Piceatannol attenuates behavioral disorder and neurological deficits in aging mice via activating the Nrf2 pathway
Yan
Zhang†
a,
Li-Hong
Zhang†
a,
Xi
Chen
 *bc,
Ning
Zhang
a and
Guang
Li
c
*bc,
Ning
Zhang
a and
Guang
Li
c
aJiamusi College, Heilongjiang University of Chinese Medicine, Jiamusi 154007, China
bInstitute of Medicinal Plant Development, Chinese Academy of Medical Sciences & Peking Union Medical College, Beijing, 100193, China. E-mail: chenxi_1999a@163.com
cYunnan Branch, Institute of Medicinal Plant Development, Chinese Academy of Medical Sciences & Peking Union Medical College, Jing Hong, 666100, China
First published on 20th November 2017
Abstract
Aging is a complex process that is accompanied by neurological damage. Chronic injection of D-galactose (D-gal) can accelerate the aging process similar to natural aging and is commonly used to build an aging model to investigate aging. In the present study, the effects of piceatannol on D-gal-induced aging in mice were evaluated. Piceatannol treatment showed an observable anti-aging effect. Results obtained in vivo showed that piceatannol retained spontaneous motor activity and enhanced spatial learning and memory abilities in mice in which aging was induced by D-gal. Morphometric analysis displayed that piceatannol prevented D-gal-induced neuronal loss, increased the number of Nissl bodies, and promoted cell proliferation in the hippocampus and cortex. Piceatannol also significantly decreased the level of MDA and elevated SOD and CAT activity in the hippocampal and cortical tissues. Furthermore, western blotting results revealed that piceatannol treatment noticeably reversed the suppression of Nrf2 nuclear translocation and increased the expressions of HO-1 and NOQ1 in mice with aging induced by D-gal. Furthermore, piceatannol activated the Nrf2 pathway in natural aging mice, whereas treatment with the Nrf2 inhibitor brusatol reversed the increased expressions of Nrf2, HO-1, and NOQ1. In conclusion, treatment with piceatannol ameliorates behavioral disorder and brain injury in an aging mouse model; this suggests that piceatannol is a promising pharmaceutical candidate for the treatment of age-associated diseases.
1. Introduction
Aging is an inevitable natural process, which is a major cause for progressive decline of brain function and gradual memory loss.1,2 With an increase in the proportion of the elderly population, exploration of the mechanisms of aging and anti-aging drugs has become an increasingly urgent requirement. The aging process involves a number of complicated factors. However, numerous studies have shown that free radical is a key contributor to the aging process. Previous studies have indicated that alteration in redox status caused by imbalance between free radical generation and antioxidant defense mechanisms in erythrocytes occurs during the aging process.3–5 In addition, a majority of the long-lifespan species have evolved ways to attenuate oxidative stress and maintain homeostasis.6,7 Therefore, resistance to oxidative stress may be a useful therapeutic strategy to delay the aging process or prevent age-related diseases.Nuclear factor erythroid 2-related factor 2 (Nrf2), which is a member of leucine zipper transcription factor family, has been regarded as one of the pivotal regulators of cytoprotective and detoxification genes to prevent oxidative stress.8 Exposed to oxidative stress, Nrf2 dissociates from Keap1 and is allowed to translocate into the nucleus; it then binds to the antioxidant response element (ARE) to induce a battery of antioxidant and phase II detoxification enzymes including NADPH quinone oxidoreductase 1 (NQO1), and heme oxygenase-1 (HO-1).9,10 It has been reported that the Nrf2 pathway can be impaired during the aging process.11 Accumulated evidence has also demonstrated that Nrf2-deficient mice are more sensitive to oxidative stress, whereas Nrf2 overexpression may have an important role in neuroprotection.12–14
Piceatannol (3,3′,4,5′-trans-tetrahydroxystilbene) is a naturally occurring stilbene found in berries, peanuts, grapes, red wine, and white tea.15 Modern pharmacological studies have reported that piceatannol has antioxidant, anti-inflammatory, anti-mutagenic, and anti-diabetic effects.16,17 Recently, it has been reported that piceatannol protects against glutamate-mediated oxidative injury in HT22 neuronal cells by inducing HO-1 expression.18 This data suggest that piceatannol may possess neuroprotective effects. However, the effects and underlying mechanisms of piceatannol in aging mice remain ambiguous.
In the present study, we estimated the neuroprotective effect of piceatannol and the possible mechanisms of its action in the aging model. We hypothesized that piceatannol might possess ameliorative effect on D-gal-induced behavioral disturbance and neurological damage, and the possible mechanisms may be the involvement of the Nrf2 pathway.
2. Materials and methods
2.1 Chemicals and reagents
Piceatannol (purity 98%) and brusatol (purity 98%) were purchased from Adamas Reagent Co., Ltd (Shanghai, China). D-Gal (purity 99%) was obtained from Aladdin Chemistry Co. (Shanghai, China). Elisa kits were purchased from Nanjing Jiancheng Biotechnology (Nanjing, China).2.2 Animals
Male Kunming mice (18–20 g, Animal Experimental Center of Wuhan University, approval number SCXK 2011-0012) were fed normally for one week to acclimatize them to the housing environment prior to the experiments. The mice were housed under standard laboratory conditions (25 ± 1 °C, a 12 h light/12 h dark cycle). Standard rodent chow and water were made available ad libitum. All the experiments and animal care were performed strictly in accordance with the Provision and General Recommendation of Chinese Experimental Animals Administration Legislation and approved by the Science and Technology Department of the Heilongjiang Province.2.3 Experimental designs
In this study, aging in the model was induced by daily subcutaneous injection of D-gal (150 mg kg−1) for 8 weeks. Moreover, normal mice were subjected to saline in the same way. Mice were randomly divided into three groups (n = 15): (1) normal group: normal mice were orally administered with 0.5% carboxymethyl cellulose (CMC) via gavage for 8 weeks; (2) model group: aging mice were orally administered with 0.5% CMC via gavage for 8 weeks; (3) piceatannol group: aging mice were orally administered with piceatannol (20 mg kg−1, dissolved in 0.5% CMC) via gavage for 8 weeks.To investigate the mechanism of piceatannol, 21-month old male C57BL/6J mice were used for further research. The naturally aging mice were randomly divided into three groups (n = 6): (1) natural aging group: natural aging mice were orally administered with 0.5% CMC via gavage for 4 weeks; (2) natural aging + piceatannol group: natural aging mice were orally administered with piceatannol (20 mg kg−1, dissolved in 0.5% CMC) via gavage for 4 weeks; (3) natural aging + piceatannol + brusatol group: natural aging mice were orally administered with piceatannol (20 mg kg−1) via gavage for 4 weeks and brusatol (2 μmol kg−1) was administered 2 h before piceatannol gavage. After 4-week treatment, the C57BL/6J mice were sacrificed, and western blot was performed to detect the expressions of various proteins using western blot in the hippocampal and cortical tissues.
2.4 Open field test
The locomotor activity and exploratory behavior of mice were estimated using the open field test.19 The open field apparatus (45 × 45 × 40 cm) was equally divided into 25 squares. After acclimatizing to the apparatus for 1 min, the relative center distance and the total moving distance of mice were determined for 5 min. After each test, the apparatus was cleaned with 70% ethanol and dried.2.5 Morris water maze test
The Morris water maze test (MWM) was used to evaluate the spatial learning and memory abilities of the all groups of mice.20 The MWM apparatus (120 cm in diameter, 50 cm in height) was filled with water (20 ± 1 °C) to a depth of 23 cm. The pool was divided into four quadrants, and a platform (10 cm in diameter, 22 cm in height) was placed at the center of the third quadrant. In brief, the Morris water maze test consists of place for navigation training and a probe test. During the training period, the mice were given four trial sessions each day for four days, and the escape latencies were determined. Once the mouse reached the platform, it was allowed to remain on it for 20 s. If the mouse was not able to reach the platform within 60 s, it was guided to the platform and permitted to stay on it for 20 s. On day 5, the probe test was performed. Each mouse was permitted to swim for 60 s after the platform was taken away.2.6 Morphometric analysis
After behavior tests, five mice were selected randomly from each group and anesthetized with ether. The mice were perfused in vivo via the left ventricle with 0.9% normal saline followed by 4% paraformaldehyde. The brains were removed with care and fixed with 4% paraformaldehyde for 48 h. A standard paraffin block from hippocampus and cortex was used for hematoxylin–eosin (H&E) staining and Nissl staining.21,222.7 BrdU labeling and immunohistochemistry
On day 63 of treatment, five mice of each group were injected with BrdU (50 mg kg−1) intraperitoneally three times with an interval of 8 hours. The mice were killed 24 hours after the last BrdU injection to investigate cell proliferation using immunofluorescence methods.The sections were dewaxed, hydrated, and then pre-treated in 50% formamide in SSC for 2 hours at 65 °C, followed by 5 minutes in SSC, then 30 minutes in 2 M HCl at 37 °C and 10 minutes in 0.1 M PBS. The endogenous peroxidase was blocked by 0.3% H2O2 and followed by incubation for 2 hours in 5% bull serum albumin (BSA). The sections were incubated overnight at 4 °C with the primary antibody anti-BrdU with a dilution of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000. Next, the sections were incubated for 1 hour with the specific goat secondary antibody conjugated with fluorescein isothiocyanate with a dilution of 1
1000. Next, the sections were incubated for 1 hour with the specific goat secondary antibody conjugated with fluorescein isothiocyanate with a dilution of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000. The nuclei were stained using DAPI.
1000. The nuclei were stained using DAPI.
2.8 Determination of MDA, SOD, and CAT
Herein, five mice from each group were sacrificed after behavior tests, and the hippocampal and cortical tissues were separated and homogenized in 50 mM cold potassium phosphate buffer (pH 7.4). Then, the mixture was centrifuged at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 10 min at 4 °C, and the supernatant was obtained for further biochemical analysis. The levels of MDA, SOD, and CAT were determined using commercial reagent kits based on manufacturers’ recommendations (Beyotime Biotechnology, China).
000 rpm for 10 min at 4 °C, and the supernatant was obtained for further biochemical analysis. The levels of MDA, SOD, and CAT were determined using commercial reagent kits based on manufacturers’ recommendations (Beyotime Biotechnology, China).
2.9 Western blotting analysis
At the end of the experiment, five mice from each group were sacrificed under anesthesia. The hippocampal and cortical tissues were obtained with care. The total protein was isolated from the frozen hippocampus tissues using a protein extraction kit (Beyotime Biotechnology, China), and the nuclear protein was isolated by the nuclear and cytoplasmic protein extraction kit according to the procedure stated on the kit (Beyotime Biotechnology, China). The protein concentrations were determined using the BCA protein assay kit (Beyotime Biotechnology, China).Equal amounts of proteins were loaded onto an SDS-polyacrylamide gel for electrophoresis and then transferred to a polyvinylidene-difluoride (PVDF) membrane using a transblotting apparatus. After blocking with a blocking buffer (5% BSA in TBS/Tween 0.1%) for 1 h at room temperature, the membrane was incubated overnight at 4 °C with primary antibodies: anti-Nrf2 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500), anti-HO-1 (1
500), anti-HO-1 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000), anti-NQO1(1
1000), anti-NQO1(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000), anti-Lamin B2, and anti-β-actin (1
1000), anti-Lamin B2, and anti-β-actin (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000) antibodies (Santa Cruz Biotechnology, USA). After being washed with TBST thrice, the membranes were incubated with secondary antibody HRP-conjugated anti-rabbit for 1 h at room temperature. The protein bands were detected using the enhanced ECL Western blot detection kit. The intensity of each band was quantified by the Image pro plus 6.0.
1000) antibodies (Santa Cruz Biotechnology, USA). After being washed with TBST thrice, the membranes were incubated with secondary antibody HRP-conjugated anti-rabbit for 1 h at room temperature. The protein bands were detected using the enhanced ECL Western blot detection kit. The intensity of each band was quantified by the Image pro plus 6.0.
2.10 Statistical analysis
All data are expressed as mean ± SEM. Data were analyzed using the SPSS 19.0 statistics software by one-way ANOVA or Student's t-test. P < 0.05 was considered as statistically significant.3. Results
3.1 Effect of piceatannol on body weight in D-gal-induced aging mice and naturally aging mice
As shown in Table 1, D-gal injection significantly inhibited the increase in the body weight of mice (P < 0.01). However, the suppression of D-gal was reversed by piceatannol (P < 0.01). In naturally aging mice, the body weight was not affected by piceatannol or brusatol (Table 2).| Group | Initial | Final |
|---|---|---|
| Data was expressed as mean ± SEM. ##P < 0.01 versus control group; **P < 0.01 versus model group. | ||
| Normal | 18.89 ± 1.63 | 34.02 ± 2.58 |
| Model | 18.92 ± 1.38 | 27.69 ± 3.24## |
| Piceatannol | 18.78 ± 1.78 | 32.26 ± 2.75** |
| Group | Initial | Final |
|---|---|---|
| Data was expressed as mean ± SEM. | ||
| Aging | 28.92 ± 2.01 | 36.92 ± 3.15 |
| Aging + piceatannol | 29.20 ± 1.89 | 37.55 ± 3.47 |
| Aging + piceatannol + brusatol | 29.14 ± 2.36 | 36.26 ± 3.21 |
3.2 Protective effect of piceatannol on spontaneous motor activity in mice with aging induced by D-gal
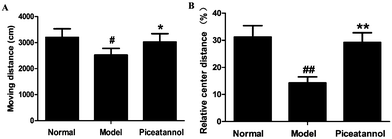
The open field test was used to evaluate the motor function and exploratory activity (Fig. 1). Compared with the normal group, distance travelled and relative center distance during this test significantly decreased (P < 0.05, P < 0.01). Moreover, mice with piceatannol treatment revealed a significant increase in the distance travelled and relative center distance as compared to the model group (P < 0.05, P < 0.01). Overall, the data demonstrated that piceatannol attenuated the impairment of spontaneous motor activity induced by chronic treatment with D-gal.3.3 Protective effect of piceatannol on learning and memory deficits in mice with aging induced by D-gal
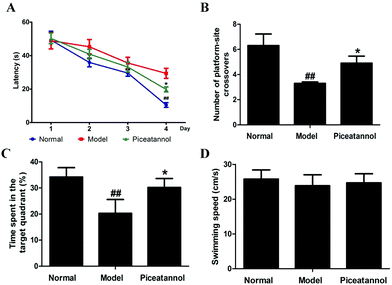
Compared with that of the normal group, the escape latency of the model group was dramatically prolonged in the training period (Fig. 2A). However, treatment with piceatannol significantly shortened the escape latency as compared to the case of the model group (P < 0.05). In the probe trial (Fig. 2B and C), the explored time in the target quadrant and the number of platform-site crossovers in the model group were significantly decreased compared to those of the normal group (P < 0.01, P < 0.01). Consistent with the abovementioned results, piceatannol supplementation reversed these poor performances in the spatial test. In addition, the swimming speed was not affected by D-gal or piceatannol. These results demonstrated that piceatannol was able to protect the mice against learning and memory deficits, which were induced by chronic treatment with D-gal.3.4 Effect of piceatannol on morphological changes in cortex and hippocampus
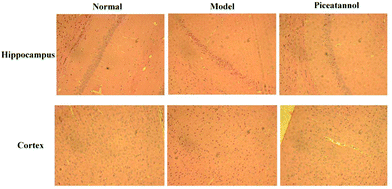
H&E staining was conducted to investigate the morphological alterations in the cortex and hippocampus CA1 region (Fig. 3). The results revealed that the morphology in the cortex and hippocampus CA1 region of the normal group was normal. However, the model group showed disordered hippocampal pyramidal cell layers and loose structures in hippocampal and cortical neurons. Furthermore, piceatannol improved these neuronal damages.3.5 Effect of piceatannol on changes in Nissl bodies in cortex and hippocampus
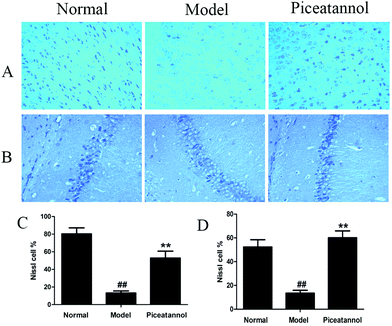
The Nissl body is considered a characteristic structure of neuron, and the number of Nissl bodies demonstrates the condition of the neuronal cell.23 As shown in Fig. 4, the Nissl bodies of the model group were lightly stained; however, the Nissl bodies of normal and piceatannol groups were deeply stained in the cortex and hippocampus CA1 region. In addition, piceatannol reversed the decrease in the number of positive Nissl cells induced by D-gal (P < 0.01).3.6 Effect of piceatannol on cell proliferation in cortex and hippocampus
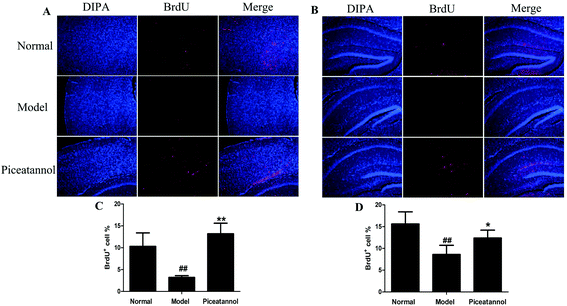
To investigate whether piceatannol induced new cell generation in cortex and hippocampus, the number of new cells was detected using immunofluorescence of BrdU. As shown in Fig. 5, the number of BrdU+ cells was lower in the cortex and hippocampus of D-gal-administered mice as compared to that in the normal group (P < 0.01, P < 0.01). On the contrary, BrdU+ cells significantly increased in the piceatannol treatment group as compared to those in the model group (P < 0.01, P < 0.05).3.7 Treatment with piceatannol decreased the oxidative stress induced by D-gal
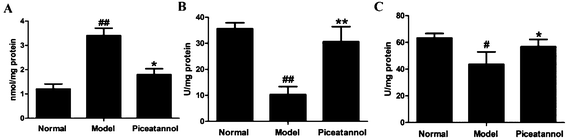
Treatment with D-gal was associated with oxidative stress in brain, as indicated by an increase in the MDA levels (P < 0.01). Administration of piceatannol significantly decreased the MDA levels as compared to the case of the model group (P < 0.05). We also detected the activities of SOD and CAT after chronic treatment with D-gal. As shown in Fig. 6B and C, piceatannol treatment observably reversed the decreased activities of SOD and CAT (P < 0.01, P < 0.05). Overall, these results indicated that piceatannol exerted antioxidant effects in mice with aging induced by chronic treatment with D-gal.3.8 Effects of piceatannol on the Nrf2 pathway in mice with aging induced by D-gal
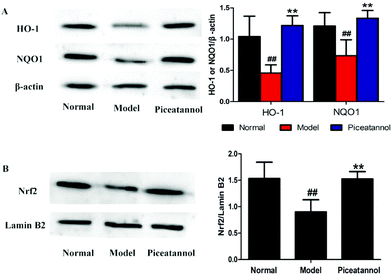
To address whether the beneficial effect of piceatannol is involved in the regulation of phase II detoxification, we investigated the expressions of HO-1 and NQO1 in the hippocampal and cortical tissues. As shown in Fig. 7A, the expressions of HO-1 and NQO1 were significantly lower in the model control group (P < 0.01, P < 0.01), whereas piceatannol reversed the decreased expressions of HO-1 and NQO1 (P < 0.01, P < 0.01).Nrf2, an important transcription factor, binds to ARE to regulate the phase II antioxidant enzymes including HO-1 and NQO1.10 To confirm whether piceatannol exerts its protective effect by regulating the Nrf2 pathway, we have measured the Nrf2 protein expression in the nuclear fraction from the hippocampal and cortical tissues. As shown in Fig. 7B, piceatannol effectively reversed the decreased Nrf2 expression (P < 0.01).
3.9 Effects of piceatannol on the Nrf2 pathway in natural aging mice
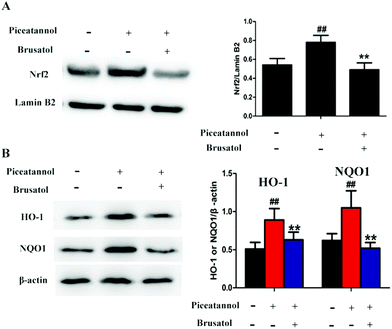
To further study the mechanism of piceatannol, naturally aging mice were treated with piceatannol and the Nrf2 inhibitor brusatol. Results (Fig. 8) showed that piceatannol significantly increased the levels of Nrf2, HO-1, and NQO1 (P < 0.01), whereas brusatol reversed piceatannol-induced increase in Nrf2, HO-1, and NQO1 expression (P < 0.01). These data suggest that the Nrf2 signaling pathway is involved in the protective effect of piceatannol in the aging mice.4. Discussion
In the present study, we investigated the effect of piceatannol on behavioral function and neurological function in mice with aging induced by D-gal. D-gal, a reducing sugar, is able to form advanced glycation end products in vivo.24 The model of aging in mice and rats induced by D-gal injection is extensively used to investigate aging.25,26 Chronic D-gal injection accelerates the aging process resulting from the rapid accumulation of free radicals.27In our study, the behavioral and neurological function of the model group confirmed that chronic D-gal treatment successfully led to aging. Our results demonstrated that piceatannol administration could maintain spontaneous motor activity and improve learning and memory ability. Additionally, piceatannol supplementation prevented neurological damage, such as reduction of Nissl body loss and promotion of cell proliferation, caused by the D-gal injection. Overall, the abovementioned results indicated that piceatannol exerted a protective effect on aging induced by D-gal in vivo.
Oxidative stress is one of most popular hypothesis resulting in aging.28D-Gal naturally exists in the body, but D-gal overload can form a superoxide anion and oxygen-derived free radicals, leading to oxidative stress.29 Furthermore, a high content of D-gal can accelerate aging of the tissues and organs, which is similar to the normal senescence process. It is reported that rats upon chronic treatment with D-gal displayed increased generation of ROS and decreased activities of antioxidant enzymes in body fluids and tissues.30,31 In our study, the increased MDA and decreased activities of antioxidant enzymes (SOD and CAT) were found, and piceatannol significantly reversed these changes in the aging mice. MDA, which is the end product of polyunsaturated fatty acid peroxidation, is extensively considered a biomarker of free radical-mediated lipid peroxidation damage. In the antioxidant defense system, SOD and CAT are important components. SOD is responsible for converting superoxide (O2) into hydrogen peroxide (H2O2), and H2O2 is decomposed into water and oxygen by the catalysis of CAT.32
Nrf2, a vital factor in oxidative stress, modulates the expressions of antioxidant proteins by interacting with the ARE. Under normal physiological conditions, Nrf2 localizes in the cytoplasm, which binds with a Kelch-like ECH-associated protein (Keap1) and is rapidly degraded by the ubiquitin-proteasome.33 Upon exposure to electrophiles or oxidative stress, Nrf2 dissociates from Keap1 and translocates into the nucleus. Next, Nrf2 in the nucleus combined with ARE facilitates transcription of downstream target genes including HO-1and NQO1, which together suppress the oxidative stress. Therefore, expressions of downstream target genes play a pivotal role in maintaining redox homeostasis.34 On analyzing the chemical structure of piceatannol, it has been observed that piceatannol is a polyphenol. Many polyphenols have been reported to be a potent Nrf2 activator, and they are easily oxidized to their quinone intermediates, which may modify the Cys residue(s) on the cytosolic inhibitory protein Keap1 to dissociate the Keap1–Nrf2 complex; this leads to the activation of Nrf2.12–14,35–37 Therefore, we speculate that piceatannol may activate Nrf2 by inhibiting protein Keap1 to dissociate the Keap1–Nrf2 complex. In our study, we found that treatment with piceatannol activated the Nrf2 signal pathway and then enhanced the downstream antioxidant protein expression in the mice with aging induced by D-gal. Moreover, piceatannol upregulated the Nrf2 signal pathway in the naturally aging mice. However, the Nrf2 inhibitor brusatol reversed the Nrf2 up-regulation of piceatannol; this suggested that the beneficial effects of piceatannol were related to Nrf2 in the aging mice.
Previous research has indicated that after oral administration of piceatannol, piceatannol reaches Cmax within 15 min. The Cmax and area under the curve (AUC) for piceatannol increased in a dose-dependent manner. A great AUC for intact piceatannol likely contributes to its important biological functions.38 Although there was no specific data to demonstrate the brain penetration of piceatannol, accumulated evidence indicated that piceatannol might possess a promising neuroprotective effect.39–41
Overall, our finding described the pharmacological effect of piceatannol on aging induced by D-gal. Oxidative stress was regarded as an important factor affecting the function of brain. In addition, the involvement Nrf2 activation may be responsible for the beneficial effects of piceatannol; this also provides a new insight into the therapeutic strategy for neurological deficits during the senescence process.
Conflicts of interest
The authors have declared no conflict of interest.Acknowledgements
This work was supported by the National Natural Science Foundation of China (no. 81673667), the CAMS Innovation Fund for Medical Science (CIFMS) (no. 2016-I2M-3-015), the Middle-aged Academic Leaders and Reserved Talent Foundation of Yunnan (no. 2013HB099), and the Science Research Fund Project of Heilongjiang University of Chinese Medicine (no. 201736).References
- N. Raz, P. Ghisletta, K. M. Rodrigue, K. M. Kennedy and U. Lindenberger, NeuroImage, 2010, 51, 501–511 CrossRef PubMed.
- J. H. Morrison and P. R. Hof, Science, 1997, 278, 412–419 CrossRef CAS PubMed.
- T. Cebe, P. Atukeren, K. Yanar, A. I. Kuruc, T. Ozan, A. Kunbaz, M. E. Sitar, R. Mirmaroufizibandeh, S. Aydin and U. Cakatay, Exp. Gerontol., 2014, 57, 132–140 CrossRef CAS PubMed.
- M. I. Waly, A. Ali, N. Guizani, A. S. Al-Rawahi, S. A. Farooq and M. S. Rahman, Asian Pac. J. Cancer Prev., 2012, 13, 4051–4055 CrossRef PubMed.
- D. Kumar and S. I. Rizvi, Rejuvenation Res., 2014, 17, 446–452 CrossRef PubMed.
- V. I. Perez, R. Buffenstein, V. Masamsetti, S. Leonard, A. B. Salmon, J. Mele, B. Andziak, T. Yang, Y. Edrey, B. Friguet, W. Ward, A. Richardson and A. Chaudhuri, Proc. Natl. Acad. Sci. U. S. A., 2009, 106, 3059–3064 CrossRef CAS PubMed.
- A. B. Salmon, V. I. Perez, A. Bokov, A. Jernigan, G. Kim, H. Zhao, R. L. Levine and A. Richardson, FASEB J., 2009, 23, 3601–3608 CrossRef CAS PubMed.
- X. L. Zhang, Y. H. Yuan, Q. H. Shao, Z. Z. Wang, C. G. Zhu, J. G. Shi, K. L. Ma, X. Yan and N. H. Chen, Toxicol. Lett., 2017, 271, 74–83 CrossRef CAS PubMed.
- Y. J. Surh, Nat. Rev. Cancer, 2003, 3, 768–780 CrossRef CAS PubMed.
- G. P. Sykiotis and D. Bohmann, Sci. Signal., 2010, 3, re3 Search PubMed.
- P. Chen, D. He, Y. Zhang, S. Yang, L. Chen, S. Wang, H. Zou, Z. Liao, X. Zhang and M. Wu, Food Funct., 2016, 7, 4576–4588 CAS.
- S. Peng, B. Zhang, X. Meng, J. Yao and J. Fang, J. Med. Chem., 2015, 58, 5242–5255 CrossRef CAS PubMed.
- S. Peng, B. Zhang, J. Yao, D. Duan and J. Fang, Food Funct., 2015, 6, 2091–2100 CAS.
- S. Peng, J. Yao, Y. Liu, D. Duan, X. Zhang and J. Fang, Food Funct., 2015, 6, 2813–2823 CAS.
- M. A. Seyed, I. Jantan, S. N. Bukhari and K. Vijayaraghavan, J. Agric. Food Chem., 2016, 64, 725–737 CrossRef CAS PubMed.
- Y. J. Surh and H. K. Na, Adv. Exp. Med. Biol., 2016, 928, 185–211 CrossRef CAS PubMed.
- H. Piotrowska, M. Kucinska and M. Murias, Mutat. Res., 2012, 750, 60–82 CAS.
- Y. Son, S. J. Byun and H. O. Pae, Amino Acids, 2013, 45, 393–401 CrossRef CAS PubMed.
- S. Sharma and S. Fulton, Int. J. Obes., 2013, 37, 382–389 CrossRef CAS PubMed.
- C. V. Vorhees and M. T. Williams, Nat. Protoc., 2006, 1, 848–858 CrossRef PubMed.
- H. Liu, Y. Deng, J. Gao, Y. Liu, W. Li, J. Shi and Q. Gong, Curr. Alzheimer Res., 2015, 12, 673–683 CrossRef CAS PubMed.
- S. Yang, Q. Gong, Q. Wu, F. Li, Y. Lu and J. Shi, Phytomedicine, 2014, 21, 712–716 CrossRef CAS PubMed.
- J. Nie, Y. Tian, Y. Zhang, Y. L. Lu, L. S. Li and J. S. Shi, PeerJ, 2016, 4, e2739 Search PubMed.
- X. Song, M. Bao, D. Li and Y. M. Li, Mech. Ageing Dev., 1999, 108, 239–251 CrossRef CAS PubMed.
- T. Ali, H. Badshah, T. H. Kim and M. O. Kim, J. Pineal Res., 2015, 58, 71–85 CrossRef CAS PubMed.
- S. Zhang, Z. Dong, Z. Peng and F. Lu, PLoS One, 2014, 9, e97573 Search PubMed.
- X. Cui, P. Zuo, Q. Zhang, X. Li, Y. Hu, J. Long, L. Packer and J. Liu, J. Neurosci. Res., 2006, 84, 647–654 CrossRef CAS PubMed.
- P. Wu, G. Ma, N. Li, Q. Deng, Y. Yin and R. Huang, Food Chem., 2015, 173, 194–202 CrossRef CAS PubMed.
- J. Lu, Y. L. Zheng, D. M. Wu, L. Luo, D. X. Sun and Q. Shan, Biochem. Pharmacol., 2007, 74, 1078–1090 CrossRef CAS PubMed.
- E. B. Kalaz, J. Coban, A. F. Aydin, I. Dogan-Ekici, S. Dogru-Abbasoglu, S. Oztezcan and M. Uysal, J. Physiol. Biochem., 2014, 70, 15–25 CrossRef CAS PubMed.
- K. V. Anand, M. S. Mohamed Jaabir, P. A. Thomas and P. Geraldine, Geriatr. Gerontol. Int., 2012, 12, 741–750 CrossRef PubMed.
- Y. N. Li, Y. Guo, M. M. Xi, P. Yang, X. Y. Zhou, S. Yin, C. X. Hai, J. G. Li and X. J. Qin, Oxid. Med. Cell. Longev., 2014, 2014, 320513 Search PubMed.
- A. Uruno and H. Motohashi, Nitric Oxide, 2011, 25, 153–160 CrossRef CAS PubMed.
- M. K. Kwak, K. Itoh, M. Yamamoto and T. W. Kensler, Mol. Cell. Biol., 2002, 22, 2883–2892 CrossRef CAS PubMed.
- J. Yao, C. Ge, D. Duan, B. Zhang, X. Cui, S. Peng, Y. Liu and J. Fang, J. Agric. Food Chem., 2014, 62, 5507–5518 CrossRef CAS PubMed.
- J. Yao, B. Zhang, C. Ge, S. Peng and J. Fang, J. Agric. Food Chem., 2015, 63, 1521–1531 CrossRef CAS PubMed.
- S. Peng, Y. Hou, J. Yao and J. Fang, Food Funct., 2017, 8, 997–1007 CAS.
- Y. Setoguchi, Y. Oritani, R. Ito, H. Inagaki, H. Maruki-Uchida, T. Ichiyanagi and T. Ito, J. Agric. Food Chem., 2014, 62, 2541–2548 CrossRef CAS PubMed.
- Y. Suzuki, Y. Nakano, K. Mishiro, T. Takagi, K. Tsuruma, M. Nakamura, S. Yoshimura, M. Shimazawa and H. Hara, Sci. Rep., 2013, 3, 3177 CrossRef PubMed.
- H. J. Kim, K. W. Lee, M. S. Kim and H. J. Lee, J. Nutr. Biochem., 2008, 19, 459–466 CrossRef CAS PubMed.
- Y. J. Jang, J. E. Kim, N. J. Kang, K. W. Lee and H. J. Lee, Ann. N. Y. Acad. Sci., 2009, 1171, 176–182 CrossRef CAS PubMed.
Footnote |
| † These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2018 |