Profiling polyphenol composition by HPLC-DAD-ESI/MSn and the antibacterial activity of infusion preparations obtained from four medicinal plants†
Borhane E. C.
Ziani
abc,
Lillian
Barros
 *a,
Ali Z.
Boumehira
b,
Khaldoun
Bachari
b,
Sandrina A.
Heleno
*a,
Ali Z.
Boumehira
b,
Khaldoun
Bachari
b,
Sandrina A.
Heleno
 a,
Maria Jose
Alves
a,
Maria Jose
Alves
 a and
Isabel C. F. R.
Ferreira
a and
Isabel C. F. R.
Ferreira
 *a
*a
aCentro de Investigacão de Montanha (CIMO), Instituto Politécnico de Bragança, Campus de Santa Apolónia, 5300-253 Bragança, Portugal. E-mail: iferreira@ipb.pt; lillian@ipb.pt; Fax: +351-273-325405; Tel: +351-273-303219 Tel: +351-273-303285
bCentre de Recherche Scientifique et Technique en Analyses Physico-Chimiques CRAPC-Bouismail-Tipaza, Algeria
cDépartement de Technologie Alimentaire et Nutrition Humaine, Ecole nationale Supérieure Agronomique ENSA-Alger, Algeria
First published on 19th October 2017
Abstract
The infusions of Thymus pallescens Noë, Saccocalyx satureioides Coss. et Dur., Ptychotis verticillata Briq. and Limoniastrum guyonianum Boiss. have been used as medicinal remedies for many diseases in Algerian folk medicine. These species have also been well documented as rich sources of phytochemicals, such as phenolic compounds with wide diversified chemical structures, which exhibit far-ranging biological activities. Thus, the phenolic compound profile of the aqueous extracts, obtained by infusing, of the mentioned species was obtained by HPLC-DAD-ESI/MS, and their antibacterial activity was evaluated against clinical isolates. Several phenolic acids were identified and quantified, particularly caffeic acid derivatives along with glycosylated flavonoids. T. pallescens and S. satureioides contain 13 phenolic compounds, where rosmarinic acid was the most abundant phenolic acid present, while L. guyonianum presented myricetin-3-O-glucoside and myricetin-O-rhamnoside as the main compounds among the eight detected molecules. P. verticillata presented a profile of ten phenolic compounds, where 5-O-caffeoylquinic acid was the most abundant phenolic acid, followed by the flavone luteolin-3-O-glucoside. The antibacterial activity of the infusions ranged between 2.5 and 20 mg mL−1 (MIC values), and L. guyonianum showed the highest activity against all of the tested bacteria, Staphylococcus aureus and Pseudomonas aeruginosa being the most sensitive and resistant strains, respectively. Thus, the studied plant species are sources of natural antibacterial substances that can be used to fight against pathogenic microorganisms.
1 Introduction
Phenolic compounds are generally part of a complex mixture isolated from plants and other matrices of biological origin.1,2 They are interesting candidates for the discovery of new antimicrobial agents to be loaded into or coated onto biomaterials.3,4 Consequently, it is essential to identify and measure all of the bioactive constituents of medicinal plants in order to ensure the reliability and repeatability of biological research as well as to enhance the quality control over the pharmacological benefits and/or hazards.5,6 Liquid chromatography coupled with different detectors, such as a diode array detector (DAD) and a mass spectrometer (MS), plays a prominent role as an analytical tool for detecting and identifying active and/or reactive metabolites.7,8 MS detection not only allows one to determine natural compounds’ chemical structures, but also offers excellent sensitivity with sufficient precision and selectivity within a reasonable time, playing an important role in the analysis of phenolic compounds (e.g. flavonoids, phenolic acids and others).7,9–11Antibiotic resistance is a serious and growing phenomenon in contemporary medicine and has emerged as one of the pre-eminent public health concerns in the 21st century.4,12 Most pathogenic bacteria have developed resistance to modern antibiotics, as a result of which we are observing multi-drug resistance among bacterial strains.4,13 Herbal medicine has been assumed to be an effective alternative to resolve this problem due to the bioactivities of herbs and many studies have been conducted across the globe to prove the antimicrobial efficiency and/or properties of several plants.3,14,15
Algerian medicinal plants contain a great number of bioactive compounds with therapeutic effects,16–18 and have been used since ancient times as herbal remedies.19,20 However, the phytochemical constituents of many of these species are still unknown.16,21Thymus pallescens Noë, Saccocalyx satureioides Coss. et Dur., Ptychotis verticillata Briq and Limoniastrum guyonianum Boiss. are some examples of unexplored medicinal plants from Algeria. T. pallescens (Lamiaceae family) is an endemic plant to the northern region of Algeria, widely used for its antitussive, antiseptic, expectorant, anti-helmintic and antispasmodic properties.22 While, S. satureioides, also from the Lamiaceae family, is an aromatic shrub (20–100 cm). This endemic plant is normally grown in septentrional Sahara, Algeria.23,24 In folk medicine, the aerial parts are commonly used in water preparations (infusions and decoctions) for the treatments of gastric disorders and spasms,25 as well as for diabetes.26 The antimicrobial properties of its essential oils have been previously studied,25,27,28 nonetheless, the polar extracts have been less explored. Ptychotis verticillata Briq (Apiaceae family), also known as Ammoides verticillata Briq or Ptychotis ammoides Koch. and commonly named Nûnkha,29,30 is an aromatic herbaceous species (10–35 cm tall), endemic to northwestern Algeria and used for its culinary and medicinal properties as a febrifuge, antispasmodic, antiseptic and antidiabetic, especially in decoctions and infusions.31,32 Moreover, the infusions and ethanolic extracts have been reported to have a wide diversity of polyphenols (flavonoids, saponins, and tannins), while its essential oils are mainly characterized by the presence of thymol and carvacrol.33L. guyonianum (Plumbaginaceae family) is a medicinal halophyte species endemic to the north of Africa; the infusion of its leaves and galls are traditionally used as an anti-dysenteric against infectious diseases or parasites responsible for painful and bloody diarrhea.34 Recent studies performed by Ziani et al.16 and Krifa et al.35 demonstrated the antioxidant and antitumor potential of this plant.
To the best of the authors’ knowledge, the phytochemical analysis of T. pallescens, S. satureioides and P. verticillata has not been previously reported. The chemical characterization and simultaneous analysis of the most prominent compounds are therefore necessary to clearly understand the biological properties of these plants. The infusion preparations of these four medicinal plant species were chosen to continue a previous study of the authors,16 where their antioxidant and cytotoxic properties were reported and different hydrophilic bioactive compounds were also quantified spectrophotometrically. Therefore, the aim of the present work was to identify those bioactive compounds by using HPLC-DAD-ESI/MSn. Furthermore, the antibacterial activity of the infusions was also tested against six Gram-negative and four Gram-positive multi-resistant bacterial strains.
2 Materials and methods
2.1. Plant material
The aerial parts of the four species (Thymus pallescens Noë, Saccocalyx satureioides Coss. et Dur., Limoniastrum guyonianum Boiss. and Ptychotis verticillata Briq) were harvested from some semi-arid and arid areas in Algeria between April and May 2014 as described in a previous study,16 taking into account local consumers’ criteria for the seasoning use of these species and the optimal growth stage and gathering period. A voucher specimen of each species was deposited in the herbarium of the Department of Botany of the National Superior School of Agronomy (ENSA), where the taxonomic criteria of the authors30,36 were used for botanical identification. After 40 days of shade-drying in the dark, all of the samples were ground for all of the subsequent analyses.For the infusion preparation, 1 g of the plant material was added to 200 mL of boiling distilled water and left to infuse at room temperature for 5 min, and then filtered through Whatman paper. The obtained infusions were frozen at −20 °C and lyophilized for further analyses.
2.2. Standards and reagents
Acetonitrile and methanol HPLC-grade and formic acids were purchased from Fisher Scientific (Lisbon, Portugal). Phenolic compound standards were obtained from Extrasynthese (Genay, France) and their purities were above 97%, as determined by HPLC-DAD analysis and stock solutions of these compounds (1 mg mL−1) were prepared in methanol/water (20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 80, v/v). The culture media Mueller–Hinton broth (MHB) and tryptic soy broth (TSB) as well as blood agar with 7% sheep blood and MacConkey agar plates were obtained from Biomerieux (Marcyl'Etoile, France). The dye p-iodonitrotetrazolium chloride (INT) was purchased from Sigma-Aldrich (St Louis, MO, USA) and was used as a microbial growth indicator. Water was treated using the Milli-Q water purification system (TGI Pure WaterSystems, Greenville, SC, USA).
80, v/v). The culture media Mueller–Hinton broth (MHB) and tryptic soy broth (TSB) as well as blood agar with 7% sheep blood and MacConkey agar plates were obtained from Biomerieux (Marcyl'Etoile, France). The dye p-iodonitrotetrazolium chloride (INT) was purchased from Sigma-Aldrich (St Louis, MO, USA) and was used as a microbial growth indicator. Water was treated using the Milli-Q water purification system (TGI Pure WaterSystems, Greenville, SC, USA).
2.3. Phenolic compound characterization by HPLC-DAD-ESI/MSn
For the LC/MS analysis, lyophilized infusion extracts were dissolved in water at a concentration of 10 mg mL−1 and filtered through a 0.22 μm disposable LC filter disk prior to the analysis. Spectral UV-Vis data from all peaks were collected in the range of 240–600 nm, and chromatograms were recorded at 370, 330 and 280 nm for phenolic compound analysis. The HPLC-DAD-ESI/MSn (Dionex Ultimate 3000 UPLC) consisted of a diode array detector (DAD) connected to a Linear Ion Trap LTQ XL mass spectrometer (Thermo Scientific, San Jose, CA, USA), following a procedure previously reported by the authors.37 Chromatographic separation was performed using a Waters Spherisorb S3ODS-2 C18 automated column (3 μm, 4.6 × 150 mm, Waters, Milford, MA, USA) and the mass detection was performed using ESI, operating in negative mode. An Xcalibur®data system (ThermoFinnigan, San Jose, CA, USA) was used for data acquisition.Phenolic compound identification was achieved by comparing retention times and UV–vis and mass spectra with those of available standard compounds. Otherwise, the available data reported in the literature were applied to identify the compounds. The quantification of these compounds was calculated from the calibration curves (2.5–100 μg mL−1) of each available phenolic standard or by using the most similar standard available. The results were expressed as mean values ± standard deviations (SD), in mg g−1 of lyophilized infusion extract.
2.4. Evaluation of the antibacterial activity
Briefly, stock solutions of 100 mg mL−1 were prepared and 100 μL of each stock solution (100 mg mL−1) was diluted in 400 μL of MHB or TSB media according to bacteria requirements (making a solution of 20 mg mL−1). Then, 200 μL of this extract solution was then added to the first well of a microplate (96-well microplate) and 100 μL from the first well were pipetted to other wells containing 100 μL of media making successive dilutions.
Afterwards, 10 μL of inoculum (1.5 × 108 CFU mL−1) of fresh overnight cultures of bacteria was added to all of the wells containing the test concentrations in the range of 20 to 0.156 mg mL−1. Three negative controls were prepared (one with MHB/TSB, another one with the extract, and the third one with the medium, inoculum and antibiotic).
The MIC of the samples was determined after adding INT (0.2 mg mL−1, 40 μL) and after incubation at 37 °C in an oven (Jouan, Berlin, Germany) for 30 min where the viable microorganisms reduced the yellow dye to pink. The MIC was defined as the lowest extract concentration that prevented this change and exhibited the complete inhibition of bacterial growth.
2.5. Statistical analysis
Three repetitions of each sample and triplicates of the infusion preparations were used in each assay. In order to determine the significant difference among samples, a Student's t-test (with p = 0.05, significant differences between samples) was applied (IBM SPSS Statistics for Windows, version 23.0, IBM Corp., Armonk, New York, USA).3 Results and discussion
3.1. HPLC-DAD-ESI/MSn analysis of phenolic compounds
The HPLC-DAD-ESI/MSn chromatographic profiles of the studied plants are shown in Fig. 1 and 2. Tables 1–3 list all of the characterized metabolites obtained by the chromatographic analysis.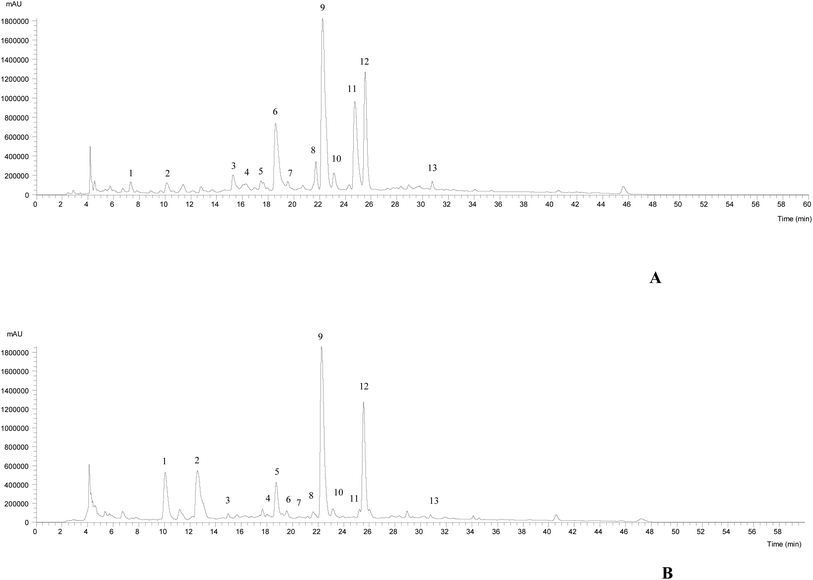 | ||
| Fig. 1 HPLC-DAD-ESI/MSn phenolic profile of Thymus pallescens (A) and Saccocalyx satureioides (B) infusions, recorded at 280 nm. | ||
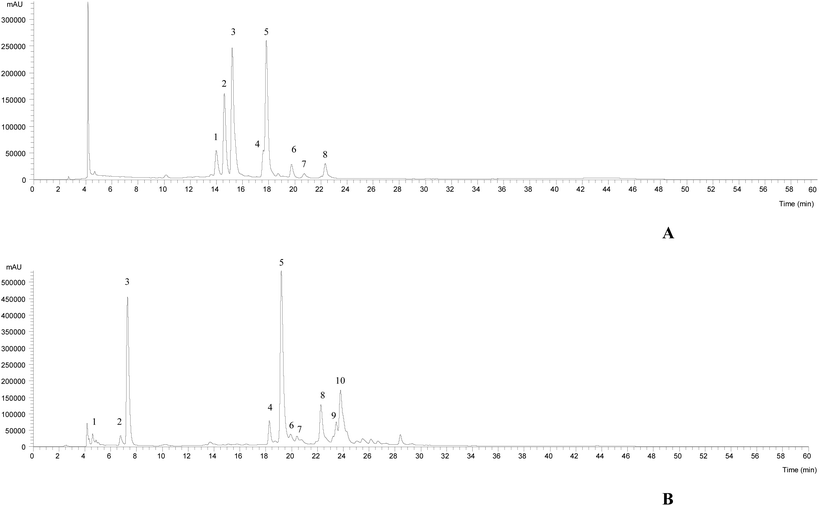 | ||
| Fig. 2 HPLC-DAD-ESI/MSn phenolic profile of Limoniastrum guyonianum (A) and Ptychotis verticillata (B) infusions, recorded at 370 nm. | ||
| Peak | R t (min) | λ max (nm) | [M − H]− (m/z) | MS2 (m/z) | Tentative identification | Quantification (mg g−1 extract) | t-Student's test p-value | |
|---|---|---|---|---|---|---|---|---|
| Thymus pallescens | Saccocalyx satureioides | |||||||
| 1 | 7.32 | 326 | 353 | 191(100), 179(5), 161(3), 135(3) | 5-O-Caffeoylquinic acid | 2.59 ± 0.04 | nd | — |
| 2 | 10.13 | 327 | 593 | 473(3), 383(15), 353(2), 311(3), 297(36) | Apigenin-6,8-C-dihexoside | 2.54 ± 0.01 | 12.2 ± 0.4 | <0.001 |
| 3 | 12.57 | 336 | 637 | 461(100), 285(7) | Luteolin-O-diglucuronide | nd | 5.0 ± 0.2 | — |
| 4 | 14.55 | 320 | 521 | 359(100), 197(4), 179(4), 161(3), 135(2) | Rosmarinic acid hexoside | nd | 1.68 ± 0.08 | — |
| 5 | 15.28 | 342 | 477 | 301(100) | Quercetin-3-O-glucoside | 2.17 ± 0.07 | nd | — |
| 6 | 16.1 | 334 | 637 | 461(100), 285(20) | Luteolin-O-diglucuronide | 1.23 ± 0.01 | nd | — |
| 7 | 17.94 | 343 | 593 | 285(100) | Luteolin-7-O-rutinoside | 1.30 ± 0.01 | 1.22 ± 0.01 | <0.001 |
| 8 | 18.58 | 347 | 461 | 285(100) | Luteolin-7-O-glucuronide | 11.5 ± 0.3 | 5.2 ± 0.2 | <0.001 |
| 9 | 19.53 | 283, 328 | 719 | 539(25), 521(20), 359(100), 297(3), 179(36), 161(2), 135(3) | Sagerinic acid | 2.78 ± 0.07 | 2.39 ± 0.01 | <0.001 |
| 10 | 20.5 | 334 | 549 | 505(30), 463(100), 301(20) | Quercetin-O-malonyhexoside | nd | 1.11 ± 0.01 | — |
| 11 | 21.71 | 288, 323 | 555 | 537(5), 511(8), 493(10), 311(100), 197(19), 179(3), 161(3), 135(12) | Salvianolic acid K | 5.72 ± 0.01 | 2.72 ± 0.02 | <0.001 |
| 12 | 22.24 | 328 | 359 | 197(27), 179(37), 161(100), 135(2) | Rosmarinic acid | 43.1 ± 0.8 | 50.1 ± 0.4 | <0.001 |
| 13 | 23.11 | 335 | 445 | 269(100) | Apigenin-O-glucuronide | 4.56 ± 0.05 | 3.3 ± 0.1 | <0.001 |
| 14 | 24.74 | 340 | 461 | 285(100) | Kaempferol-O-glucuronide | 29.1 ± 0.5 | 1.35 ± 0.03 | <0.001 |
| 15 | 25.54 | 329 | 537 | 493(8), 359(100), 313(11), 295(4), 197(2), 179(3), 161(2) | Lithospermic acid A isomer I | 20.9 ± 0.9 | 21.69 ± 0.02 | 0.107 |
| 16 | 30.76 | 329 | 537 | 493(9), 359(100), 313(9), 295(3), 197(3), 179(3), 161(4) | Lithospermic acid A isomer II | 2.7 ± 0.1 | 2.22 ± 0.03 | <0.001 |
| Total phenolic acid | 78 ± 2 | 80.8 ± 0.5 | 0.020 | |||||
| Total flavonoids | 52.4 ± 0.9 | 29.3 ± 0.9 | <0.001 | |||||
| Total phenolic compounds | 130 ± 3 | 110 ± 1 | <0.001 | |||||
| Peak | R t (min) | λ max (nm) | [M − H]− (m/z) | MS2 (m/z) | Tentative identification | Quantification (mg g−1 extract) |
|---|---|---|---|---|---|---|
| 1Lg | 13.99 | 353 | 631 | 479(5), 317(100) | Myricetin-hexosyl-gallate | 5.73 ± 0.05 |
| 2Lg | 14.61 | 353 | 493 | 317(100) | Myricetin-3-O-glucuronide | 6.90 ± 0.08 |
| 3Lg | 15.21 | 356 | 479 | 317(100) | Myricetin-3-O-glucoside | 8.2 ± 0.2 |
| 4Lg | 17.5 | 354 | 535 | 317(100) | Myricetin-O-acetylglucoronide | 5.41 ± 0.02 |
| 5Lg | 17.83 | 349 | 463 | 317(100) | Myricetin-3-O-rhamnoside | 8.12 ± 0.05 |
| 6Lg | 19.75 | 355 | 535 | 317(100) | Myricetin-O-acetylglucoronide | 5.36 ± 0.06 |
| 7Lg | 20.72 | 352 | 659 | 493(12), 479(100), 317(51) | Myricetin derivative | 5.17 ± 0.03 |
| 8Lg | 22.33 | 353 | 535 | 317(100) | Myricetin-O-acetylglucoronide | 5.36 ± 0.01 |
| Total phenolic compounds | 50.3 ± 0.5 | |||||
| Peak | R t (min) | λ max (nm) | [M − H]− (m/z) | MS2 (m/z) | Tentative identification | Quantification (mg g−1 extract) |
|---|---|---|---|---|---|---|
| 1Pv | 4.94 | 323 | 353 | 191(100), 179(45), 161(3), 135(8) | 3-O-Caffeoylquinic acid | 3.04 ± 0.10 |
| 2Pv | 6.77 | 325 | 353 | 191(11), 179(2), 173(100), 161(1), 135(4) | 4-O-Caffeoylquinic acid | 3.42 ± 0.15 |
| 3Pv | 7.31 | 326 | 353 | 191(100), 179(2), 161(3), 135(4) | 5-O-Caffeoylquinic acid | 40.90 ± 1.32 |
| 4Pv | 18.28 | 347 | 593 | 285(100) | Luteolin-7-O-rutinoside | 1.49 ± 0.03 |
| 5Pv | 19.21 | 347 | 447 | 285(100) | Luteolin-7-O-glucoside | 5.99 ± 0.22 |
| 6Pv | 19.93 | 347 | 609 | 285(100) | Kaempferol-O-dihexoside | 0.75 ± 0.03 |
| 7Pv | 20.42 | 347 | 505 | 463(25), 301(100) | Quercetin-O-acetylhexoside | 1.20 ± 0.03 |
| 8Pv | 23.46 | 332 | 431 | 269(100) | Apigenin-7-O-glucoside | 2.67 ± 0.03 |
| 9Pv | 23.79 | 347 | 447 | 285(100) | Kaempferol-O-glucuronide | 5.44 ± 0.32 |
| 10Pv | 24.2 | 337 | 489 | 285(100) | Kaempferol-O-acetylhexoside | 0.74 ± 0.01 |
| Total phenolic acid | 47.35 ± 1.57 | |||||
| Total flavonoids | 18.28 ± 0.67 | |||||
| Total phenolic compounds | 65.63 ± 2.25 | |||||
Regarding the phenolic composition of Thymus pallescens and Saccocalyx satureioides, as both species belong to the Lamiaceae family, the profiles were very similar to other species belonging to this family.41–43 Thirteen compounds (six phenolic acid derivatives and seven flavonoids) were identified in both species, based on their chromatographic behavior and mass spectra, in comparison with the literature. Table 1 summarizes the identified compounds, their retention times and m/z values for the parent ion and fragment ions. Both samples presented flavonol derivatives (quercetin, kaempferol glycoside derivatives), flavone derivatives (apigenin and luteolin glycoside derivatives) and phenolic acid derivatives, mainly caffeic acid derivatives, such as caffeic acid dimers, trimers, and tetramers (dimers of rosmarinic acid). To the best of the authors’ knowledge, this is the first report regarding the phenolic composition of these two species.
Compounds 1, 5, 7, 8 and 12 were identified as 5-O-caffeoylquinic acid, quercetin-3-O-glucoside, luteolin-7-O-rutinoside, luteolin-7-O-glucuronide and rosmarinic acid, respectively, according to their DAD spectra, mass characteristics and retention times when compared with commercial standards. With the exception of compounds 1 and 5, which were only present in T. pallescens and compound 4 that was only present in S. satureioides, the other compounds were found in both samples.
Compounds 4, 9, 11, 13, 15 and 16 were identified as caffeic acid derivatives. Compound 4 ([M − H]− at m/z 521) fragmented at m/z 359 (rosmarinic acid, loss of −162 mu, hexoside moiety), which allowed its identification as rosmarinic acid hexoside. Compound 9 ([M − H]− at m/z 719) released a main MS2 fragment at m/z 359 ([M − 2H]2−, rosmarinic acid), allowing its identification as sagerinic acid.44 Compounds 15 and 16 ([M − H]− at m/z 537) revealed a similar UV spectrum and fragmentation pattern, being identified as a caffeic acid trimer, lithospermic acid A, taking into account literature findings.44–47 Compound 11 ([M − H]− at m/z 555) was tentatively assigned to salvianolic acid K, due to a similar fragmentation pattern described by Hauck et al.48
The remaining molecules (peaks 2, 3, 6, 10, 13 and 14) correspond to flavonoid derivatives. Compound 2 ([M − H]− at m/z 593) released the MS2 fragment ions at m/z 473 and 383 (loss of 120 and 90 mu characteristic of C-hexosyl flavones) and at m/z 353 corresponding to apigenin aglycone (apigenin + 83 mu, bearing some sugar residues),49 which allowed the identification of this compound as apigenin-C-hexoside C-hexoside. Thus, this compound was tentatively identified as apigenin-6,8-C-diglucoside. Compounds 3 and 6 presented a UV spectrum characteristic of luteolin (λmax at 350 nm) and the same pseudomolecular ion [M − H]− at m/z 637, releasing two fragments at m/z 461 and 285 ([M − 176 − 176]−, loss of two glucuronyl moieties), being identified as luteolin-O-diglucuronide. Similar findings were taken into account to identify compounds 13 and 14, which were assigned to apigenin-O-glucuronide and kaemferol-O-glucuronide, respectively. Compound 3 was only detected in S. satureioides, while compound 6 was identified in T. pallescens. Finally, compound 10 ([M − H]− at m/z 549), releasing MS2 fragments at m/z 301 ([M − H − 162 − 86]−, loss of a malonylhexoside moiety) was assigned to quercetin-O-malonylhexoside, being only present in S. satureioides. Rosmarinic acid was the most abundant phenolic acid present in both samples, while luteolin-7-O-glucuronide was the most abundant flavonoid present in T. pallescens and apigenin-6,8-C-dihexoside was predominant in S. satureioides.
In the case of L. guyonianum infusion, only flavonol glycoside derivatives were detected, related to myricetin (λmax around 354 nm, an MS2 fragment at m/z 317). They presented MS2 fragments corresponding to distinct losses of glucuronyl (−176 mu), glucosyl (−162 mu) and rhamnosyl (−146 mu) moieties, and an elution order coherent with the type of substituent sugar, according to their expected polarity, being assigned to myricetin-O-glucuronide (peak 2Lg), myricetin-3-O-glucoside (peak 3Lg, positively identified with the commercial standards), and myricetin-O-rhamnoside (peak 5Lg), respectively. Myricetin and its derivatives have been previously described in Limoniastrum feei (Girard) Batt from Algeria50 as myricetin-3-O-beta-galactopyranoside and myricetin-3-O-alpha-rhamnopyranoside. Taking into account the position and nature of the sugar moieties, peaks 2Lg and 5Lg were assumed to be myricetin-3-O-glucuronide and myricetin-3-O-rhamnoside, respectively.
Furthermore, compound 1Lg was identified as a hexosyl derivative of myricetin that also appeared to be attached to a gallic acid moiety (−152 mu), yielding a deprotonated ion at m/z 631, which consisted of a myricetin-hexosyl-gallate. Compounds 4Lg, 6Lg and 8Lg presented the same pseudomolecular ion [M − H]− at m/z 505, releasing an MS2 fragment at m/z 317 (myricetin; [M − H − 42 − 162]−, loss of an acetylhexoside moiety), being assigned to myricetin-O-acetylhexoside. No further identification was possible to obtain for peak 7Lg ([M − H]− at m/z 659), being assigned to a myricetin derivative. The major myricetin derivatives present in this sample were myricetin-3-O-glucoside and myricetin-O-rhamnoside, respectively.
The phenolic profile of P. verticillata infusion, determined by HPLC-DAD-ESI/MSn, presented three chlorogenic acids, being compounds 1, 2 and 3 ([M − H]− at m/z 353) identified as 3-O-caffeoylquinic acid, 4-O-caffeoylquinic acid and 5-O-caffeoylquinic acid, respectively, according to their similar fragmentation pattern previously described by Clifford et al.51,52 The remaining compounds were identified as flavonols (peaks 6Pv, 7Pv, 9Pv and 10Pv) and flavones (4Pv, 5Pv and 8Pv). The latter compounds 4Pv (luteolin-3-O-rutinoside), 5Pv (luteolin-3-O-glucuronide) and 8Pv (apigenin-3-O-glucoside) were positively identified with commercial standards. Peak 7Pv ([M − H]− at m/z 505) released two fragments, at m/z 463 (−42, loss of a acetyl moiety) and m/z 301 (−162 u, loss of a hexosyl moiety), thus being tentatively assigned to quercetin-O-acetylhexoside. Compounds 6Pv ([M − H]− at m/z 609), 9Pv ([M − H]− at m/z 447) and 10Pv ([M − H]− at m/z 489) were identified as kaempferol glycosides based on their UV spectra (λmax around 348 nm) and the production of an MS2 fragment ion at m/z 285, and could be assumed as kaempferol-O-dihexoside, kaempferol-O-glucuronide and kaempferol-O-acetylhexoside, respectively. 5-O-Caffeoylquinic acid was the most abundant compound, followed by the flavone luteolin-3-O-glucoside.
3.2. Antibacterial activity
The search for natural antimicrobial compounds in clinical microbiology is incited by the need to thwart the increasing infectious diseases caused by multiple drug resistant (MDR) and total drug resistant (TDR) strains.4 In the biomedical field, the microbial antibiotic-resistance leads to a growing need for new, effective anti-infective materials for the prevention and delay of implant and device-associated infections.53 In the present work, the antibacterial activity of the samples was tested against ten bacteria, some of them being multiresistant. The infusion of L. guyonianum showed the highest activity against E. coli, E. coli ESBL, MRSA and MSSA (MICs = 2.5 mg mL−1), followed by K. pneumoniae, K. pneumoniae ESBL, E. faecalis, L. monocytogenes (MICs = 5 mg mL−1), and P. aeruginosa and M. morganii (MICs ranging between 10–20 mg mL−1). The infusions of T. pallescens and S. satureioides showed almost similar values and were found to be less active against K. pneumoniae, K. pneumoniae ESBL, E. faecalis, L. monocytogenes, P. aeruginosa and M. morganii strains (MICs ranging from 2.5 to >20 mg mL−1) (Table 4). P. verticillata infusion showed the weakest activity against all of the bacteria presenting MIC values in the highest tested concentration or even presenting no activity at the maximum tested concentration: 20 mg mL−1. These results showed that the plant infusions can inhibit bacterial strains irrespective of their mechanisms of resistance. No significant differences were observed between the strains showing well-known mechanisms of resistance and the susceptible ones, because one-dilution differences in this kind of analysis are taken for granted.| Antimicrobial activity MIC values (mg mL−1) | Thymus pallescens | Saccocalyx satureioides | Limoniastrum guyonianum | Ptychotis verticillata |
|---|---|---|---|---|
| MRSA, methicillin-resistant Staphylococcus aureus; MSSA, methicillin-susceptible Staphylococcus aureus; MIC, minimal inhibitory concentration; ESBL, extended-spectrum beta-lactamases. | ||||
| Gram-negative bacteria | ||||
| Escherichia coli | 10 | 5 | 2.5 | 20 |
| Escherichia coli ESBL | 10 | 5 | 2.5 | 20 |
| Klebsiella pneumoniae | 10 | 10 | 5 | 20 |
| Klebsiella pneumoniae ESBL | 10 | 10 | 5 | 20 |
| Morganella morganii | 20 | >20 | 20 | >20 |
| Pseudomonas aeruginosa | 20 | 20 | 10 | >20 |
| Gram-positive bacteria | ||||
| Enterococcus faecalis | 10 | 10 | 5 | 20 |
| Listeria monocytogenes | 10 | 10 | 5 | 20 |
| MRSA | 5 | 5 | 2.5 | 10 |
| MSSA | 5 | 5 | 2.5 | 10 |
Many research groups have gone one step further and isolated and identified the structure of flavonoids that possess antibacterial activity. The studied infusions were found to be rich sources of phenolic components that could exert antibacterial activity. For instance, flavonols and their derivatives, as they were found abundantly in the infusion of T. pallescens (quercetin and kaempferol) and L. guyonianum (myricetin), are characterized by a remarkable antibacterial activity against both Gram-positive and Gram-negative bacteria, such as S. aureus, Lactobacillus acidophilus, Porphyromonas gingivalis, Prevotella melaninogenica, S. epidermidis, E. coli, Proteus vulgaris, P. aeruginosa, Enterococcus aerogenes and Enterobacter sakazakii.42,54 Quercetin, 3-O-methylquercetin and several quercetin glycosides,55–59 and also kaempferol and its glycoside derivatives,55,57,60 were also reported to have antimicrobial activity. Other categories of compounds with known antibacterial activity are phenolic acids, such as chlorogenic, protocatechuic, p-coumaric, caffeic, syringic, p-hydroxybenzoic, ferulic, vanillic, gentisic and gallic acids,12 their derivatives,42 and flavones (e.g. apigenin and luteolin glycoside derivatives),12,55,56,61–65 isoflavones,66–69 flavanones,56,70 and other flavonol glycosides.56,71–74
To the best of the authors’ knowledge, no data are available on the antibacterial activity of these plants against drug-resistant bacteria in the literature. The obtained results showed that the antibacterial activity of all of the plant infusions is moderate, especially against Gram-positive bacteria. Moreover, whilst methicillin-resistant strains usually display resistance to several drugs, no relevant differences were observed between methicillin-susceptible and -resistant strains. These data are also in agreement with earlier studies carried out on other species belonging to the genus of the studied plants. Aqueous extracts obtained from Thymus vulgaris showed antibacterial activity against many ATCC bacterial strains.42,75,76 Moreover, many solvent fractions of L. guyonianum from Tunisia showed antibacterial activity against several human pathogenic strains such as S. aureus, E. feacalis, E. coli, Salmonella typhi and P. aeruginosa;77 also, the Tunisian L. guyonianum methanolic, chloroformic and petroleum extracts showed a potent action against P. aeruginosa and S. aureus with MIC values of 23 and 46 μg mL−1 as the best values, respectively.78 Concerning the antimicrobial activity of S. satureioides, only the essential oil was previously studied by the authors,25 and showed strong potential against ATCC bacterial strains (Bacillus subtilis, S. aureus, L. monocytogenes, E. faecalis, E. coli, K. pneumoniae, Proteus mirabilis, P. aeruginosa and Salmonella typhimurium).
According to the results obtained from the HPLC-DAD-ESI/MSn analysis, the appreciable antibacterial activity of the extracts against MDR strains could be explained by the wide spectrum of polyphenols identified in the infusions (previous section), which act as antimicrobial substances via different mechanisms of action (MA). In fact, many MA are ascribed to polyphenols, such as cytoplasmic membrane damage, and inhibition of nucleic acid, cell wall and cell membrane synthesis.3,55 Moreover, in addition to their direct antibacterial activity, a growing body of evidence suggests that polyphenols may interfere with some bacterial virulence factors such as enzymes, toxins and signal receptors.3,55,56,73
4 Conclusion
Overall, this work focuses on the determination of the phenolic compound profile as well as on the investigation of the antibacterial activity of four medicinal plant species from the Algerian flora. The identification and quantification of phenolic compounds in T. pallescens, S. satureioides, L. guyonianum and P. verticillata have been accomplished. The results indicate that the infusion preparation of T. pallescens and S. satureioides contained considerable amounts of rosmarinic acid, lithospermic acid A, luteolin-7-O-glucuronide and apigenin-6,8-C-dihexoside, while L. guyonianum was rich in myricetin derivatives (glucosides) and P. verticillata was rich in caffeic acid derivatives particularly 5-O-caffeoylquinic acid. The findings of this study showed that all four species exhibited a broad spectrum of antibacterial activity against clinical isolates (MIC values between 2.5 and 20 mg mL−1), which could be used as an alternative source of antibiotics. However, pharmacological testing is necessary following the isolation of the bioactive compounds. The infusions of these plants should be furthermore investigated in vivo to better understand their efficacy and medicinal properties.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors are grateful to the Foundation for Science and Technology (FCT, Portugal) and FEDER under Programme PT2020 for financial support to CIMO (UID/AGR/00690/2013), S. Heleno (SFRH/BPD/101413/2014) grant and L. Barros contract. The authors are also grateful to the Interreg España-Portugal for financial support through the project 0377_Iberphenol_6_E.References
- D. Lin, M. Xiao, J. Zhao, Z. Li, B. Xing, X. Li, M. Kong, L. Li, Q. Zhang, Y. Liu, H. Chen, W. Qin, H. Wu and S. Chen, An overview of plant phenolic compounds and their importance in human nutrition and management of Type 2 Diabetes, Molecules, 2016, 21, 2–19 Search PubMed.
- S. Maqsood, S. Benjakul, A. Abushelaibi and A. Alam, Phenolic compounds and plant phenolic extracts as natural antioxidants in prevention of lipid oxidation in seafood : a detailed Review, Compr. Rev. Food Sci. Food Saf., 2014, 13, 1125–1140 CrossRef CAS.
- M. Wink, Modes of action of herbal medicines and plant secondary metabolites, Medicines, 2015, 251–286 CrossRef CAS PubMed.
- P. Vadhana, B. R. Singh, M. Bharadwaj and S. V. Singh, Pharmaceutical emergence of herbal antimicrobial drug resistance in clinical bacterial isolates, Pharm. Anal. Acta, 2015, 6, 434 Search PubMed.
- A. O. Matei, F. Gatea and G. L. Radu, Analysis of phenolic compounds in some medicinal herbs by LC – MS, J. Chromatogr. Sci., 2015, 12, 1–8 Search PubMed.
- P. Terpinc, C. Blaz, T. Polak, J. Hribar and T. Pozrl, LC – MS analysis of phenolic compounds and antioxidant activity of buckwheat at different stages of malting, Food Chem., 2016, 210, 9–17 CrossRef CAS PubMed.
- G. Chen, X. Li, F. Saleri and M. Guo, Analysis of flavonoids in rhamnus davurica and its antiproliferative activities, Molecules, 2016, 21, 1–14 Search PubMed.
- M. Tasioula-margari and E. Tsabolatidou, Extraction, separation, and identification of phenolic compounds in virgin olive oil by HPLC-DAD and HPLC-MS, Antioxidants, 2015, 4, 548–562 CrossRef CAS PubMed.
- D. Benedec, L. Vlase, I. Oniga, A. C. Mot, R. Silaghi-Dumitrescu, D. Hanganu, B. Tiperciuc and G. Crişan, LC-MS analysis and antioxidant activity of phenolic compounds from two indigenous species of Mentha. Note I, Farmacia, 2013, 61, 262–267 CAS.
- W. Rached, M. Bennaceur, L. Barros, R. C. Calhelha, S. Heleno, M. J. Alves, M. Carvalho, A. M. Abderrazak and I. C. F. R. Ferreira, Detailed phytochemical characterization and bioactive properties of Myrtus nivelii, Batt & Trab., Food Funct., 2017, 8, 3111–3119 CAS.
- I. Jabeur, N. Martins, L. Barros, R. C. Calhelha, J. Vaz, L. Achour, C. Santos-Buelga and I. C. F. R. Ferreira, Contribution of phenolic composition to the antioxidant, anti-inflammatory and antitumor potential of Equisetum giganteum L. and Tilia platyphyllos Scop., Food Funct., 2017, 8, 975–984 CAS.
- M. Kozyra, A. Biernasiuk and A. Malm, Natural drugs analysis of phenolic acids and antibacterial activity of extracts obtained from the flowering herbs, Acta Pol. Pharm. Drug Res., 2017, 74, 161–172 Search PubMed.
- P. C. Carvajal, E. Coppo, A. Di Lorenzo, D. Gozzini, F. Bracco, G. Zanoni, S. M. Nabavi, A. Marchese, C. R. Arciola and M. Daglia, Chemical characterization and in vitro antibacterial activity of Myrcianthes hallii, (O. Berg) McVaugh (Myrtaceae), a traditional plant growing in ecuador, Materials, 2016, 9, 1–14 Search PubMed.
- N. Amin, R. Shawahna, A. Mustafa, R. Al-ramahi, M. Kasem and A. Naser, Herbal remedies use by breast cancer patients in the West Bank of Palestine, J. Ethnopharmacol., 2016, 178, 1–8 CrossRef PubMed.
- S. A. Heleno, L. Barros, A. Martins, P. Morales, V. Fernandez-Ruiz, J. Glamoclija, M. Sokovic and I. C. F. R. Ferreira, Nutritional value, bioactive compounds, antimicrobial activity and bioaccessibility studies with wild edible mushrooms, LWT–Food Sci. Technol., 2015, 63, 799–806 CrossRef CAS.
- B. E. C. Ziani, R. C. Calhelha, J. C. M. Barreira, L. Barros, M. Hazzit and I. C. F. R. Ferreira, Bioactive properties of medicinal plants from the Algerian flora : Selecting the species with the highest potential in view of application purposes, Ind. Crops Prod., 2015, 77, 582–589 CrossRef CAS.
- A. Djeridane, M. Yousfi, B. Nadjemi, D. Boutassouna, P. Stocker and N. Vidal, Antioxidant activity of some algerian medicinal plants extracts containing phenolic compounds, Food Chem., 2006, 97, 654–660 CrossRef CAS.
- W. Rached, R. C. Calhelha, M. Bennaceur, A. Marouf, L. Barros, C. Santos-buelga and I. C. F. R. Ferreira, Phytochemical characterization and bioactive properties of Osyris quadripartita, Salzm. ex Decne. leaves from Algeria, RCS Advances, 2016, 6, 72768–72776 CAS.
- L. Reguieg, Using medicinal plants in Algeria, Am. J. Food Nutr., 2011, 1, 126–127 CrossRef.
- B. Benarba, Use of medicinal plants by breast cancer patients in Algeria, EXCLI J., 2015, 14, 1164–1166 Search PubMed.
- F. Ramdane, M. H. Mahammed, M. D. O. Hadj, A. Chanai, R. Hammoudi, N. Hillali, H. Mesrouk, I. Bouafia and C. Bahaz, Ethnobotanical study of some medicinal plants from Hoggar, Algeria, J. Med. Plants Res., 2015, 9, 820–827 CrossRef.
- M. Hazzit, A. Baaliouamer, A. R. Veríssimo, M. L. Faleiro and M. G. Miguel, Chemical composition and biological activities of Algerian Thymus oils, Food Chem., 2009, 116, 714–721 CrossRef CAS.
- P. Ozenda, in Flore du Sahara septentrional et central, CNRS, Paris, France, 3rd edn, 2004 Search PubMed.
- P. Quezel and S. Santa, Nouvelles flores de l'Algérie et des régions désertiques méridionales, CNRS, Paris, France, 1983, vol. T. II Search PubMed.
- M. Bendahou, M. Benyoucef, A. Muselli, J. Desjobert, J. Paolini, A. Bernardini and J. Costa, Antimicrobial activity and chemical composition of Saccocalyx satureioides Coss. et Dur. Essential oil and extract obtained by microwave extraction. comparison with hydrodistillation, J. Essent. Oil Res., 2014, 20, 174–178 CrossRef.
- N. Belmekki and N. Bendimerad, Antioxidant activity and phenolic content in methanol crude extracts from three Lamiaceae grown in southwestern Algeria, J. Nat. Prod. plant Resour., 2012, 2, 175–181 CAS.
- M. M. Zerroug, H. Laouer, R. Strange and J. Nicklin, The effect of essential oil of Saccocalyx satureioides Coss. Et Dur. on the growth of and the production of solanapyrone a by Ascochyta rabiei (Pass.) Labr, Adv. Environ. Biol., 2011, 5, 501–506 Search PubMed.
- D. M. Biondi, M. Sari, Z. A. Ghani and G. Ruberto, Essential oil of Algerian Saccocalyx satureioides, Coss. et Durieu, Flavour Fragrance J., 2006, 21, 546–548 CrossRef CAS.
- L. Bellakhdar, La Pharmacopée Marocaine Traditionnelle. Médecine Arabe Ancienne et Savoirs Populaires, Ibis Press, Paris, 1997 Search PubMed.
- P. Quezel and S. Santa, in Nouvelle flore de l'Algérie et des régions désertiques méridionales, CNRS, Paris, 1963, vol. Tome II, pp. 350–1170 Search PubMed.
- E. O. El Mokhtar, P. Tomi, A. Bouyanzer, B. Hammouti, J. Desjobert, J. Costa and J. Paolini, Chemical composition and antioxidant activity of essential oils and solvent extracts of Ptychotis verticillata from Morocco, Food Chem. Toxicol., 2011, 49, 533–536 CrossRef PubMed.
- M. Bnouham, W. Benalla, A. Asehraou and M. Berrabah, Antibacterial activity of essential oil from Ptychotis verticillata, Spatula DD, 2012, 2, 69–73 CrossRef.
- O. Toubal, A. Djahoudi, C. Henchiri and M. Bouazza, Phytochemical screening and antimicrobial evaluation of the aqueous extracts of Ammoides verticillata, an Endemic Species, J. Life Sci., 2012, 6, 243–247 Search PubMed.
- M. Chaieb and M. Boukhris, Flore succincte et illustrée des zones arides et sahariennes de Tunisie, Association pour la protection de la nature et de l'environnement, Sfax, 1998 Search PubMed.
- M. Krifa, I. Skandrani, A. Pizzi, N. Nasr, Z. Ghedira, N. Mustapha, K. Ghedira and L. Chekir-ghedira, An aqueous extract of Limoniastrum guyonianum gall induces anti-tumor effects in melanoma-injected mice via modulation of the immune response, Food Chem. Toxicol., 2014, 69, 76–85 CrossRef CAS PubMed.
- R. Maire, Flore de l'Afrique de Nord, Paul lechevalier, paris (VI), 1962, vol. VIII Search PubMed.
- S. M. F. Bessada, J. C. M. Barreira, L. Barros, I. C. F. R. Ferreira and M. B. P. P. Oliveira, Phenolic profile and antioxidant activity of Coleostephus myconis (L.) Rchb.f.: An underexploited and highly disseminated species, Ind. Crops Prod., 2016, 89, 45–51 CrossRef CAS.
- CLSI, Performance Standards for Antimicrobial Susceptibility Testing, 18th Informational Supplement. CLSI Document M100-S18, 2008.
- EUCAST, ESCMID: European Society of Clinical Microbiology and Infectious Diseases, http://www.eucast.org/ast of bacteria/ previous versions of documents/.
- B. Svobodova, L. Barros, R. C. Calhelha, S. Heleno, M. Jose, S. Walcott, M. Bittova, V. Kuban and I. C. F. R. Ferreira, Bioactive properties and phenolic profile of Momordica charantia, L. medicinal plant growing wild in Trinidad and Tobago, Ind. Crops Prod., 2016, 95, 365–373 CrossRef.
- M. B. Hossain, D. K. Rai, N. P. Brunton, A. B. Martin-Diana and C. Barry-Ryan, Characterization of phenolic composition in lamiaceae spices by LC-ESI-MS/MS, J. Agric. Food Chem., 2010, 58, 10576–10581 CrossRef CAS PubMed.
- N. Martins, L. Barros, C. Santos-Buelga, S. Silva, M. Henriques and I. C. F. R. Ferreira, Decoction, infusion and hydroalcoholic extract of cultivated thyme: Antioxidant and antibacterial activities, and phenolic characterisation, Food Chem., 2014, 167, 131–137 CrossRef PubMed.
- P. Nocera, A. Lettieri and M. Catauro, A polyphenol complex from Thymus vulgaris L. plants cultivated in the Campania Region (Italy): New perspectives against neuroblastoma, J. Funct. Foods, 2016, 20, 253–266 CrossRef.
- L. Barros, M. Dueñas, M. D. Inês, M. Sousa João, C. Santos-buelga and I. C. F. R. Ferreira, Phenolic profiles of cultivated, in vitro cultured and commercial samples of Melissa officinalis L. infusions, Food Chem., 2013, 136, 1–8 CrossRef CAS PubMed.
- H. Chen, Q. Zhang, X. Wang and Q. Wang, Qualitative analysis and simultaneous quantification of phenolic compounds in the aerial parts of salvia miltiorrhiza by HPLC- DAD and ESI/MSn, Phytochem. Anal., 2011, 22, 247–257 CrossRef CAS PubMed.
- M. Ruan, Y. Li, X. Li, J. Luo and L. Kong, Qualitative and quantitative analysis of the major constituents in Chinese medicinal preparation Guan-Xin-Ning injection by HPLC-DAD-ESI-MSn, J. Pharm. Biomed. Anal., 2012, 59, 184–189 CrossRef CAS PubMed.
- G. Zeng, H. Xiao, J. Liu and X. Liang, Identification of phenolic constituents in Radix Salvia miltiorrhizae by liquid chromatography/electrospray ionization mass spectrometry, Rapid Commun. Mass Spectrom., 2006, 20, 499–506 CrossRef CAS PubMed.
- B. Hauck, J. A. Gallagher, S. M. Morris, D. Leemans and A. L. Winters, Soluble phenolic compounds in fresh and ensiled orchard grass (Dactylis glomerata, L.), a common species in permanent pastures with potential as a biomass feedstock, Agric. Food Chem., 2014, 62, 468–475 CrossRef CAS PubMed.
- F. Ferreres, B. M. Silva, P. B. Andrade, R. M. Seabra and M. A. Ferreira, Approach to the study of C-Glycosyl Flavones by Ion Trap HPLC-PAD-ESI/MS/MS: Application to seeds of Quince (Cydonia oblonga), Phytochem. Anal., 2003, 14, 352–359 CrossRef CAS PubMed.
- M. Chaabi, N. Beghidja, S. Benayache and A. Lobstein, Activity-guided isolation of antioxidant principles from Limoniastrum feei (Girard) Batt., Z. Naturforsch., 2008, 63, 801–807 CAS.
- M. N. Clifford, K. L. Johnston, S. Knight and N. Kuhnert, Hierarchical scheme for LC-MSn identification of chlorogenic acids, J. Agric. Food Chem., 2003, 51, 2900–2911 CrossRef CAS PubMed.
- M. N. Clifford, S. Knight and N. Kuhnert, Discriminating between the six isomers of dicaffeoylquinic acid by LC-MSn, J. Agric. Food Chem., 2005, 53, 3821–3832 CrossRef CAS PubMed.
- D. Campoccia, L. Montanaro and R. C. Arciola, A review of the biomaterials technologies for infection-resistant surfaces, Biomaterials, 2013, 34, 8533–8554 CrossRef CAS PubMed.
- T. P. Cushnie, V. E. S. Hamilton, D. G. Chapman, P. W. Taylor and A. J. Lamb, Aggregation of Staphylococcus aureus following treatment with the antibacterial flavonol galangin, J. Appl. Microbiol., 2007, 103, 1562–1567 CrossRef CAS PubMed.
- T. P. T. Cushnie and A. J. Lamb, Antimicrobial activity of flavonoids, Int. J. Antimicrob. Agents, 2005, 26, 343–356 CrossRef CAS PubMed.
- T. P. T. Cushnie and A. J. Lamb, Agents recent advances in understanding the antibacterial properties of flavonoids, Int. J. Antimicrob. Agents, 2011, 38, 99–107 CrossRef CAS PubMed.
- R. Hendra, S. Ahmad, A. Sukari and M. Y. Shukor, Flavonoid analyses and antimicrobial activity of various parts of Phaleria macrocarpa (Scheff.) Boerl Fruit, Int. J. Mol. Sci., 2011, 12, 3422–3431 CrossRef CAS PubMed.
- M. Li and Z. Xu, Quercetin in a lotus leaves extract may be responsible for antibacterial activity, Arch. Pharmacal Res., 2008, 31, 640–644 CrossRef CAS PubMed.
- G. Mandalari, R. N. Bennett, G. Bisignano, D. Trombetta, A. Saija, C. B. Faulds and M. J. Gasson, Antimicrobial activity of flavonoids extracted from bergamot (Citrus bergamia, Risso) peel, a byproduct of the essential oil industry, J. Appl. Microbiol., 2007, 103, 2056–2064 CrossRef CAS PubMed.
- L. S. Teffo, M. A. Aderogba and J. N. Eloff, Antibacterial and antioxidant activities of four kaempferol methyl ethers isolated from Dodonaea viscosa, Jacq. var. angustifolia leaf extracts, S. Afr. J. Bot., 2010, 76, 25–29 CrossRef CAS.
- H. B. Nayaka, R. L. Londonkar, M. K. Umesh and A. Tukappa, Antibacterial attributes of apigenin, isolated from Portulaca oleracea L., Int. J. Bacteriol., 2014, 2014, 1–8 CrossRef PubMed.
- Y. Sato, S. Suzaki, T. Nishikawa and M. Kihara, Phytochemical flavones isolated from Scutellaria barbata and antibacterial activity against methicillin-resistant Staphylococcus aureus, J. Ethnopharmacol., 2000, 72, 483–488 CrossRef CAS PubMed.
- M. Sato, S. Fujiwara, H. Tsuchiya, T. Fujii, M. Iinuma, H. Tosa and Y. Ohkawa, Flavones with antibacterial activity against cariogenic bacteria, J. Ethnopharmacol., 1996, 54, 171–176 CrossRef CAS PubMed.
- L. E. Alcaraz, S. E. Blanco, O. N. Puig, F. Tomas and F. H. Ferretti, Antibacterial activity of flavonoids against methicillin-resistant Staphylococcus aureus strains, J. Theor. Biol., 2000, 205, 231–240 CrossRef CAS PubMed.
- W. F. Zheng, R. X. Tan, L. Yang and Z. L. Liu, Two Flavones from Artemisia giraldil and their antimicrobial activity, Planta Med., 1996, 62, 160–162 CrossRef CAS PubMed.
- S. G. Dastidar, A. Manna, K. A. Kumar, K. Mazumdar, N. K. Dutta, A. N. Chakrabarty, N. Motohashi and Y. Shirataki, Studies on the antibacterial potentiality of isoflavones, Int. J. Antimicrob. Agents, 2004, 23, 99–102 CrossRef CAS PubMed.
- A. P. Mukne, V. Viswanathan and A. G. Phadatare, Structure pre-requisites for isoflavones as effective antibacterial agents, Pharmacogn. Rev., 2011, 5, 13–19 CrossRef CAS PubMed.
- H. Hong, M. R. Landauer, M. A. Foriska and G. D. Ledney, Antibacterial activity of the soy isoflavone genistein, J. Basic Microbiol., 2006, 46, 329–335 CrossRef CAS PubMed.
- C. Morel, F. R. Stermitz, G. Tegos and K. Lewis, Isoflavones as potentiators of antibacterial activity, Agric. Food Chem., 2003, 51, 5677–5679 CrossRef CAS PubMed.
- M. Iinuma, H. Tsuchiya, M. Satot, J. Yokoyama, M. Ohyama, Y. Ohkawa, T. Tanaka, S. Fujiwarax and T. Fujiix, Flavanones with potent antibacterial activity against methicillin-resistant Staphylococcus aureus, J. Pharm. Pharmacol., 1994, 46, 892–895 CrossRef CAS PubMed.
- K. Simin, Z. Ali, S. M. Khaliq-uz-zaman and A. Viqar uddin, Structure and biological activity of a new rotenoid from Pongamia pinnata, Nat. Prod. Lett., 2010, 16, 351–357 CrossRef PubMed.
- H. Xu and S. F. Lee, Activity of plant flavonoids against antibiotic- resistant bacteria activity of plant flavonoids against Antibiotic- Resistant Bacteria, Phyther. Res., 2001, 15, 39–43 CrossRef CAS.
- M. Daglia, Polyphenols as antimicrobial agents, Curr. Opin. Biotechnol., 2012, 23, 174–181 CrossRef CAS PubMed.
- Y. Liu, N. Murakami, H. Ji, P. Abreu, S. Zhang, N. Murakami, H. Ji, P. Abreu, S. Z. Antimalarial, Y. Liu, N. Murakami, H. Ji, P. Abreu and S. Zhang, Antimalarial flavonol glycosides from Euphorbia hirta, Pharm. Biol., 2007, 45, 278–281 CrossRef CAS.
- I. Ben, E. Hadj, M. Chaouachi, R. Bahri and I. Chaieb, Chemical composition and antioxidant, antibacterial, allelopathic and insecticidal activities of essential oil of Thymus algeriensis Boiss. et Reut., Ind. Crops Prod, 2015, 77, 631–639 CrossRef.
- F. Guesmi, M. Ben Farhat, M. Mejri and A. Landoulsi, In-vitro assessment of antioxidant and antimicrobial activities of methanol extracts and essential oil of Thymus hirtus, sp. algeriensis, Lipids Health Dis., 2014, 13, 1–12 CrossRef PubMed.
- N. Trabelsi, S. Oueslati, C. Henry-vitrac, P. Waffo-téguo, F. Medini, J. Mérillon, C. Abdelly and R. Ksouri, Phenolic contents and biological activities of Limoniastrum guyonianum fractions obtained by centrifugal partition chromatography, Ind. Crops Prod., 2013, 49, 740–746 CrossRef CAS.
- A. Bouzidi, A. Benzarti, A. El Arem, A. Mahfoudhi, S. Hammami, M. Gorcii, M. Mastouri, A. N. Hellal and M. Zine, Chemical composition, antioxidant and antimicrobial effects of Tunisian Limoniastrum guyonianum, Durieu ex Boiss extracts, Pak, J. Pharm. Sci., 2016, 29, 1299–1305 CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7fo01315a |
| This journal is © The Royal Society of Chemistry 2018 |
