Bioavailability of hydroxycinnamates in an instant green/roasted coffee blend in humans. Identification of novel colonic metabolites†
Miren
Gómez-Juaristi
,
Sara
Martínez-López
,
Beatriz
Sarria
,
Laura
Bravo
 * and
Raquel
Mateos
* and
Raquel
Mateos
 *
*
Department of Metabolism and Nutrition, Institute of Food Science, Technology and Nutrition (ICTAN-CSIC). Spanish National Research Council (CSIC), José Antonio Novais 10, 28040 Madrid, Spain. E-mail: lbravo@ictan.csic.es; Tel: +34 915492300
First published on 16th November 2017
Abstract
Roasting greatly reduces the phenolic content in green coffee beans. Considering the beneficial effects of coffee polyphenols, blends containing green coffee beans are being consumed as a healthier alternative to roasted coffee. This study was aimed at assessing the absorption and metabolism of hydroxycinnamates in an instant green/roasted (35/65) coffee blend in healthy humans. Twelve fasting men and women consumed a cup of coffee containing 269.5 mg (760.6 μmol) of chlorogenic acids. Blood and urine samples were taken before and after coffee consumption at different times and analyzed by LC-MS-QToF. Up to 25 and 42 metabolites were identified in plasma and urine, respectively, mainly in the form of sulfate and methyl derivatives, and to a lower extent as glucuronides. Un-metabolized hydroxycinnamate esters (caffeoyl-, feruloyl-, and coumaroylquinic acids), hydroxycinnamic acids (caffeic, ferulic and coumaric acids) and their phase II metabolites, in addition to phase II derivatives of lactones, represented a minor group of metabolites (16.3% of the metabolites excreted in urine) with kinetics compatible with small intestine absorption. Dihydrohydroxycinnamic acids and their phase II derivatives, in addition to feruloylglycine, showed delayed kinetics due to their colonic origin and represented the most abundant group of metabolites (75.7% of total urinary metabolites). Dihydrohydroxycinnamate esters (dihydroferuloyl-, dihydrocaffeoyl- and dihydrocoumaroylquinic acids) have been identified for the first time in both plasma and urine, with microbial origin (excreted 8–12 h after coffee intake) amounting to 8% of total urinary metabolites. In conclusion, coffee polyphenols are partially bioavailable and extensively metabolized, mainly by the colonic microbiota.
1. Introduction
Coffee is the most widely consumed beverage with over 2 billion cups daily consumed in industrialized countries due to its unique aroma and stimulatory effects.1 This makes coffee a key beverage at breakfast, mid morning or after-meals. Considering the high intake of this beverage, coffee may have a relevant impact on the health of widespread populations.Although coffee consumption is frequently restricted in patients suffering from hypertension, arrhythmia or other kind of cardiovascular condition, recent findings point to an inverse association between coffee consumption and certain chronic disorders such as cardiovascular diseases and related pathologies.2,3 Recently, Bravo et al.4 showed that balancing the divergent results of observational and intervention studies, the detrimental effects of moderate coffee consumption on cardiovascular diseases risk are not supported. In fact, there is a protective effect associated to moderate consumption (around 3–4 cups per day) of filtered coffee, reducing the risk of coronary and cerebrovascular heart diseases and heart failure, with no negative impact on blood pressure.
A cup of coffee (ranging from 25 mL in espresso coffee to 180 mL in American type filter coffee, otherwise 100 mL is taken as a reference) is a wide and interesting source of bioactive compounds that, synergistically or not, produce health benefits after acute or regular consumption. Depending on the type of bean, the content in hydroxycinnamic acids varies between 15–325 mg,5 and methylxanthines from 60–85 mg, mainly caffeine and minor amounts of theobromine and theophylline.5–7 Coffee is also an important source of the diterpenoids cafestol and kahweol (from 0.1 to 10 mg for filtered and unfiltered coffee, respectively8), trigonelline (40–110 mg9), soluble dietary fiber (0.14–0.75 g10), and melanoidins, which are formed during green coffee roasting (0.25–0.81 g per 100 mL in brewed dark coffee11).
Polyphenols are considered the healthiest constituents of coffee, with estimated intakes up to 1–2 g day−1 following regular coffee intake.5 Polyphenols contained in coffee are hydroxycinnamate esters, collectively known as hydroxycinnamic acids or more commonly chlorogenic acids, considering the abundance of the latter. Hydroxycinnamates derived from the binding of quinic acid with one or two hydroxycinnamic acid moieties (caffeic, ferulic and/or p-coumaric acid) lead to different isomers of caffeoylquinic, feruloylquinic and p-coumaroylquinic acids, respectively, along with dicaffeoylquinic and caffeoylferuloylquinic acids as the main phenolic compounds identified in green coffee beans. In addition, glycosides and amides derivatives of hydroxycinnamic acids complete the complex phenolic profile of green coffee beans.12,13 However, part of the hydroxycinnamic acids are lost during roasting, with reductions of over 50% of the initial phenolic content.14,15 Hydroxycinnamic acid derivatives undergo isomerization and transformation into lactones during roasting, with different potential bioactivity than the precursor hydroxycinnamic acids in the green coffee bean.15 Therefore, the benefits associated with the consumption of coffee depend on the type of coffee consumed, either green, roasted or green/roasted coffee blends.
The biological activity of phenolic compounds depends on their bioavailability and metabolic fate, as well as on their digestive accessibility, which is determined by the release from the food matrix and efficiency in trans-epithelial passage. Recently Del Rio et al.16 reviewed the rate and extent of absorption of coffee polyphenols in humans, as well as the metabolic pathways involved, showing that hydroxycinnamic acids are extensively metabolized and partially absorbed in healthy subjects. These polyphenols are partially absorbed in the upper gastrointestinal tract, being hydrolyzed by intestinal esterases into free caffeic, ferulic and coumaric acids and conjugated by phase II enzymes into methylated, sulfated and/or glucuronidated metabolites. Maximum plasma concentrations of these metabolites appear 1–3 h after coffee intake in nM concentrations.17,18 Polyphenols not absorbed in the small intestine reach the colon where they are metabolized by the intestinal microbiota mainly to reduced forms of hydroxycinnamic acids, which are absorbed through the colonic epithelium and conjugated by phase II enzymes. The colonic metabolites appear in plasma 5–9 h after coffee consumption at higher concentrations in the μM range, although some in the nM range.17,18
Since roasting greatly reduces the content of hydroxycinnamic acids in coffee beans, consuming a green/roasted coffee blend is a healthier option to roasted coffee. In fact, a soluble coffee product containing 35% of green coffee and 65% of roasted beans, which presented the distinct aroma and taste of roasted coffee, has shown interesting health benefits. In a previous study by our research group, healthy and hypercholesterolemic subjects who regularly consumed 3 cups of this coffee blend (providing 496 mg day−1 of hydroxycinnamic derivatives) distributed along the day showed positive effects on blood pressure, blood glucose and triglyceride levels, also improving the lipid profile in hypercolesterolemic subjects.19,20 In order to further understand the results derived from the intervention studies, it is important to study the bioavailability and metabolism of polyphenols in coffee and this may also enable the identification of the biological mechanisms associated to their beneficial health effects. In addition, the results here presented could be extrapolated to the biotransformation of polyphenols contained in other green/roasted coffee blends, or green coffee consumed as an infusion or as a nutraceutical, which consumption is increasing. Up to date, most of the studies on the bioavailability of coffee polyphenols have been carried out with roasted coffee.17,18,21–24 Therefore, the aim of the present work was to evaluate the bioavailability of hydroxycinnamates after consuming a realistic amount of a soluble green/roasted (35/65) coffee blend in healthy humans. In addition, an important effort has been made to identify new metabolites derived from the microbiota, showing the importance of gut bacteria on polyphenols absorption and metabolism.
2. Materials and methods
2.1. Chemical reagents and materials
A commercial brand of soluble green/roasted coffee blend (Nescafe Green Blend©) was purchased in a local supermarket in Madrid (Spain). All solvents and reagents were of analytical grade unless otherwise stated. Ascorbic acid, 5-caffeoylquinic acid, p-coumaric acid, ferulic acid, caffeic acid, 3,4-dihydroxyphenylpropionic acid, 4-hydroxyphenylpropionic acid, 4-hydroxy-3-metoxyphenylpropionic acid, 3,4-dihydroxyphenylacetic acid, 4-hydroxyphenylacetic acid, 3,4-dihydroxyphenylbenzoic acid and 4-hydroxyphenylbenzoic acid were from Sigma-Aldrich (Madrid, Spain). 3,5-Dicaffeoylquinic acid was purchased from PhytoLab (Vestenbergsgreuth, Germany). Methanol, formic acid, and acetonitrile (HPLC grade) were acquired from Panreac (Madrid, Spain).2.2. Characterization and quantification of the phenolic content of the soluble green/roasted coffee blend by HPLC-MS and HPLC-DAD
Soluble coffee was prepared according to manufacturer's instructions. 3.5 g of green/roasted coffee blend was dissolved in 240 mL of boiling water. After shaking for 5 min, the sample was made up to 250 mL and filtered through filter paper. Then, an aliquot of the coffee beverage was filtered through a PVDF 0.45 μm filter prior to analysis. Phenolic compounds in green/roasted coffee blend infusion were analysed using an Agilent 1200 series liquid chromatographic system equipped with an autosampler, quaternary pump, diode array detector (DAD) and simple quadrupole (sQ) mass spectrometer (Agilent Technologies, Waldrom, Germany). Samples (20 μL) were injected into a Superspher RP18 column (4.6 mm × 250 mm i.d., 4 μm; Agilent Technologies) preceded by an ODS RP18 guard column kept in a thermostatic oven at 30 °C. Elution was performed at a flow rate of 1 mL min−1 using a binary system consisting of 1% formic acid in deionized water (solvent A) and 1% formic acid in acetonitrile (solvent B). The solvent gradient changed from 10% to 20% solvent B over 5 min, 20% to 25% solvent B over 30 min, 25% to 35% solvent B over 10 min, then maintained isocratically for 5 min, and returning to the initial conditions over 10 min. Chromatograms were recorded at 320 nm. The mass spectrometer was fitted to an atmospheric pressure electrospray ionization (ESI) source, which operated in negative ion mode. Capillary voltage was set to 3500 V, with nebulizing gas flow rate of 12 h L−1, drying gas temperature of 350 °C and nebulizer pressure of 45 psi. Mass spectrometry data were acquired in scan mode (mass range m/z 100–1000). Data acquisition and analysis were carried out in an Agilent ChemStation.An Agilent 1200 series liquid chromatographic system (Agilent Technologies) equipped with a quaternary pump, column oven, autosampler and DAD was used to quantify the identified polyphenols in the green/roasted coffee blend by high-performance mass spectrometry (HPLC-MS). The chromatographic conditions (column, guard column, binary gradient, injection volume, etc.) were as described above. For quantitative analysis the external standard method was used. Due to the lack of standards for certain polyphenols, they were tentatively quantified by using the calibration curves corresponding to their phenolic precursors. Thus, 5-caffeoylquinic acid and 3,5-dicaffeoylquinic acids were used to calculate the mono- and di-acylcinnamate esters content, respectively. Samples were prepared and analyzed in triplicate and the results were expressed as the mean value.
2.3. Subjects and study design
The study protocol was conducted in accordance with the ethical recommendations of the Declaration of Helsinki and approved by the Ethics Committee of Hospital Universitario Puerta de Hierro in Majadahonda (Madrid, Spain). Volunteers recruitment was carried out through placing advertisements at the Institute of Food Science, Technology and Nutrition (ICTAN).The study was carried out in twelve healthy subjects (7 men and 5 women); men's average age and body mass index were 27.86 ± 3.48 years and 23.42 ± 2.52 kg m−2, respectively, and women's 28.88 ± 3.56 years and 22.43 ± 3.33 kg m−2, respectively. They were non-smoker, non-vegetarian, non-pregnant women, who were not taking any medication or nutritional supplements, not suffering from any chronic pathology or gastrointestinal disorder. The sample size was estimated attending to similar previous bioavailability studies.17,21,25 The volunteers gave their informed consent prior to participation.
The study was carried out at the Human Nutrition Unit of ICTAN. After an overnight fast, volunteers consumed 3.5 g of the instant soluble green/roasted coffee blend in 250 mL of hot water. A polyphenol-free breakfast, lunch and afternoon snack were provided 2 h, 6 h and 10 h after consumption of coffee by the volunteers, who remained at the Human Nutrition Unit throughout the duration of the study. Water and isotonic beverages were available ad libitum. On the three days previous to the intervention, participants were also instructed not to consume coffee, other infusions (yerba mate, chamomile, etc.), tea, beer, red wine or their derived products, whole grain cereals (white bread was allowed), as well as fruits, fruit juices and vegetables, except banana, watermelon, cantaloupes and potatoes. In addition, consumption of legumes, virgin olive oil, vinegar and dried fruits was also restricted. Volunteers were asked to complete a 24 h food intake recall the day before each intervention in order to control any possible food restriction incompliance.
Prior to coffee intake, a nurse inserted a cannula in the cubital vein of the non-prevailing arm of the volunteers and blood samples were collected into EDTA-coated tubes at baseline (t = 0) and 0.5, 1, 1.5, 2, 3, 4, 5, 6, 8, 9, 10, and 12 h after consuming a cup of coffee. Plasma was separated by centrifugation (10 minutes, 3000 rpm, 4 °C) and stored at −80 °C until further analysis. Urine samples were collected in 24 h urine collection containers, which contained 5 mg of ascorbic acid, at different time intervals (t = −2–0, 0–2, 2–5, 5–8, 8–12, 12–24 h), and were aliquoted and frozen at −20 °C until analysis.
2.4. Extraction of phenolic metabolites from biological samples
A liquid–liquid extraction and protein precipitation with acetonitrile and methanol was used to isolate metabolites from plasma following the procedure described by Day et al.26 with some modifications. A 400 μL defrosted plasma sample was acidified with 12 μL of 50% (v/v) aqueous formic acid. After vortexing the aqueous mixture, it was added drop wise to 1000 μL of cold acetonitrile, containing 50 μL of 10% (m/v) ascorbic acid, and vortexed three times for 20 s before centrifuging at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 10 min at 4 °C. The supernatant was separated, and the pellet was re-extracted with 1000 μL of methanol. After centrifugation (10
000 rpm for 10 min at 4 °C. The supernatant was separated, and the pellet was re-extracted with 1000 μL of methanol. After centrifugation (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm, 10 min), the two supernatants were combined and reduced to dryness under a stream of nitrogen at 30 °C. The dried samples were resuspended in 100 μL of aqueous formic acid (0.1%) containing 10% acetonitrile acidified with 0.1% formic acid and centrifuged at 4 °C for 20 min at 14
000 rpm, 10 min), the two supernatants were combined and reduced to dryness under a stream of nitrogen at 30 °C. The dried samples were resuspended in 100 μL of aqueous formic acid (0.1%) containing 10% acetonitrile acidified with 0.1% formic acid and centrifuged at 4 °C for 20 min at 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm. The final supernatant was collected, filtered (0.45 μm pore-size, cellulose-acetate membrane filters, Albet) and 20 μL were analyzed by LC-MS-QToF. Recoveries of the standards chlorogenic, caffeic and ferulic acids ranged from 95 to 99%.
000 rpm. The final supernatant was collected, filtered (0.45 μm pore-size, cellulose-acetate membrane filters, Albet) and 20 μL were analyzed by LC-MS-QToF. Recoveries of the standards chlorogenic, caffeic and ferulic acids ranged from 95 to 99%.
Urine samples were diluted with an equivalent volume of Milli-Q water (50%) and centrifuged at 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm (10 min, 4 °C). Supernatants were filtered (0.45 μm pore-size cellulose-acetate membrane filters) and a 5 μL aliquot was directly injected into the LC-MS-QToF equipment.
000 rpm (10 min, 4 °C). Supernatants were filtered (0.45 μm pore-size cellulose-acetate membrane filters) and a 5 μL aliquot was directly injected into the LC-MS-QToF equipment.
2.5. Metabolite identification by HPLC-ESI-QToF analysis
Analysis were performed on an Agilent 1200 series LC system coupled to an Agilent 6530A Accurate-Mass Quadrupole Time-Of-Flight (Q-ToF) with ESI-Jet Stream Technology (Agilent Technologies). Compounds were separated on a reverse-phase Ascentis Express C18 (15 cm × 3 mm, 2.7 μm) column (Sigma-Aldrich Química, Madrid) preceded by a Supelco 55215-U guard column at 30 °C. The test samples, either 30 μL of plasma or 5 μL of urine, were injected and separated using a mobile phase consisting of Milli-Q water (phase A) and acetonitrile (phase B), both containing 0.1% formic acid, at a flow rate of 0.3 mL min−1. The mobile phase was initially programmed with 90% of solvent A and 10% of B. The elution program increased to 30% of solvent B in 10 min, 40% solvent B in 5 min and 50% solvent B in 5 min. Then, the initial conditions (10% solvent B) were recovered in 2 min and maintained for 8 min. The Q-ToF acquisition conditions were as follows: drying gas flow (nitrogen, purity > 99.9%) and temperature were 10 L min−1 and 325 °C, respectively; sheath gas flow and temperature were 6 L min−1 and 250 °C, respectively; nebulizer pressure was 25 psi; cap voltage was 3500 V and nozzle voltage was 500 V. Mass range selected was from 100 up to 970 m/z in negative mode and fragmentor voltage of 150 V. Data were processed in a Mass Hunter Workstation Software.Due to the lack of standards for some phase II metabolites, they were tentatively quantified using the calibration curves of their corresponding phenolic precursors. Thus, 5-caffeoylquinic acid was used to quantify monohydroxycinnamoyl derivatives; caffeic, ferulic, dihydrocaffeic, dihydroferulic and dihydrocoumaric acids were used to quantify their respective free hydroxycinnamic acids and phase II metabolites. The rest of microbial metabolites identified, derivatives of hydroxyphenylacetic and hydroxyphenylbenzoic acids, were quantified using their respective commercially available standards. Urine concentration of excreted metabolites was normalized by the volume excreted in each studied interval. A linear response was obtained for all the standard curves (from 1 to 1000 nM), as checked by linear regression analysis. Calibration curves were freshly prepared in a pool of both plasma and urine due to matrix effects. Limits of detection and quantification in plasma ranged from 1 to 3 nM and from 2 to 9 nM, respectively, while limits of detection and quantification in urine ranged from 3 to 20 nM and from 30 to 80 nM, respectively. The inter- and intra-day precision of the assay (as the coefficient of variation, ranging from 2.1 to 10%) were considered acceptable and allowed the quantification of phenolic compounds and their metabolites (quantified as equivalents of the respective parent molecules). The recovery ranged between 95% and 105% in plasma and between 93% and 98% in urine samples.
2.5. Nutrikinetic analysis
Statistical analyses were carried out using the program SPSS (version 19.0, SPSS, Inc., IBM Company). To determine the absorption and elimination of hydroxycinnamic metabolites after consumption of the soluble green/roasted coffee blend, metabolie nutrikinetics were studied using the pharmacokinetic functions of Microsoft Excel. Maximum concentration (Cmax), area under curve (AUC) and time to reach maximum concentration (Tmax) were calculated. Data are given as mean ± standard deviation.3. Results
3.1. Phenolic content of instant green/roasted coffee blend
The phenolic constituents present in the instant green/roasted coffee blend were analyzed by diode-array detection. A representative HPLC-DAD chromatogram of the phenolic fraction of soluble green/roasted coffee blend at 320 nm has been included in the ESI (ESI Table 1†). The 250 mL serving of coffee was prepared from 3.5 g of the green/roasted coffee blend providing 760.6 μmol (269.5 mg) of hydroxycinnamic acids (Table 1). The most abundant phenolic compounds in this coffee were monoacylquinic derivatives (83.5% of the total polyphenols in coffee), constituted mainly by caffeoylquinic acids (66.8%), feruloylquinic acids (13.5%), coumaroylquinic acids (2.2%), and dimethoxycinnamoylquinic acids (1.0%). The next most abundant group was diacylquinic acids (12.3%), headed by dicaffeoylquinic acids (10.9%), and followed by minor amounts of caffeoylquinic lactones (2.2% of total polyphenols), caffeoyl-N-tryptophan (1.7% of total polyphenols) and caffeoylshikimic acids (0.3% of total polyphenols).| Polyphenols | (mg g−1, d.m.) |
|---|---|
| Caffeoylquinic acids | 51.2 ± 0.7 (66.8%) |
| Coumaroylquinic acids | 1.7 ± 0.1 (2.2%) |
| Feruloylquinic acids | 10.4 ± 0.5 (13.5%) |
| Dimethoxycinnamoylquinic acids | 0.8 ± 0.1 (1.0%) |
| Dicaffeoylquinic acids | 8.3 ± 0.6 (10.9%) |
| Caffeoylferuloylquinic acids | 1.0 ± 0.1 (1.4%) |
| Caffeoylshikimic acids | 0.26 ± 0.02 (0.3%) |
| Caffeoylquinic lactones | 1.70 ± 0.08 (2.2%) |
| Caffeoyl-N-tryptophan (mg g−1) | 1.3 ± 0.1 (1.7%) |
| Total hydroxycinnamic acids | 77 ± 2 |
3.2. LC-QToF identification of hydroxycinnamates and metabolites in plasma and urine
Table 2 shows the retention time (RT), molecular formula, accurate mass of the quasimolecular ion [M − H]− after negative ionization, MS2 fragments and location (U: urine or P: plasma) of the main compounds identified in plasma and urine samples by LC-QToF. The characterization of the identified compounds was supported by commercial standards and/or previously published results.| Identified compound | RT (min) | Molecular formula | [M − H]− | Fragment MS2 | Location |
|---|---|---|---|---|---|
| Caffeic acid metabolites | |||||
| 3-Caffeoylquinic acid | 4.0 | C16H18O9 | 353.0878 | 191 | U |
| 5-Caffeoylquinic acid | 6.2 | C16H18O9 | 353.0878 | 191 | P, U |
| 4-Caffeoylquinic acid | 10.1 | C16H18O9 | 353.0878 | 191 | U |
| Caffeic acid | 7.9 | C9H8O4 | 179.0350 | 135 | U |
| Caffeic acid 3-sulfate | 10.7 | C9H8O7S | 258.9918 | 179; 135 | U |
| Dihydrocaffeic acid | 7.0 | C9H10O4 | 181.0506 | 137 | P, U |
| Dihydrocaffeic acid 3-glucuronide | 6.5 | C15H18O10 | 357.0827 | 181; 137 | U |
| Dihydrocaffeic acid 4-sulfate | 7.8 | C9H10O7S | 261.0074 | 181 | P, U |
| Dihydrocaffeic acid 3-sulfate | 10.4 | C9H10O7S | 261.0074 | 181 | U |
| 3-Dihydrocaffeoylquinic acid | 8.5 | C16H20O9 | 355.1035 | 181 | U |
| 5-Dihydrocaffeoylquinic acid | 13.1 | C16H20O9 | 355.1035 | 181 | U |
| 4-Dihydrocaffeoylquinic acid | 14.8 | C16H20O9 | 355.1035 | 181 | U |
| Dihydrocaffeoylquinic acid glucuronide | 11.3 | C22H28O15 | 531.1355 | 355; 181 | U |
| Ferulic acid metabolites | |||||
| 3-Feruloylquinic acid | 7.1 | C17H20O9 | 367.1035 | 191 | U |
| 5-Feruloylquinic acid | 9.6 | C17H20O9 | 367.1035 | 191 | P, U |
| 4-Feruloylquinic acid | 10.2 | C17H20O9 | 367.1035 | 191 | P, U |
| Ferulic acid | 11.4 | C10H10O4 | 193.0506 | 134 | P |
| Isoferulic acid | 12.9 | C10H10O4 | 193.0506 | 134 | P |
| Ferulic acid 4-glucuronide | 8.4 | C16H18O10 | 369.0827 | 193; 134 | P, U |
| Isoferulic acid 3-glucuronide | 9.2 | C16H18O10 | 369.0827 | 193; 134 | U |
| Ferulic acid 4-sulfate | 8.7 | C10H10O7S | 273.0074 | 193; 134 | P, U |
| Isoferulic acid 3-sulfate | 10.4 | C10H10O7S | 273.0074 | 193; 134 | U |
| Dihydroferulic acid | 10.8 | C10H12O4 | 195.0663 | 136 | P, U |
| Dihydroisoferulic acid | 11.6 | C10H12O4 | 195.0663 | 136 | P |
| Dihydroferulic acid 4-glucuronide | 6.5 | C16H20O10 | 371.0984 | 195; 136 | P, U |
| Dihydroisoferulic acid 3-glucuronide | 8.4 | C16H20O10 | 371.0984 | 195; 136 | P, U |
| Dihydroferulic acid 4-sulfate | 8.8 | C10H12O7S | 275.0231 | 195; 136 | P, U |
| Dihydroisoferulic acid 3-sulfate | 10.4 | C10H12O7S | 275.0231 | 195; 136 | P, U |
| 3-Dihydroferuloylquinic acid | 15.1 | C17H22O9 | 369.1191 | 195 | P, U |
| 5-Dihydroferuloylquinic acid | 15.4 | C17H22O9 | 369.1191 | 195 | P, U |
| 4-Dihydroferuloylquinic acid | 15.6 | C17H22O9 | 369.1191 | 195 | U |
| Feruloylglycine | 8.8 | C12H13O5N | 250.0721 | 191; 134 | P, U |
| Isoferuloylglycine | 9.5 | C12H13O5N | 250.0721 | 191; 134 | U |
| Coumaric acid metabolites | |||||
| Coumaroylquinic acid | 16.4 | C16H18O8 | 337.0929 | 163 | U |
| Coumaric acid glucuronide | 12.3 | C15H16O9 | 339.0722 | 163 | P, U |
| Coumaric acid glucuronide | 12.9 | C15H16O9 | 339.0722 | 163 | U |
| Coumaric acid sulfate | 18.5 | C9H8O6S | 242.9969 | 163 | P |
| Dihydrocoumaric acid | 8.5 | C9H10O3 | 165.0557 | 121 | U |
| Dihydrocoumaric acid glucuronide | 6.8 | C15H18O9 | 341.0878 | 165 | U |
| Dihydrocoumaric acid sulfate | 10.6 | C9H10O6S | 245.0125 | 165 | U |
| Dihydrocoumaroylquinic acid | 14.8 | C16H20O8 | 339.1085 | 165 | P, U |
| Dihydrocoumaroylquinic acid | 15.3 | C16H20O8 | 339.1085 | 165 | P, U |
| Dimethoxycinnamic acid metabolites | |||||
| Dimethoxycinnamic acid | 15.1 | C11H12O4 | 207.0652 | 103 | P |
| Dihydrodimethoxycinnamic acid | 13.8 | C11H14O4 | 209.0819 | P, U | |
| Lactone derivatives | |||||
| Caffeoylquinic lactone sulfate | 11.8 | C16H16O11S | 415.0330 | 335 | U |
| Caffeoylquinic lactone sulfate | 12.3 | C16H16O11S | 415.0330 | 335 | U |
| Caffeoylquinic lactone sulfate | 13.5 | C16H16O11S | 415.0330 | 335 | U |
| Feruloylquinic lactone glucuronide | 12.0 | C23H26O14 | 525.1261 | 349 | P |
| Phenolic acids | |||||
| 3-Hydroxyphenylpropionic acid | 11.1 | C9H10O3 | 165.0557 | 121 | P, U |
| 3,4-Dihydroxyphenylacetic acid | 5.6 | C8H8O4 | 167.0350 | 123 | P, U |
| Methoxy-hydroxyphenylacetic acid | 6.5 | C9H10O4 | 181.0506 | 137 | P, U |
| Methoxy-hydroxyphenylacetic acid | 8.8 | C9H10O4 | 181.0506 | 137 | P, U |
| 3-Hydroxyphenylacetic acid | 7.4 | C8H8O3 | 151.0401 | 107 | P, U |
| 3,4-Dihydroxybenzoic acid | 3.8 | C7H6O4 | 153.0193 | 109 | P, U |
| 3-Methoxy-4-hydroxybenzoic acid | 6.3 | C8H8O4 | 167.0350 | 123 | P, U |
| Hydroxybenzoic acid | 6.1 | C7H6O3 | 137.0244 | 93 | P, U |
| 3-Hydroxyhippuric acid | 11.0 | C9H9O4N | 194.0459 | 100 | P, U |
| 4-Hydroxyhippuric acid | 14.2 | C9H9O4N | 194.0459 | 150 | P, U |
| Phloroglucinol | 6.5 | C6H6O3 | 125.0244 | 79 | P, U |
Un-metabolized compounds originally present in the coffee beverage were detected in both plasma and urine samples. 3-, 4- and 5-caffeoylquinic acids were identified in urine and only 5-caffeoylquinic acid in plasma. Likewise, 3-, 4- and 5-feruloylquinic acids were detected in urine and 4- and 5-feruloylquinic acids were also found in plasma. In addition, coumaroylquinic acid was detected in urine (Table 2).
Caffeic and dimethoxycinnamic acids in urine and trans- and isoferulic acids in plasma were characterized, which were formed from their respective monohydroxycinnamoylquinic acid. Phase II derivatives of these free hydroxycinnamic acids (caffeic acid and iso- and trans-ferulic acids) were also detected in biological fluids. In particular, caffeic acid 3-sulfate, ferulic acid 3-glucuronide and ferulic acid 3-sulfate were detected in urine while ferulic acid 4-glucuronide and ferulic acid 4-sulfate were observed in both plasma and urine samples. No free coumaric acid was detected in contrast to their phase II derivatives. Specifically, a sulfated derivative of coumaric acid was detected in plasma and two isomers of coumaric acid glucuronide were detected in different samples; the isomer with shorter retention time appeared in both plasma and urine while that with longer retention time only in urine.
After green/roasted coffee intake, an important group of metabolites formed were reduced hydroxycinnamate esters, which were extensively transformed into phase II derivatives. Dihydrocaffeic, dihydro-trans-ferulic and dihydrodimethoxycinnamic acids were identified in both plasma and urine, whereas dihydrocoumaric acid was only found in urine and dihydroisoferulic acid was only observed in plasma. In addition, phase II derivatives of these reduced forms were also detected. Dihydrocaffeic acid 3-glucuronide, dihydrocaffeic acid 4-sulfate and dihydrocaffeic acid 3-sulfate were detected in urine, and dihydrocaffeic acid 4-sulfate in both urine and plasma. Additionally, two isomers of each glucuronidated and sulfated derivatives of dihydroferulic acid were detected in plasma and urine samples; in contrast, an isomer of glucuronidated and sulfated dihydrocoumaric acid were detected only in urine.
A new group of reduced forms resulting from the microbial metabolism of hydroxycinnamates, namely dihydrohydroxycinnamoylquinic acids, has been identified for the first time. Thus, 3-, 4- and 5-dihydrocaffeoylquinic acids were identified in urine with quasimolecular ion at m/z 355.1035 and fragment ion at m/z 181 corresponding to dihydrocaffeic acid. Likewise, 3-, 4- and 5-dihydroferuloylquinic acids were identified based on their [M − H]− at m/z 369.1191 and fragment ion at m/z 195 corresponding to dihydroferulic acid. Three isomers of dihydroferuloylquinic acid were observed in urine whereas the 3- and 5-isomers were detected in plasma. Lastly, two chromatographic peaks at 14.8 and 15.3 min showed a quasimolecular ion at m/z 339.1085 and fragment ion at m/z 165, which were compatible with dihydrocoumaroylquinic acid, detected in both plasma and urine samples.
Feruloylglycine has been previously described after coffee intake.17 However, in the present study two chromatographic peaks at 8.8 and 9.5 min showed a chemical structure compatible with this compound ([M − H]− at m/z 250.0721 and fragment ions at m/z 191 and 134), being tentatively assigned as feruloylglycine and isoferuloylglycine, respectively, according to the order of elution of their precursors (ferulic and isoferulic acids). While feruloylglycine was detected in both plasma and urine, isoferuloylglycine was only detected in urine.
Phase II derivatives of lactones were also characterized. Although hydroxycinnamoyl lactones were detected in biological samples, up to three sulfated derivatives of caffeoylquinic lactone and one glucuronidated derivative of feruloylquinic lactone were observed in urine and plasma, respectively, in the present study.
Lastly, derivatives of hydroxyphenylpropionic, hydroxyphenylacetic, hydroxybenzoic and hydroxyhippuric acids were detected in plasma and urine samples.
3.3. Quantification of plasma metabolites and nutrikinetic parameters
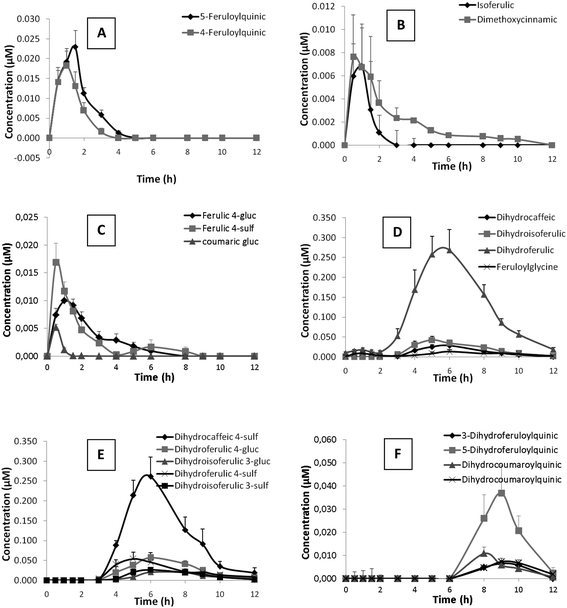
Out of the 48 metabolites identified after coffee consumption, 25 were detected in plasma although only 20 showed levels above limit of quantification. The kinetics of plasma appearance and clearance of these metabolites up to 12 h post-intake are represented in Fig. 1. Nutrikinetic parameters are summarized in Table 3.| Metabolite | C max (μM) | T max (h) or rangea | AUC (μM min−1) |
|---|---|---|---|
| a Range where the metabolite showed the highest value. b No pharmacokinetic parameters of these metabolites were determined because their content was at trace levels. | |||
| Intestinal absorption | |||
| 5-Caffeoylquinic acid | Tracesb | (1.0–1.5)a | — |
| 5-Feruloylquinic acid | 0.02 ± 0.01 | 1.2 ± 0.4 | 0.04 ± 0.02 |
| 4-Feruloylquinic acid | 0.02 ± 0.01 | 0.9 ± 0.3 | 0.03 ± 0.02 |
| Ferulic acid | Tracesb | (1.0–1.5)a | — |
| Isoferulic acid | 0.008 ± 0.001 | 0.9 ± 0.2 | 0.1 ± 0.2 |
| Dimethoxycinnamic acid | 0.008 ± 0.002 | 0.7 ± 0.3 | 0.02 ± 0.02 |
| Ferulic acid 4-glucuronide | 0.011 ± 0.004 | 1.1 ± 0.5 | 0.03 ± 0.02 |
| Ferulic acid 4-sulfate | 0.02 ± 0.01 | 0.7 ± 0.2 | 0.03 ± 0.02 |
| Coumaric acid glucuronide | 0.005 ± 0.002 | 0.5 ± 0.1 | 0.003 ± 0.001 |
| Coumaric acid sulfate | Tracesb | (1.0–1.5)a | — |
| Feruloylquinic lactone glucuronide | Tracesb | (1.0–1.5)a | — |
| Microbial metabolites | |||
| Dihydrocaffeic acid | 0.03± 0.01 | 6 ± 1 | 0.12 ± 0.08 |
| Dihydroferulic acid | 0.3 ± 0.1 | 6 ± 1 | 1.3 ± 0.6 |
| Dihydroisoferulic acid | 0.05 ± 0.03 | 5 ± 1 | 0.2 ± 0.1 |
| Dihydrodimethoxycinnamic acid | Tracesb | (6.0–8.0)a | 0.013 ± 0.004 |
| Dihydrocaffeic acid 4-sulfate | 0.26 ± 0.09 | 6.0 ± 0.1 | 1.1 ± 0.4 |
| Dihydroferulic acid 4-glucuronide | 0.06 ± 0.04 | 6 ± 1 | 0.3 ± 0.2 |
| Dihydroisoferulic acid 3-glucuronide | 0.02 ± 0.01 | 7 ± 1 | 0.10 ± 0.08 |
| Dihydroferulic acid 4-sulfate | 0.09 ± 0.05 | 5.6 ± 0.7 | 0.4 ± 0.2 |
| Dihydroisoferulic 3-sulfate | 0.03 ± 0.02 | 5.8 ± 0.5 | 0.1 ± 0.2 |
| Feruloylglycine | 0.02 ± 0.01 | 7 ± 2 | 0.09 ± 0.06 |
| 3-Dihydroferuloylquinic acid | 0.009 ± 0.004 | 9.2 ± 0.8 | 0.02 ± 0.01 |
| 5-Dihydroferuloylquinic acid | 0.04 ± 0.02 | 9.3 ± 0.5 | 0.11 ± 0.08 |
| Dihydrocoumaroylquinic acid | 0.011 ± 0.008 | 8.4 ± 0.7 | 0.03 ± 0.02 |
| Dihydrocoumaroylquinic acid | 0.009 ± 0.005 | 9.4 ± 0.7 | 0.03 ± 0.01 |
Un-metabolized hydroxycinnamate esters such as caffeoyl- and feruloylquinic acids were detected in plasma. While 5-caffeoylquinic acid was at trace levels (Table 3), the concentrations of 4- and 5-feruloylquinic acids showed a rapid increase between 1 and 2 h after coffee consumption and complete clearance at 5 h (Fig. 1A). Hydroxycinnamic acids such as isoferulic and dimethoxycinnamic acids (Fig. 1B) along with trace levels of ferulic acid were detected in plasma, together with phase II derivatives of hydroxycinnamic acids such as ferulic acid 4-glucuronide, ferulic acid 4-sulfate, coumaric acid glucuronide (Fig. 1C), and coumaric acid sulfate at trace levels (Table 3). Hydroxycinnamic acids and their phase II derivatives showed similar kinetic profiles with a rapid increase in concentration between 1 and 2 h after coffee consumption and slow clearance, maintaining or even showing a second maxima between 4 and 5 h in the particular cases of dimethoxycinnamic acid (Fig. 1B) and ferulic acid 4-glucuronide, or even at 6–7 h in the case of ferulic acid 4-sulfate (Fig. 1C), with subsequent clearance at 10–12 h post-intake. Additionally, trace amounts of feruloylquinic lactone glucuronide was detected in plasma. All these compounds were present in low concentrations in plasma, showing Cmax ranging from traces to 20 nM (Table 3). The time to reach maximum concentration (Tmax) ranged between 0.5 and 1.2 h, which pointed to absorption taking place in the small intestine.
Reduced forms of hydroxycinnamic acids, dihydrocaffeic, dihydroferulic, dihydroisoferulic acid (Fig. 1D) and dihydrodimethoxycinnamic acid, along with their phase II derivatives, dihydrocaffeic acid 4-sulfate, dihydroferulic acid 4-glucuronide, dihydroisoferulic acid 3-glucuronide, dihydroferulic acid 4-sulfate and dihydroisoferulic acid 3-sulfate (Fig. 1E), formed the main group of metabolites detected in plasma (Table 3). The plasmatic profile of these metabolites showed maxima concentrations between 5.6 and 7.0 h (Tmax) post-intake and minor second maxima between 8 and 9 h in the particular case of dihydroisoferulic acid, dihydrocaffeic acid 4-sulfate, dihydroferulic acid 4-sulfate and dihydroisoferulic 3-sulfate, compatible with a biphasic kinetic. Dihydroferulic acid and dihydrocaffeic acid 4-sulfate were the predominant metabolites, showing Cmax values of 300 and 260 nM, respectively, followed by sulfated and glucuronidated derivatives of ferulic acid and dihydroisoferulic acid with Cmax ranging from 90 to 50 nM. Dihydrocaffeic acid, phase II derivatives of isoferulic acid (sulfated and glucuronidated derivatives) and dihydrodimethoxycinnamic acids were less abundant, with Cmax values ranging from 30 nM to trace levels.
Feruloylglycine, as a ferulic acid derivative, was detected with a plasmatic profile similar to that of the reduced forms of hydroxycinnamic acids (Fig. 1D), as its concentration rapidly increased with Tmax at 7 h decreasing afterwards, although not recovering baseline levels 12 h after coffee intake (Fig. 1D). Their presence in plasma was relatively low (Cmax 20 nM, Table 3).
Furthermore, for the first time reduced forms of hydroxycinnamate esters (3- and 5-dihydroferuloylquinic acids and two isomers of dihydrocoumaroylquinic acid) have been identified in plasma. These compounds showed the most delayed kinetic of all the mentioned metabolites, with maxima concentration about 9 h (Tmax 8.4–9.4 h) and partial clearance 12 h post-intake (Fig. 1F). Among these microbial metabolites, 5-dihydroferuloylquinic acid was the most abundant (Cmax 40 nM), followed by 3-dihydroferuloylquinic acid and the two isomers of dihydrocoumaroylquinic acids with Cmax values ranging from 9 to 11 nM (Table 3).
Lastly, metabolites related with hydroxyphenylpropionic, hydroxyphenylacetic and hydroxybenzoic acids were also detected in plasma, although no differences were observed in their concentrations along the 12 h blood sampling period (data not shown).
3.4. Quantification of urinary metabolites
Table 4 shows the metabolites quantified in 24 h urine samples, where 42 metabolites of the 48 metabolites identified after coffee consumption were identified. Additionally, eleven microbial metabolites derivatives of hydroxyphenylpropionic, hydroxyphenylacetic, and hydrophenylbenzoic acids along with hydroxylhippuric acid and phloroglucinol were also detected in urine.| Metabolites | −2–0 h | 0–2 h | 2–5 h | 5–8 h | 8–12 h | 12–24 h | TOTAL (μmol) |
|---|---|---|---|---|---|---|---|
| Intestinal absorption | |||||||
| 3-Caffeoylquinic acid | N.D. | 0.014 ± 0.003 | 0.023 ± 0.003 | 0.007 ± 0.003 | N.D. | N.D. | 0.044 ± 0.009 |
| 5-Caffeoylquinic acid | N.D. | 0.056 ± 0.009 | 0.14 ± 0.05 | 0.013 ± 0.006 | N.D. | N.D. | 0.21 ± 0.07 |
| 4-Caffeoylquinic acid | N.D. | 0.04 ± 0.01 | 0.032 ± 0.005 | 0.017 ± 0.009 | 0.02 ± 0.01 | N.D. | 0.11 ± 0.04 |
| 3-Feruloylquinic acid | N.D. | 0.24 ± 0.04 | 0.27 ± 0.06 | 0.13 ± 0.02 | 0.09 ± 0.03 | 0.15 ± 0.02 | 0.9 ± 0.2 |
| 5-Feruloylquinic acid | N.D. | 0.41 ± 0.07 | 0.7 ± 0.1 | 0.21 ± 0.04 | 0.16 ± 0.02 | 0.008 ± 0.008 | 1.5 ± 0.3 |
| 4-Feruloylquinic acid | N.D. | 0.44 ± 0.05 | 0.56 ± 0.07 | 0.15 ± 0.03 | N.D. | N.D. | 1.2 ± 0.1 |
| Coumaroylquinic acid | N.D. | N.D. | 0.041 ± 0.008 | 0.057 ± 0.008 | 0.094 ± 0.004 | 0.07 ± 0.02 | 0.26 ± 0.04 |
| Caffeic acid | N.D. | 0.02 ± 0.01 | 0.04 ± 0.02 | 0.10 ± 0.04 | 0.17 ± 0.01 | 0.107 ± 0.008 | 0.45 ± 0.09 |
| Caffeic acid 3-sulfate | N.D. | 0.4 ± 0.1 | 1.2 ± 0.2 | 0.7 ± 0.1 | 0.30 ± 0.05 | N.D. | 2.6 ± 0.5 |
| Ferulic acid 4-glucuronide | N.D. | 0.32 ± 0.06 | 0.6 ± 0.1 | 0.7 ± 0.1 | 0.63 ± 0.04 | 0.01 ± 0.01 | 2.3 ± 0.3 |
| Isoferulic acid 3-glucuronide | N.D. | 0.17 ± 0.03 | 0.07 ± 0.02 | N.D. | N.D. | N.D. | 0.24 ± 0.06 |
| Ferulic acid 4-sulfate | N.D. | 0.9 ± 0.3 | 2.1 ± 0.5 | 2.2 ± 0.2 | 2.3 ± 0.3 | 0.04 ± 0.04 | 8 ± 1 |
| Isoferulic acid 3-sulfate | N.D. | 1.6 ± 0.2 | 0.05 ± 0.03 | N.D. | N.D. | N.D. | 1.7 ± 0.2 |
| Coumaric acid glucuronide | N.D. | 0.11 ± 0.02 | 0.11 ± 0.03 | 0.02 ± 0.02 | N.D. | N.D. | 0.23 ± 0.06 |
| Coumaric acid glucuronide | N.D. | 0.07 ± 0.02 | 0.03 ± 0.02 | N.D. | N.D. | N.D. | 0.11 ± 0.04 |
| Caffeoylquinic lactone sulfate | N.D. | 0.18 ± 0.07 | 0.1 ± 0.1 | N.D. | N.D. | N.D. | 0.3 ± 0.2 |
| Caffeoylquinic lactone sulfate | N.D. | 0.34 ± 0.06 | 0.36 ± 0.08 | N.D. | N.D. | N.D. | 0.7 ± 0.1 |
| Caffeoylquinic lactone sulfate | N.D. | 0.13 ± 0.02 | 0.16 ± 0.03 | N.D. | N.D. | N.D. | 0.29 ± 0.04 |
| Total – intestinal absorption | N.D. | 5 ± 1 | 7 ± 2 | 4.3 ± 0.6 | 3.8 ± 0.5 | 0.4 ± 0.1 | 21 ± 4 |
| Colonic absorption | |||||||
| Dihydrocaffeic acid | 0.06 ± 0.01 | 0.061 ± 0.009 | 0.24 ± 0.07 | 1.2 ± 0.2 | 0.8 ± 0.2 | 0.06 ± 0.02 | 2.5 ± 0.5 |
| Dihydroferulic acid | 0.06 ± 0.03 | 0.09 ± 0.03 | 1.2 ± 0.4 | 5.5 ± 0.9 | 2.5 ± 0.7 | 0.03 ± 0.03 | 9 ± 2 |
| Dihydrocoumaric acid | 0.01 ± 0.01 | 0.04 ± 0.02 | 0.16 ± 0.04 | 0.30 ± 0.05 | 0.23 ± 0.08 | 0.02 ± 0.02 | 0.7 ± 0.2 |
| Dihydrodimethoxycinnamic acid | N.D. | N.D. | N.D. | N.D. | <L.C. | N.D. | Traces |
| Dihydrocaffeic acid 3-glucuronide | N.D. | N.D. | 0.05 ± 0.02 | 0.23 ± 0.04 | 0.34 ± 0.03 | 0.01 ± 0.01 | 0.6 ± 0.1 |
| Dihydrocaffeic acid 4-sulfate | 0.36 ± 0.07 | 0.14 ± 0.04 | 0.06 ± 0.03 | N.D. | N.D. | N.D. | 0.6 ± 0.1 |
| Dihydrocaffeic 3-sulfate | N.D. | 0.15 ± 0.07 | 1.4 ± 0.4 | 13 ± 3 | 15 ± 3 | 3 ± 1 | 31 ± 8 |
| Dihydroferulic acid 4-glucuronide | 0.02 ± 0.01 | 0.02 ± 0.02 | 0.5 ± 0.2 | 3.5 ± 0.8 | 3.5 ± 0.5 | 0.22 ± 0.05 | 8 ± 2 |
| Dihydroisoferulic acid 3-glucuronide | N.D. | 0.010 ± 0.008 | 0.4 ± 0.2 | 1.5 ± 0.5 | 1.8 ± 0.2 | 0.16 ± 0.03 | 4 ± 1 |
| Dihydroferulic acid 4-sulfate | N.D. | 0.4 ± 0.3 | 1.3 ± 0.5 | 6 ± 1 | 5 ± 1 | 0.10 ± 0.07 | 13 ± 3 |
| Dihydroisoferulic acid 3-sulfate | N.D. | N.D. | 0.11 ± 0.06 | 1.0 ± 0.2 | 1.5 ± 0.2 | 0.20 ± 0.07 | 2.8 ± 0.5 |
| Dihydrocoumaric glucuronide | N.D. | 0.09 ± 0.02 | N.D. | N.D. | N.D. | N.D. | 0.09 ± 0.02 |
| Dihydrocoumaric sulfate | N.D. | 0.05 ± 0.05 | 0.19 ± 0.04 | 0.7 ± 0.2 | 3.1 ± 0.8 | 4 ± 1 | 8 ± 2 |
| Dihydrocaffeoylquinic acid glucur | N.D. | N.D. | N.D. | 0.010 ± 0.005 | 0.089 ± 0.006 | 0.003 ± 0.003 | 0.10 ± 0.01 |
| 3-Dihydrocaffeoylquinic acid | N.D. | N.D. | 0.05 ± 0.02 | 0.13 ± 0.02 | 0.31 ± 0.04 | 0.15 ± 0.03 | 0.6 ± 0.1 |
| 5-Dihydrocaffeoylquinic acid | N.D. | N.D. | N.D. | 0.008 ± 0.006 | 0.14 ± 0.01 | 0.035 ± 0.007 | 0.19 ± 0.03 |
| 4-Dihydrocaffeoylquinic acid | N.D. | N.D. | N.D. | 0.01 ± 0.01 | 0.24 ± 0.01 | 0.04 ± 0.01 | 0.29 ± 0.03 |
| 3-Dihydroferuloylquinic acid | N.D. | N.D. | N.D. | 0.04 ± 0.04 | 0.8 ± 0.3 | 0.02 ± 0.02 | 0.9 ± 0.3 |
| 5-Dihydroferuloylquinic acid | N.D. | N.D. | N.D. | 0.08 ± 0.05 | 5 ± 3 | 0.45 ± 0.08 | 6 ± 3 |
| 4-Dihydroferuloylquinic acid | N.D. | N.D. | N.D. | 0.03 ± 0.02 | 0.18 ± 0.04 | 0.01 ± 0.01 | 0.22 ± 0.08 |
| Dihydrocoumaroylquinic acid | N.D. | N.D. | 0.13 ± 0.08 | 0.13 ± 0.05 | 0.7 ± 0.3 | 0.05 ± 0.02 | 1.0 ± 0.4 |
| Dihydrocoumaroylquinic acid | N.D. | N.D. | N.D. | 0.06 ± 0.05 | 0.51 ± 0.07 | 0.16 ± 0.03 | 0.7 ± 0.1 |
| Feruloylglycine | 0.34 ± 0.07 | 0.54 ± 0.09 | 1.4 ± 0.2 | 9 ± 2 | 2 ± 1 | 0.63 ± 0.08 | 14 ± 4 |
| Isoferuloylglicine | N.D. | N.D. | 0.006 ± 0.005 | 0.09 ± 0.02 | 0.111 ± 0.007 | N.D. | 0.21 ± 0.04 |
| Total – colonic absorption | 0.8 ± 0.2 | 1.6 ± 0.6 | 7 ± 2 | 42 ± 10 | 45 ± 12 | 9 ± 3 | 106 ± 28 |
| Total – intestinal + colonic absorp. | 0.8 ± 0.2 | 7 ± 2 | 14 ± 4 | 47 ± 10 | 49 ± 12 | 10 ± 3 | 127 ± 32 |
| Other microbial metabolites | |||||||
| 3-Hydroxyphenylpropionic acid | N.D. | 0.024 ± 0.002 | 0.036 ± 0.004 | 0.10 ± 0.02 | 0.26 ± 0.06 | 0.08 ± 0.01 | 0.5 ± 0.1 |
| 3,4-Dihydroxyphenylacetic acid | 0.04 ± 0.01 | 0.05 ± 0.02 | 0.05 ± 0.03 | 0.03 ± 0.01 | 0.04 ± 0.02 | 0.06 ± 0.03 | 0.3 ± 0.1 |
| 3-Methoxy-4-hydroxyphenylacetic acid | N.D. | 0.03 ± 0.01 | 0.2 ± 0.1 | 1.4 ± 0.4 | 0.9 ± 0.3 | N.D. | 2.6 ± 0.8 |
| 4-Methoxy-3-hydroxyphenylacetic acid | 0.14 ± 0.04 | 0.18 ± 0.03 | 0.16 ± 0.04 | 0.19 ± 0.02 | 0.34 ± 0.03 | 0.16 ± 0.03 | 1.2 ± 0.2 |
| 3-Hydroxyphenylacetic acid | 0.19 ± 0.04 | 0.21 ± 0.03 | 0.23 ± 0.04 | 0.38 ± 0.08 | 0.65 ± 0.08 | 0.35 ± 0.07 | 2.0 ± 0.3 |
| 3,4-Dihydroxybenzoic acid | 0.06 ± 0.02 | 0.05 ± 0.01 | 0.04 ± 0.01 | 0.03 ± 0.01 | 0.07 ± 0.02 | 0.08 ± 0.02 | 0.34 ± 0.09 |
| 3-Methoxy-4-hydroxybenzoic acid | 0.17 ± 0.03 | 0.10 ± 0.02 | 0.11 ± 0.02 | 0.14 ± 0.02 | 0.5 ± 0.2 | 0.34 ± 0.06 | 1.3 ± 0.4 |
| Hydroxybenzoic acid | 0.10 ± 0.03 | 0.06 ± 0.01 | 0.11 ± 0.02 | 0.10 ± 0.01 | 0.15 ± 0.01 | 0.29 ± 0.08 | 0.8 ± 0.2 |
| 4-Hydroxyhippuric acid | N.D. | N.D. | N.D. | N.D. | 0.09 ± 0.01 | N.D. | 0.09 ± 0.01 |
| 3-Hydroxyhippuric acid | N.D. | N.D. | N.D. | N.D. | 0.08 ± 0.01 | N.D. | 0.08 ± 0.01 |
| Phloroglucinol | 0.10 ± 0.01 | 0.04 ± 0.02 | 0.06 ± 0.02 | 0.09 ± 0.02 | 0.17 ± 0.02 | 0.22 ± 0.05 | 0.69 ± 0.14 |
| Total other microbial metabolites | 0.8 ± 0.2 | 0.8 ± 0.1 | 1.0 ± 0.3 | 2.5 ± 0.6 | 3.3 ± 0.8 | 1.6 ± 0.6 | 10 ± 2 |
Monoacylquinic acids (3-, 4- and 5-caffeoylquinic acids, 3-, 4- and 5-feruloylquinic acids, and coumaroylquinic acid) were excreted unmetabolized in small amounts in the urine, accounting for 3.3% of the total metabolites quantified. Among these acids, feruloylquinic acids were the most abundant compounds, accounting for 84.9% of the total un-metabolized compounds, followed by caffeoylquinic acids (8.8%) and coumaroylquinic acid (6.3%).
Phase II derivatives of free hydroxycinnamic acids (caffeic, trans-ferulic, isoferulic and coumaric acids) along with caffeic acid accounted for 12.0% of the total metabolites excreted in urine. Ferulic acid 4-sulfate was the most abundant hydroxycinnamic acid (8 μmol per 24 h), followed by caffeic acid 3-sulfate, ferulic acid 4-glucuronide and isoferulic acid 3-sulfate with urinary excretions as high as 2.6, 2.3 and 1.7 μmol, respectively (Table 4). The concentration of isoferulic acid 3-glucuronide and two isomers of coumaric acid glucuronide ranged from 0.11 to 0.24 μmol in 24 h. Additionally, three isomers of caffeoylquinic lactone sulfate were excreted in urine, recovering up to 1290 nmol in 24 h urine, equivalent to 1% of the metabolites excreted (Table 4).
All the aforementioned metabolites, hydroxycinnamates, hydroxycinnamic acids and their phase II derivatives along with sulfated lactone derivatives, were preferentially excreted between 2 and 5 h after coffee ingestion and amounted to 16.3% of the total urinary metabolites excreted in 24 h. It is worth noting that glucuronidated and sulfated ferulic acids were also largely excreted between 8 and 12 h, in agreement with the biphasic profile observed in plasma (Fig. 1C).
However, reduced forms of hydroxycinnamic acids and their phase II derivatives were the most important group of metabolites quantified in urine after coffee ingestion, which were largely excreted between 5 and 12 h post-intake, representing 64.4% of the total urinary metabolites. Excretion of dihydrocaffeic acid and its phase II derivatives added up to 35 μmol in 24 h, being dihydrocaffeic acid 3-sulfate the most abundant urinary metabolite (31 μmol). Similarly, excretion of dihydroferulic acid and its phase II derivatives amounted up to 37 μmol in 24 h, in line with the higher excretion of unmetabolized feruloylquinic acids compared to caffeoylquinic acids previously described (Table 4). Among dihydroferulic acid metabolites, the 4-sulfate and 4-glucuronide derivatives were the main urinary metabolites (13 and 8 μmol in 24 h, respectively). In accordance with the lower presence of coumaroylquinic acids in the coffee beverage, excretion of dihydrocoumaric acid and its sulfated and glucuronidated metabolites amounted to 9 μmol in 24 h, with an important concentration of dihydrocoumaric acid sulfate (8 μmol in 24 h).
Interestingly, reduced forms of hydroxycinnamates were also characterized in urine for the first time; 3-, 4- and 5-dihydrocaffeoylquinic acids, 3-, 4- and 5-dihydroferoylquinic acids, and two isomers of dihydrocoumaroylquinic acid. These novel metabolites were quantified in noticeable amounts, accounting for 8% of the total urinary metabolites, and were specially excreted between 8 and 12 h after coffee intake, in agreement with their delayed appearance in plasma (Fig. 1F).
Feruloylglycine and isoferuloylglycine were also extensively excreted in the 5–8 h period. Their contribution to the total amount of identified metabolites was relevant (11.2%), mainly constituted by feruloylglycine (14 μmol in 24 h), thus actually being the second most abundant urinary metabolite after dihydrocaffeic acid-3 sulfate (Table 4).
Finally, the majority of microbial metabolites such as derivatives of hydroxyphenylpropionic, hydroxyphenylacetic and hydrophenylbenzoic acids, along with hydroxyhippuric acid and phloroglucinol, were present in basal urine before coffee intake and their levels increased minimally after coffee consumption (0–24 h), peaking between 8 and 12 h compared to baseline values (Table 4). These compounds were not included in the calculation of the recovery of polyphenols after coffee consumption since they are not exclusively derived from the biotransformation of hydroxycinnamic acids.
Taken together, the total amount of hydroxycinnamate metabolites excreted in urine 24 h after the intake of a single serving of the green/roasted coffee blend added to 127 μmol, which represents only 16.7% of the 760.6 μmol of polyphenols consumed.
3. Discussion
Although absorption and metabolism of hydroxycinammate esters in coffee have been previously reported,17,18,21–24 the novelty of the present work consisted in evaluating the bioavailability of the phenolics in a green/roasted coffee blend (35![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 65, v/v) using a realistic dose of coffee, namely 3.5 g of the instant soluble green/roasted coffee blend added to 250 mL of boiling water, following manufacturer's instructions. This yielded a beverage containing 269.5 mg (760.6 μmol) of hydroxycinnamic acids. A higher dose of coffee would have altered the kinetics of appearance, clearance and biotransformation pathways, not reflecting the response to nutritional doses. Furthermore, special attention was given to searching for novel microbial metabolites, considering that the identification of these metabolites could contribute to shed light on the biotransformation pathway of hydroxycinnamate esters in coffee and, therefore, to understand the bioactivity associated to coffee consumption.
65, v/v) using a realistic dose of coffee, namely 3.5 g of the instant soluble green/roasted coffee blend added to 250 mL of boiling water, following manufacturer's instructions. This yielded a beverage containing 269.5 mg (760.6 μmol) of hydroxycinnamic acids. A higher dose of coffee would have altered the kinetics of appearance, clearance and biotransformation pathways, not reflecting the response to nutritional doses. Furthermore, special attention was given to searching for novel microbial metabolites, considering that the identification of these metabolites could contribute to shed light on the biotransformation pathway of hydroxycinnamate esters in coffee and, therefore, to understand the bioactivity associated to coffee consumption.
Results showed that hydroxycinnamate esters present in the green/roasted blend were partially absorbed and extensively metabolized, so that most of the metabolites were produced by the gut microbiota. Reduced forms of hydroxycinnamic acids (dihydrohydroxycinnamic acids), mainly as phase II conjugated metabolites formed after absorption in the colon, were the predominant metabolites in plasma and urine, underlying the importance of the microbiota in the metabolism of hydroxycinnamic compounds.
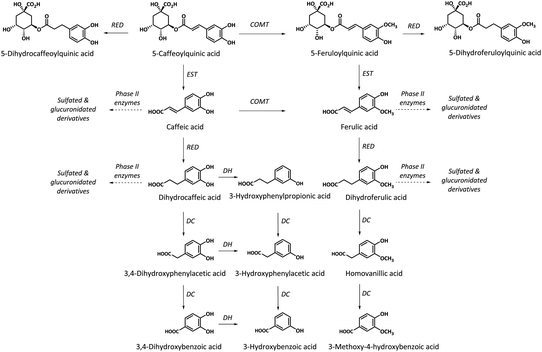
Whereas no free dicaffeoylquinic and caffeoylferuloylquinic acids were detected in the collected biological samples, small amounts of monoacylquinic acids (caffeoyl-, feruloyl- and p-coumaroylquinic acids) were quantified, reaching 3.3% of the metabolites excreted in urine. The characterized caffeoyl- and feruloylquinic acids in both plasma and urine, and p-coumaroylquinic acids in urine would derive from the green/roasted coffee blend after their direct absorption without being metabolized in the small intestine, although part of the excreted monohydroxycinnamoylquinic acids may also result from hydrolysis by intestinal esterases of diacyl derivatives of hydroxycinnamic acids (dicaffeoylquinic and caffeoylferuloylquinic acids)27 (Fig. 2). These results are in agreement with other recent studies in humans, that failed to observe diacylquinic derivatives and reported low amounts of monoacylquinic acids (Cmax < 0.1 μM at Tmax 0.5–2.1) after the consumption of 300 mg of hydroxycinnamates in a green coffee extract,28 146 mg of hydroxycinnamates in roasted coffee,17 400 mL (the amount of hydroxycinnamates was not specified) of instant roasted coffee,21 and three doses of roasted coffee providing 176, 352 and 704 mg of hydroxycinnamates.18 These results contrast with earlier bioavailability studies in roasted coffee where high plasmatic levels of both monoaylquinic acids (3-, 4- and 5-caffeoylquinic acids) and diacylquinic derivatives (3,4-, 3,5- and 4,5-dicaffeoylquinic acids) with Cmax of 1.0–3.14 μM and 0.9–1.11 μM, respectively, were reported after the ingestion of 1200 mg of hydroxycinnamates,29 and Cmax of 0.9–5.9 μM and 1.5–2.5 μM, respectively, after the ingestion of 170 mg of hydroxycinnamates.30 However, these authors did not detect diacyl derivatives of hydroxycinnamic acids in urine. Matsui et al.28 had already observed a dicaffeoylquinic acid isomer in plasma after the ingestion of 300 mg of hydroxycinnamates from a green coffee extract although in trace amount. Additionally, to our knowledge, this is the first study reporting the presence of coumaroylquinic acid in urine.
Most of the compounds identified in plasma and urine were metabolites derived from the hydrolysis of mono- and diacyl derivatives of hydroxycinnamic acids by esterases present in the small intestinal wall,27 yielding free hydroxycinnamic acids (caffeic, ferulic and p-coumaric acids). The hydroxycinnamic acids followed two different pathways; a minor ratio of metabolites was subsequently metabolized by phase II enzymes into sulfated, glucuronidated and methylated derivatives in the intestinal epithelium afterwards entering the bloodstream, whereas most hydroxycinnamic derivatives reached the colon and were substrates for microbial reductases prior to absorption and conjugation into phase II metabolites.
Free hydroxycinnamic acids and their phase II derivatives accounted for 12.0% of total urinary metabolites. It is important to note the biphasic kinetics observed for some phase II derivatives of hydroxycinnamic acids, namely sulfated and glucuronidated derivatives of ferulic acid, showing a second although less intense maximum in plasma and higher urinary excretion between 5 and 12 h. This outcome may extend the in vivo bioactivity of polyphenols and may be due to either enterohepatic recirculation31 or colonic absorption, both pathways would explain the longer permanence of polyphenols in the body, and therefore extended bioactivity. Among this type of metabolites, ferulic acid 4-sulfate and ferulic acid 4-glucuronide were the most abundant. The aforementioned results on chemical nature, kinetics and relative abundance of the detailed metabolites are in line with those reported previously.17,18,21
Special attention deserves the content of dimethoxycinnamic acid in plasma (Cmax 8 nM, Tmax 0.7 h), which contrasts with the high concentration in plasma (Cmax 380 nM) at 60 min after consuming 400 mL of 1% (w/v) soluble coffee described by Nagy et al.32 The same research group explained that the result could be due to dimethoxycinnamic acid being preferentially formed by dimethoxycinnamoylquinic acid hydrolysis and absorbed at the intestinal level by passive diffusion.33 These researchers emphasized the importance of considering the abundant 3,4-dimethoxycinnamic acid metabolite in future coffee bioavailability and metabolic studies. However, other recent bioavailability studies have not reported the presence of these derivatives in biological fluids after coffee consumption,17,21,23 in line with the low amount of dimethoxycinnamic acid determined in the present study.
Lactone derivatives were also absorbed in the small intestine without previous hydrolysis and were transformed into phase II derivatives, representing 1.0% of the total urinary metabolites. Stalmach et al.17 characterized for the first time the presence of two isomers of caffeoylquinic lactone sulfate in both plasma and urine with similar nutrikinetic profile as here reported. Likewise, Redeuil et al.21 characterized phase II derivatives of caffeoyl- and feruloylquinic lactone in plasma (urine analysis was not carried out), and Marmet et al.23 characterized caffeoyl- and feruloylquinic lactones in plasma after oral ingestion of soluble coffee, observing early appearance in both plasma and urine in accordance with the results reported here.
In all, after consumption of the green/roasted coffee blend, the low levels of metabolites of hydroxycinnamic acids, hydroxycinnamate esters and lactone derivatives in plasma and urine, accounting for 16.3% of the urinary metabolites, confirm the low bioavailability in the upper gastrointestinal tract.
It is well known the importance of the colonic microbiota in the metabolism of hydroxycinnamates, already demonstrated by Stalmach et al.34 in a study carried out in ileostomist individuals. In the present study, dihydrocaffeic, dihydroferulic and dihydrocoumaric acids, derived from microbial metabolism, and their phase II metabolites were identified, accounting for 64.4% of the total urinary metabolites (Tables 3 and 4).
Another microbial metabolite derived from ferulic acid extensively excreted in urine was feruloylglycine, which was also detected in plasma (Cmax 20 nM). Additionally, an isomer of this compound, isoferuloylglycine, was identified in low amounts only in urine. Both compounds (iso- and trans-feruloylglycine) were formed by CoA enzyme in the colon, which is consistent with the nutrikinetic parameters observed. Feruloylglycine had already been reported in other studies after the consumption of coffee rich in hydroxycinnamic acids, although only in urine.17,22 Therefore, to our knowledge, this is the first time that feruloylglycine has been detected in plasma and urine, together with isoferuloylglycine in urine. Noticeably, feruloylglycine was the second most abundant urinary metabolite (14 μmol in 24 h) after dihydrocaffeic acid 3-sulfate (31 μmol in 24 h). Consequently, the presence of dihydrocaffeic acid-3-sulfate and feruloylglycine in urine could be very sensitive biomarkers of green/roasted coffee intake, considering that the excreted amount of both metabolites reached 24.4% and 11.0% of the excreted metabolites, respectively. Urinary excretion of iso- and trans-feruloylglycine together accounted for 11.3% of the total metabolites.
An important and novel contribution of the present work compared to previous reports on the bioavailability of coffee hydroxycinnamate studies was the identification in plasma and urine of reduced forms of monoacylquinic acids, namely dihydrocaffeoyl-, dihydroferuloyl- and dihydrocoumaroylquinic acids (Tables 3 and 4). Additionally, the presence of low amounts of dihydrocaffeoylquinic acid glucuronide in urine was also identified. Only one in vitro study on the microbial biotransformation of chlorogenic acid had reported the formation of dihydrocaffeoylquinic acid.35 In the present in vivo study, relevant amounts of dihydromonoacylquinic acids were detected in plasma (Cmax 9–40 nM) and urine (10 μmol excreted in 24 h), amounting to 8% of the total urinary metabolites with the most delayed kinetic out of all the characterized metabolites.
Reduced forms of hydroxycinnamic acids and their phase II derivatives, together with reduced forms of hydroxycinnamates and feruloylglycine, accounted for 83.7% of the total excretion, highlighting the relevance of the colon in the metabolism of hydroxycinnamates.
In general, ferulic acid derivatives were formed in higher amounts than those derived from caffeic acid due to the double biotransformation pathway followed by hydroxycinnamates: minimally by hydrolysis of their precursors and preferentially by methylation of caffeoylquinic acids via catechol-O-methyltransferase (COMT). Additionally, it is worth noting that sulfation was the predominant phase II transformation, followed by methylation and, to a lower extent, glucuronidation, in agreement with Marmet et al.23 and Sanchez-Bridge et al.24
Finally, derivatives of hydroxyphenylacetic, hydroxybenzoic, and hydroxyhippuric acids were also characterized in both plasma and urine, amounting up to 10 μmol in 24 h urine (Table 4). However, these metabolites were not considered in the recovery of the ingested green/roasted coffee blend phenols, since they are not exclusively formed during the biotransformation of hydroxycinnamic acids. In all, the total urinary excretion of coffee phenol metabolites presented a recovery of just 16.7% of the phenols ingested, pointing to a low bioavailability of the green/roasted coffee blend polyphenols. Previous studies also showed a limited bioavailability of coffee phenolics, with recoveries up to 29% of the ingested hydroxycinnamtes (146 mg, 412 μmol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 17). The same research group evaluated the impact of dose of chlorogenic acid in coffee on the bioavailability of this phenol in humans,22 and observed recoveries of 24%, 25% and 16% after the intake of single servings of coffee containing low (412 μmol ∼ 146 mg), medium (635 μmol ∼ 225 mg) and high (795 μmol ∼ 282 mg) contents of the studied phenol, respectively, and plasmatic kinetics coherent with that observed in urine. Considering that in the present study the ingested amount of polyphenols (760.6 μmol ∼ 269.5 mg) was in line with the highest dose reported by Stalmach et al.,22 both studies are in agreement and suggest that hydroxycinnamates absorption saturation may take place with high intake doses. Contrary to these results, Renouf et al.18 and later Sanchez-Bridge et al.24 showed a dose–response appearance of metabolites in plasma (urine was not analyzed). The highest doses used in both studies (970 μmol ∼ 344 mg and 1485 μmol ∼ 526 mg, respectively) were higher than the almost 761 μmol used in the present study and in Stalmach et al.22 Moreover, Sanchez-Bridge et al.,24 evaluated the influence of roasting on the extent of conjugation by comparing three types of coffees (high, low and unroasted coffees), observing similar extent of conjugation with roasted or unroasted coffees.
17). The same research group evaluated the impact of dose of chlorogenic acid in coffee on the bioavailability of this phenol in humans,22 and observed recoveries of 24%, 25% and 16% after the intake of single servings of coffee containing low (412 μmol ∼ 146 mg), medium (635 μmol ∼ 225 mg) and high (795 μmol ∼ 282 mg) contents of the studied phenol, respectively, and plasmatic kinetics coherent with that observed in urine. Considering that in the present study the ingested amount of polyphenols (760.6 μmol ∼ 269.5 mg) was in line with the highest dose reported by Stalmach et al.,22 both studies are in agreement and suggest that hydroxycinnamates absorption saturation may take place with high intake doses. Contrary to these results, Renouf et al.18 and later Sanchez-Bridge et al.24 showed a dose–response appearance of metabolites in plasma (urine was not analyzed). The highest doses used in both studies (970 μmol ∼ 344 mg and 1485 μmol ∼ 526 mg, respectively) were higher than the almost 761 μmol used in the present study and in Stalmach et al.22 Moreover, Sanchez-Bridge et al.,24 evaluated the influence of roasting on the extent of conjugation by comparing three types of coffees (high, low and unroasted coffees), observing similar extent of conjugation with roasted or unroasted coffees.
A limitation of the present study was the reduced urinary collection time. Most of the microbial metabolites showed relevant amounts in the 12–24 h interval, not returning to basal levels, and therefore it would have been interesting to extend the collection to at least 48 h. It is likely that the amount of urinary metabolites has been underestimated and thus a higher bioavailability of coffee polyphenols cannot be ruled out. Another limitation was the lack of certain metabolite standards, mainly phase II derivatives, forcing to express the results as equivalents of the corresponding precursor compound. Therefore, the results here indicated did not accurately measure the concentrations of the metabolites described in the biological samples. Nevertheless, the results are in line with other studies on the bioavailability of coffee hydroxycinnamates.17,22
In summary, polyphenols contained in a commercial instant green/roasted coffee blend were partially absorbed and extensively metabolized. The predominant metabolites were phase II derivatives of reduced forms of hydroxycinnamic acids, sulfated, methyl derivatives and to a lower extent glucuronidated conjugates, in addition to feruloylglycine. Reduced forms of hydroxycinnamates were identified for the first time in an in vivo bioavailability study of phenols in coffee, accounting for 8% of the total urinary metabolites, completing the knowledge on the coffee polyphenol bio-transformations. Attending to their high urinary elimination, dihydrocaffeic acid-3-sulfate and feruloylglycine can be used as biomarkers of intake of hydroxycinnamate-rich foods.
In conclusion, the bioavailability of hydroxycinnamates in green/roasted coffee blend is discreet but should not be disregarded considered the high intake of coffee. The phenols in this beverage are extensively metabolized, mainly by the microbiota, and remain for long time in the body of coffee consumers which favors the possible bioactivity of these compounds.
Conflicts of interest
The authors declare no conflicts of interest.Acknowledgements
We are grateful to volunteers participating in the study. Authors thank I. Alvarez and M. A. Martinez at the Analytical Techniques Service (USTA) facilities of ICTAN for technical assistance on mass spectrometry analyses. We also are grateful to the Spanish Ministry of Economy and Competitivity (MINECO-FEDER), projects AGL2010-18269 and AGL2015-69986-R for financial support. S. M.-L. was a predoctoral fellow of the JAE Program (JAEPre097) co-funded by CSIC and the European Social Fund. M. G.-J. was a predoctoral FPI fellow BES2008-007138 of the Spanish Ministry of Science and Innovation. All authors revised and approved the final version of the manuscript.References
- M. S. Butt and M. T. Sultan, Crit. Rev. Food Sci. Nutr., 2011, 51, 363–373 CrossRef CAS PubMed.
- E. Lopez-Garcia, P. Guallar-Castillon, L. Leon-Muñoz, A. Graciani and F. Rodriguez-Artalejo, Clin. Nutr., 2014, 33, 1–149 CrossRef PubMed.
- I. A. Ludwig, M. N. Clifford, M. E. Lean, H. Ashihara and A. Crozier, Food Funct., 2014, 5, 1695–1717 CAS.
- L. Bravo and R. Mateos and B. Sarria, in Coffee: Chemistry, Quality and Health Implications, ed. A. Farah, Royal Society of Chemistry, Cambridge, 2016 Search PubMed.
- T. W. Crozier, A. Stalmach, M. E. Lean and A. Crozier, Food Funct., 2012, 3, 30–33 CAS.
- N. M. De Aragao, M. C. Veloso, M. S. Bispo, S. L. Ferreira and de J. B. Andrade, Talanta, 2005, 67, 1007–1013 CrossRef CAS PubMed.
- M. A. Heckman, J. Weil and E. Gonzalez de Mejia, J. Food Sci., 2010, 75, R77 CrossRef CAS PubMed.
- N. Naidoo, C. Chen, S. A. Rebello, K. Speer, E. S. Tai, J. Lee, S. Buchmann, I. Koelling-Speer and R. M. van Dam, Nutr. J., 2011, 15, 10–48 Search PubMed.
- J. Zhou, L. Chan and S. Zhou, Curr. Med. Chem., 2012, 19, 3523–3531 CrossRef CAS PubMed.
- M. E. Diaz-Rubio and F. Saura-Calixto, J. Agric. Food Chem., 2007, 55, 1999–2003 CrossRef CAS PubMed.
- V. Fogliano and F. J. Morales, Food Funct., 2011, 2, 117–123 CAS.
- R. M. Alonso-Salces, F. Serra, F. Reniero and K. Heberger, J. Agric. Food Chem., 2009, 57, 4224–4235 CrossRef CAS PubMed.
- G. Baeza, B. Sarria, L. Bravo and R. Mateos, J. Agric. Food Chem., 2016, 64, 9663–9674 CrossRef CAS PubMed.
- A. Farah, T. de Paulis, L. C. Trugo and P. R. Martin, J. Agric. Food Chem., 2005, 53, 1505–1513 CrossRef CAS PubMed.
- R. Jaiswal, M. F. Matei, P. Subedi and N. Kuhnert, Food Res. Int., 2014, 61, 214–227 CrossRef CAS.
- D. Del Rio, A. Rodriguez-Mateos, J. P. E. Spencer, M. Tognolini, G. Borges and A. Crozier, Antioxid. Redox Signaling, 2013, 18, 1818–1892 CrossRef CAS PubMed.
- A. Stalmach, W. Mullen, D. Barron, K. Uchida, T. Yokota, C. Cavin, H. Steiling, G. Williamson and A. Crozier, Drug Metab. Dispos., 2009, 37, 1749–1758 CrossRef CAS PubMed.
- M. Renouf, C. Marmet, F. Giuffrida, M. Lepage, D. Barron, M. Beaumont, G. Williamson and F. Dionisi, Mol. Nutr. Food Res., 2014, 58, 301–309 CAS.
- B. Sarriá, S. Martínez-López, R. Mateos and L. Bravo-Clemente, Food Res. Int., 2016, 89, 1023–1028 CrossRef.
- B. Sarriá, S. Martínez-López, J. L. Sierra-Cinos, L. García-Diz, R. Mateos and L. Bravo-Clemente, Regularly consuming a green/roasted coffee blend reduces the risk of metabolic syndrome, Eur. J. Nutr., 2016 DOI:10.1007/s00394-016-1316-8.
- K. Redeuil, C. Smarrito-Menozzi, P. Guy, S. Rezzi, F. Dionisi, G. Williamson, K. Nagy and M. Renouf, J. Chromatogr., A, 2011, 1218, 4678–4688 CrossRef CAS PubMed.
- A. Stalmach, G. Williamson and A. Crozier, Food Funct., 2014, 5, 1727–1737 CAS.
- C. Marmet, L. Actis-Goretta, M. Renouf and F. Giuffrida, J. Pharm. Biomed. Anal., 2014, 88, 617–625 CrossRef CAS PubMed.
- B. Sanchez-Bridge, M. Renouf, J. Sauser, M. Beaumont and L. Actis-Goretta, Biofactors, 2016, 42, 259–267 CAS.
- R. Mateos, S. Martínez-López, G. Baeza Arévalo, M. Amigo-Benavent, B. Sarria and L. Bravo-Clemente, Food Chem., 2016, 205, 248–256 CrossRef CAS PubMed.
- A. J. Day, F. Mellon, D. Barron, G. Sarrazin, M. R. Morgan and G. Williamson, Free Radical Res., 2001, 35, 941–952 CrossRef CAS PubMed.
- M. F. Andreasen, P. A. Kroon, G. Williamson and M. T. Garcia-Conesa, J. Agric. Food Chem., 2001, 49, 5679–5684 CrossRef CAS PubMed.
- Y. Matsui, S. Nakamura, N. Kondou, Y. Takasu, R. Ochiai and Y. Masukawa, J. Chromatogr. B: Anal. Technol. Biomed. Life Sci., 2007, 858, 96–105 CrossRef CAS PubMed.
- M. Monteiro, A. Farah, D. Perrone, L. C. Trugo and C. Donangelo, J. Nutr., 2007, 37, 2196–2201 Search PubMed.
- A. Farah, M. Monteiro, C. M. Donangelo and S. Lafay, J. Nutr., 2008, 138, 2309–2315 CrossRef CAS PubMed.
- A. Rodríguez-Mateos, D. Vauzour, C. G. Krueger, D. Shanmuganayagam, J. Reed, L. Calani, P. Mena, D. Del Río and A. Crozier, Arch. Toxicol., 2014, 88, 1803–1853 CrossRef PubMed.
- K. Nagy, K. Redeuil, G. Williamson, S. Rezzi, F. Dionisi, K. Longet, F. Destaillats and M. Renouf, J. Chromatogr., A, 2011, 1218, 491–497 CrossRef CAS PubMed.
- T. L. Farrell, M. Gómez-Juaristi, L. Poquet, K. Redeuil, K. Nagy, M. Renouf and G. Williamson, Mol. Nutr. Food Res., 2012, 56, 1413–1429 CAS.
- A. Stalmach, H. Steiling, G. Williamson and A. Crozier, Arch. Biochem. Biophys., 2010, 501, 98–105 CrossRef CAS PubMed.
- F. Tomás-Barberán, R. García-Villalba, A. Quartieri, S. Raimondi, A. Amaretti, A. Leonardi and M. Rossi, Mol. Nutr. Food Res., 2014, 58, 1122–1131 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7fo01553d |
| This journal is © The Royal Society of Chemistry 2018 |