioavailability of fruit and vegetable antioxidants, J. Food Sci., 2011, 76(1), R6–R15 CrossRef CAS PubMed.
- J. W. DeVries, Dietary fiber: the influence of definition on analysis and regulation, J. AOAC Int., 2004, 87(3), 682–706 CAS.
- A. E. Quirós-Sauceda, J. F. Ayala-Zavala, S. G. Sáyago-Ayerdi, R. Vélez-de la Rocha, J. A. Sañudo-Barajas and G. A. González-Aguilar, Added dietary fiber affects antioxidant capacity and phenolic compounds content extracted from tropical fruit, J. Appl. Bot. Food Qual., 2014, 87, 227–233 Search PubMed.
- A. Scalbert and G. Williamson, Dietary intake and bioavailability of polyphenols, J. Nutr., 2000, 130(8S Suppl), 2073S–2085S CAS.
- F. Cebeci and N. Şahin-Yeşilçubuk, The matrix effect of blueberry, oat meal and milk on polyphenols, antioxidant activity and potential bioavailability, Int. J. Food Sci. Nutr., 2013, 65(1), 69–78 CrossRef PubMed.
- J. Correa-Betanzo, E. Allen-Vercoe, J. McDonald, K. Schroeter, M. Corredig and G. Paliyath, Stability and biological activity of wild blueberry (Vaccinium angustifolium) polyphenols during simulated in vitro gastrointestinal digestion, Food Chem., 2014, 165, 522–531 CrossRef CAS PubMed.
- A. Kosinska-Cagnazzo, S. Diering, D. Prim and W. Andlauer, Identification of bioaccessibility and up taken phenolic compounds from strawberry fruits in in vitro digestion/Caco-2 absorption model, Food Chem., 2015, 170, 88–294 CrossRef PubMed.
- K. Fernández and J. Labra, Simulated digestion of proanthocyanidins in grape skin and seed extracts and the effects of digestion on the angiotensin I-converting enzyme (ACE) inhibitory activity, Food Chem., 2013, 139, 196–202 CrossRef PubMed.
- G. L. Chen, S. G. Chen, F. Chen, Y. Q. Xie, M. D. Han, C. X. Luo, Y. Y. Zhao and Y. Q. Gao, Nutraceutical potential and antioxidant benefits of selected fruit seeds subjected to an in vitro digestion, J. Funct. Foods, 2016, 20, 317–331 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2018 |