Brønsted acid-catalyzed metal- and solvent-free quinoline synthesis from N-alkyl anilines and alkynes or alkenes†
Waqar
Ahmed‡
a,
Sheng
Zhang‡
a,
Xiaoqiang
Yu
a,
Yoshinori
Yamamoto
ab and
Ming
Bao
 *ac
*ac
aState Key Laboratory of Fine Chemicals, Dalian University of Technology, Dalian 116023, China. E-mail: mingbao@dlut.edu.cn; Fax: +86-0411-84986181; Tel: +86-0411-84986180
bWPI-AIMR (WPI-Advanced Institute for Materials Research), Tohoku University, Sendai 980-8577, Japan and Research Organization of Science and Technology, Ritsumeikan University, Kusatsu, Shiga 525-8577, Japan
cSchool of Petroleum and Chemical Engineering, Dalian University of Technology, Panjin 124221, China
First published on 28th November 2017
Abstract
Brønsted acid-catalyzed cyclization reactions of N-alkyl anilines with alkynes or alkenes in the presence of oxygen gas as an oxidant under metal- and solvent-free conditions are described. Various quinoline derivatives are obtained in satisfactory to excellent yields. Different groups, such as methyl, fluoro, chloro, bromo, methoxy, and ester linked on benzene rings, are tolerated under optimized reaction conditions.
Introduction
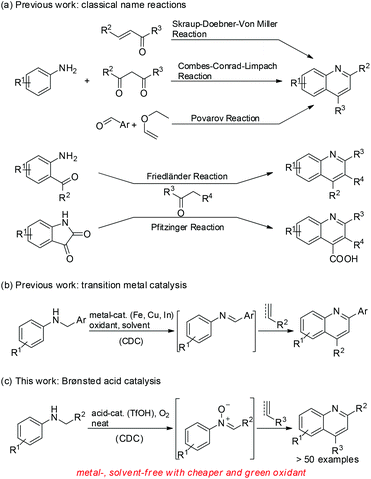
Quinoline motifs have attracted considerable interest due to their potent applications1 in photoelectric materials2 and pharmaceuticals,3 including antimalarial, antibacterial, antileishmanial, anti-inflammatory, and insecticidal agents.4 Many methods have been established for the conversion of anilines to various substituted quinolines in the presence of different reaction partners since the late 1800s. Various classical name reactions are identified for quinoline synthesis; these reactions include Skraup, Doebner–von Miller, Combes, Conrad–Limpach, Povarov, Friedländer, and Pfitzinger reactions (Scheme 1a).5 These methods either use adverse conditions, such as high temperature of approximately 200 °C, or hazardous reagents in stoichiometric amounts, which adversely affect the environment-friendly chemistry and atom economy. Moreover, some methods rely on reagents for quinoline synthesis that needs additional steps.Recently, transition metal-catalyzed coupling reactions have been used as potent tools for the synthesis of heterocyclic compounds, especially quinolines.6 Fe, Cu, In, Bi, Pd, and Ag salts have been used as catalysts for quinoline synthesis. Furthermore, a transition-metal-catalyzed quinoline synthesis uses N-alkyl anilines and arylacetylenes or arylethylenes through direct cross-dehydrogenative coupling (CDC) of a C–H bond to construct a new C–C bond (Scheme 1b).7 Given its high atom economy and environment-friendly nature, the CDC reaction has frequently been used to construct quinoline motifs. Liu8 and Mancheño9 groups independently reported the iron-catalyzed synthesis of quinolines using either N-benzylanilines or N-aryl glycine esters and arylacetylenes or arylethylenes as reaction partners in the presence of di-tertiary-butyl peroxide or TEMPO oxoammonium salt as an oxidant in a stoichiometric amount through the direct CDC method. Liu and coworkers recently reported about the copper-catalyzed synthesis of quinolines via the CDC reaction of N-aryl glycine esters with olefins in the presence of either K2S2O8 (in stoichiometric amount) or N-hydroxyphthalimide combined with O2 as oxidants.10 Jia and Wang revealed that the CDC reaction of N-aryl glycine esters with olefins or arylacetylenes for the synthesis of quinolines can be promoted in the presence of catalytic InCl3 and tris(4-bromophenyl)ammonium hexachloroantimonate (TBPA˙+) using O2 as the terminal oxidant.11 Quinoline synthesis via transition-metal-catalyzed CDC reaction commonly requires the use of costly inorganic or organic oxidants. Notably, Jia and coworkers recently succeeded on the metal-free CDC reaction of N-benzylanilines with arylethylenes for quinoline synthesis in the presence of catalytic radical cation salt, namely, TBPA˙+ using O2 as the oxidant.12
Although these existing methods can provide easy access to quinoline derivatives, the development of remarkably efficient, environment-friendly, and economic organic synthetic process is still desirable. In the course of our research on the development of efficient methods for heterocyclic synthesis,13 we found that quinolines can readily be obtained from the Brønsted acid-catalyzed metal- and solvent-free CDC reaction of N-alkyl anilines with arylacetylenes or arylethylenes using O2 as the oxidant (Scheme 1c). The results are reported in this paper.
Results and discussion
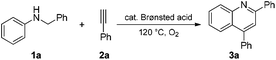
The cyclization of N-benzyl aniline (1a) with phenylacetylene (2a) was selected as a model reaction to optimize the reaction conditions. The results are shown in Table 1. Brønsted acid catalysts, including trifluoroacetic acid (TFA), p-toluene sulfonic acid (PTSA), methanesulfonic acid, benzoic acid (BzOH), sulphuric acid (H2SO4) and trifluoromethanesulfonic acid (TfOH), were initially screened in 1,2-dichloroethane (DCE) in air at 120 °C for 15 h. A relatively high yield (57%) of quinoline 3a was obtained when TfOH was used as the catalyst (entry 6 vs. entries 1–5). The solvent was subsequently screened, but the 3a yield could not be improved (entry 6 vs. entries 7–10).| Entry | Acid cat. | Solvent | O2 | Time (h) | Yieldb (%) |
|---|---|---|---|---|---|
| a Reaction conditions: N-Benzyl aniline (1a, 0.2 mmol, 36.7 mg), phenylacetylene (2a, 1.0 mmol, 102.1 mg), Brønsted acid (15 mol%), and solvent (2.0 mL), 120 °C. b Isolated yield. c No reaction; the starting materials were recovered. d The reaction occurred at 110 °C. e The reaction was performed in the presence of 10 mol% TfOH. | |||||
| 1 | TFA | DCE | O2 in air | 15 | 48 |
| 2 | PTSA | DCE | O2 in air | 15 | 45 |
| 3 | CH3SO3H | DCE | O2 in air | 15 | 40 |
| 4 | BzOH | DCE | O2 in air | 15 | NRc |
| 5 | H2SO4 | DCE | O2 in air | 15 | Trace |
| 6 | TfOH | DCE | O2 in air | 15 | 57 |
| 7 | TfOH | Toluene | O2 in air | 15 | 43 |
| 8 | TfOH | 1,4-Dioxane | O2 in air | 15 | 54 |
| 9 | TfOH | DMF | O2 in air | 15 | 43 |
| 10 | TfOH | CH3CN | O2 in air | 15 | NRc |
| 11 | TfOH | DCE | O2 balloon | 15 | 62 |
| 12 | TfOH | None | O2 balloon | 15 | 77 |
| 13 | TfOH | None | O2 balloon | 24 | 83 |
| 14 | TfOH | None | O2 balloon | 24 | 76d |
| 15 | TfOH | None | O2 balloon | 24 | 71e |
| 16 | TfOH | None | N2 | 24 | NRc |
| 17 | none | None | O2 balloon | 24 | NRc |
Additionally, an increased 3a yield (62%) was observed when the model reaction was performed under a pure oxygen atmosphere (O2 balloon) in DCE for 15 h (entry 11 vs. entry 6). The further increased yield was observed under solvent-free conditions (entry 12, 77%). The highest 3a yield was finally obtained when the model reaction was carried out for a prolonged time (entry 13, 24 h, 83%). The 3a yield decreased with the reduced reaction temperature and acid catalyst loading (entries 14 and 15). No reaction was observed in the absence of oxygen gas or an acid catalyst (entries 16 and 17). Therefore, we performed the subsequent reactions of the N-alkyl anilines with arylacetylenes or arylethylenes in the presence of TfOH as a catalyst at 120 °C under solvent-free conditions for 24 h. During optimization, starting materials were recovered along with the product in case of low yield (Table 1).
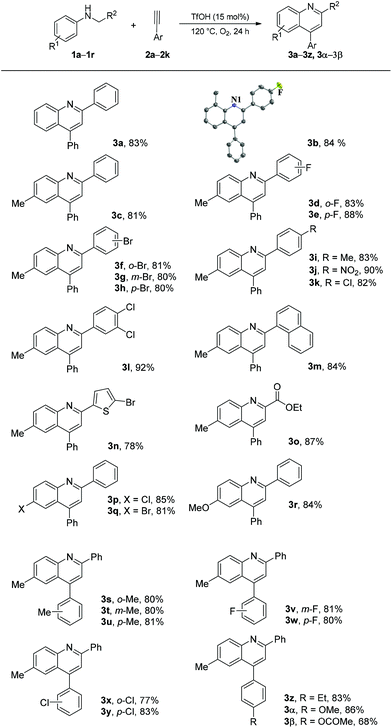
On the basis of the optimized reaction conditions, we explored the scope and limitation of this type of cyclization reaction. The results are summarized in Scheme 2. Initially, alkyne 2a was used as a reaction partner to investigate the scope of N-alkyl anilines. As described in Table 1, the desired product 3a was obtained in 83% yield. The reactions of N-(4-fluorobenzyl)-2-methylaniline (1b) and N-benzyl-4-methylaniline (1c) proceeded well to produce the corresponding quinoline products 3b and 3c in good yields (84% and 81%, respectively). Good to excellent yields were obtained in the reactions of N-benzyl-4-methylanilines 1d–1k bearing a substituent (F, Br, Me, NO2, or Cl) on the ortho-, meta-, or para-position of the benzyl group (1d–1k, 80%–90%). The reactions of N-(3,4-dichlorobenzyl)-4-methylaniline (1l), 4-methyl-N-(naphthalen-1-ylmethyl)aniline (1m), N-((5-bromothiophen-2-yl)methyl)-4-methylaniline (1n), and ethyl 2-(p-tolylamino)acetate (1o) also proceeded well and produced quinoline products 3l–3o in 78%–92% yields. Moreover, the reaction of N-benzylanilines 1p–1r having substituents (Cl, Br, and MeO) at the para-position of the aniline ring also produced the corresponding quinoline products 3p–3r in 81%–85% yields. Afterward, the reactions of N-benzyl-4-methylaniline (1c) with various arylacetylenes 2b–2k were examined to explore the scope of alkyne substrates. Quinolines 3s–3β were obtained in 68%–86% yields. These results indicated that different groups, such as methyl, fluoro, chloro, bromo, methoxy, nitro, and ester linked on benzene rings, were tolerated under the optimized reaction conditions. The notably maintained Br and Cl atoms in the structures of products should make the products considerably useful in organic transformation.
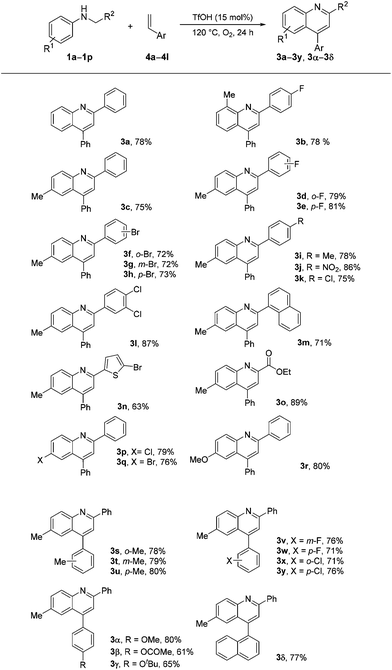
We found that arylethylenes, instead of arylacetylenes, can be used for quinoline synthesis under the optimized reaction conditions. The results are summarized in Scheme 3. Similar good results were obtained, as shown in Scheme 2. Quinoline products 3a–3y and 3α–3δ were obtained in satisfactory to good yields (61%–89%).
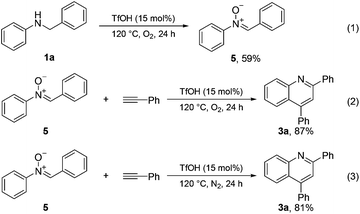
Control experiments were conducted to gain insights into the mechanism of this type of cyclization reaction (Scheme 4). An imine-N-oxide 5 (59%) was obtained when the N-benzyl aniline substrate 1a was treated solely under the optimized reaction conditions (eqn (1)). Subsequently, imine-N-oxide 5 was used as a starting material to react with phenylacetylene under the standard conditions; 87% of quinoline 3a was obtained (eqn (2)). The quinoline 3a was also obtained in good yield (81%) when the imine-N-oxide 5 was treated with phenylacetylene under an N2 atmosphere (eqn (3)). These results suggested that the current cyclization reaction might involve an imine-N-oxide intermediate.
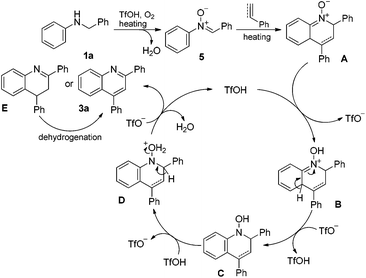
On the basis of our experimental outcomes and previous reports,8,14 a plausible catalytic cycle is proposed to account for the present Brønsted acid-catalyzed cyclization reaction (Scheme 5). N-Benzylaniline substrate 1a was oxidized in the presence of O2 and an acid to produce imine-N-oxide 5. The imine-N-oxide 5 subsequently underwent the Diels–Alder-type cyclization reaction in the presence of phenylacetylene or styrene as a dienophile under heating conditions to generate an imine-N-oxide intermediate A. The intermediate A abstracted a proton from TfOH to form intermediate B, which underwent rearomatization reaction to produce intermediate C. Protonation of N-OH in intermediate C occurred to generate intermediate D. Dehydration and aromatization of intermediate D finally occurred to yield quinoline product 3a (generated from phenylacetylene) or an intermediate E (generated from styrene) and regenerated acid catalyst TfOH. The intermediate E subsequently underwent dehydrogenation reaction in the presence of O2 gas under heating conditions to generate the target product 3a.
Conclusions
A convenient and efficient method for quinoline synthesis was developed using simple and readily available N-alkyl anilines and arylacetylenes or arylethylenes as the starting materials. The Brønsted acid-catalyzed metal- and solvent-free cyclization proceeded well under an O2 atmosphere to furnish quinolines in satisfactory to excellent yields. The widespread availability of the starting materials, cheap and environment-friendly oxidants, and experimental simplicity make the present methodology considerably useful in organic synthesis.Experimental
General information
The reactions were performed in clean, oven-dried reactors fitted with air-tight stoppers. All the chemicals were used without further purification. The solvents were used after drying according to the given procedure. 1H and 13C NMR spectra were obtained with a Bruker Avance II-400 spectrometer (400 MHz for 1H, 100 MHz for 13C) or a Varian Inova-500 spectrometer (500 MHz for 1H, 125 MHz for 13C); CDCl3 and TMS were used as the solvent and internal standard, respectively. The chemical shifts are reported in ppm downfield (δ) from TMS and the coupling constants J are given in Hz. The peak patterns are indicated as follows: s, singlet; d, doublet; t, triplet; q, quartet; m, multiplet. High-resolution mass spectra were recorded on either a Q-TOF mass spectrometer or a GC-TOF mass spectrometer. TLC was used with SiO2 (silica gel 60 F254, Merck) as a stationary phase. Spots were viewed under UV light. Flash column chromatography was performed with SiO2 (80 mesh) as a stationary phase.General procedure for the synthesis of quinolines 3
N-Substituted arylamines 1a–1r (0.20 mmol), phenylacetylene 2a (110 μL, 1 mmol, 5 equiv.), or styrenes (115 μL, 1 mmol, 5 equiv.) were added to a clean, oven-dried reactor and stirred for 1 min. Trifluoromethanesulfonic acid (2.7 μL, 0.03 mmol, 15 mol%) was then added. Oxygen was purged directly from the cylinder such that the environment was completely saturated with it for 24 h at 120 °C. Afterward, the reaction mixture was purified by flash column chromatography on silica gel (eluent: petroleum ether/DCM = 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) to yield quinoline 3.
2) to yield quinoline 3.
Procedure for synthesis of imine-oxide 5
1a was added into a 100 mL oven-dried round bottom flask (366.4 mg, 2.0 mmol). Trifluoromethanesulfonic acid (27.0 μL, 0.3 mmol) was also added to the above flask after heating, until 1a was melted. Oxygen was purged directly from the cylinder at 120 °C for 24 h. The acid catalyst and water generated were evaporated on a rotary evaporator. The crude mixture was dissolved in a diethyl ether![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DCM mixture (9.5
DCM mixture (9.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5), washed two times with K2CO3 solution and subsequently with distilled water, and dried over anhydrous Na2SO4. The solvent was evaporated, which resulted in a yellow feathery solid. This solid was washed with ice-cold diethyl ether, which yielded white crystals of the title compound.
0.5), washed two times with K2CO3 solution and subsequently with distilled water, and dried over anhydrous Na2SO4. The solvent was evaporated, which resulted in a yellow feathery solid. This solid was washed with ice-cold diethyl ether, which yielded white crystals of the title compound.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
We are grateful to the National Natural Science Foundation of China (No. 21372035, 21573032, and 21602026) for their financial support. This work was also supported by the China Postdoctoral Science Foundation (No. 2016 M590226).Notes and references
- For selected reviews, see: (a) V. Oliveri and G. Vecchio, Mini-Rev. Med. Chem., 2016, 16, 1185 CrossRef CAS PubMed; (b) M. J. Mphahlele and L. G. Lesenyeho, J. Heterocycl. Chem., 2013, 50, 1 CrossRef CAS; (c) V. Prachayasittikul, S. Prachayasittikul, S. Ruchirawat and V. Prachayasittikul, Drug Des., Dev. Ther., 2013, 7, 1157 CrossRef PubMed; (d) R. G. Anderson and G. Nickless, Analyst, 1967, 92, 207 RSC.
- For selected reviews, see: (a) H. Xu, R. Chen, Q. Sun, W. Lai, Q. Su, W. Huang and X. Liu, Chem. Soc. Rev., 2014, 43, 3259 RSC; (b) A. Danel, E. Gondek and I. Kityk, Opt. Mater., 2009, 32, 267 CrossRef CAS; (c) H. D. Willauer and G. E. Collins, Electrophoresis, 2003, 24, 2193 CrossRef CAS PubMed.
- For selected reviews, see: (a) K. Gopaul, S. A. Shintre and N. A. Koorbanally, Anticancer Agents Med. Chem., 2015, 15, 631 CrossRef CAS PubMed; (b) O. Afzal, S. Kumar, M. R. Haider, M. R. Ali, R. Kumar, M. Jaggi and S. Bawa, Eur. J. Med. Chem., 2015, 97, 871 CrossRef CAS PubMed; (c) M. Orhan Puskullu, B. Tekiner and S. Suzen, Mini-Rev. Med. Chem., 2013, 13, 365 CAS.
- For selected reviews, see: (a) P.-Y. Chung, Z.-X. Bian, H.-Y. Pun, D. Chan, A. S.-C. Chan, C.-H. Chui, J. C.-O. Tang and K.-H. Lam, Future Med. Chem., 2015, 7, 947 CrossRef CAS PubMed; (b) Y. Zhang, T. Han, Q. Ming, L. Wu, K. Rahman and L. Qin, Nat. Prod. Commun., 2012, 7, 963 CAS; (c) M. A. ElSohly and W. Gul, Recent Pat. Antiinfect. Drug Discovery, 2007, 2, 222 CrossRef CAS.
- For selected reviews, see: (a) M. Fallah-Mehrjardi, Mini-Rev. Org. Chem., 2017, 14, 187 CAS; (b) G. A. Ramann and B. J. Cowen, Molecules, 2016, 21, 986 CrossRef PubMed; (c) A. Majumder, R. Gupta and A. Jain, Green Chem. Lett. Rev., 2013, 6, 151 CrossRef CAS; (d) J. Marco-Contelles, E. Pérez-Mayoral, A. Samadi, M. d. C. Carreiras and E. Soriano, Chem. Rev., 2009, 109, 2652 CrossRef CAS PubMed.
- For selected reviews, see: (a) S. M. Prajapati, K. D. Patel, R. H. Vekariya, S. N. Panchal and H. D. Patel, RSC Adv., 2014, 4, 24463 RSC; (b) Y. Yamamoto, Chem. Soc. Rev., 2014, 43, 1575 RSC; (c) J. Barluenga, F. Rodriguez and F. J. Fananas, Chem. – Asian J., 2009, 4, 1036 CrossRef CAS PubMed; (d) D. M. D'Souza and T. J. Mueller, Chem. Soc. Rev., 2007, 36, 1095 RSC.
- P. Liu, Z. Wang, J. Lin and X. Hu, Eur. J. Org. Chem., 2012, 1583 CrossRef CAS.
- P. Liu, Y. Li, H. Wang, Z. Wang and X. Hu, Tetrahedron Lett., 2012, 53, 6654 CrossRef CAS.
- H. Richter and O. G. Mancheño, Org. Lett., 2011, 13, 6066.
- (a) G. Liu, J. Qian, J. Hua, F. Cai, X. Li and L. Liu, Org. Biomol. Chem., 2016, 14, 1147 RSC; (b) Z. Xie, J. Jia, X. Liu and L. Liu, Adv. Synth. Catal., 2016, 358, 919 CrossRef CAS.
- X. Jia, F. Peng, C. Qing, C. Huo and X. Wang, Org. Lett., 2012, 14, 4030 CrossRef CAS PubMed.
- J. Liu, F. Liu, Y. Zhu, X. Ma and X. Jia, Org. Lett., 2015, 17, 1409 CrossRef CAS PubMed.
- (a) X. Yu, J. Wang, Z. Xu, Y. Yamamoto and M. Bao, Org. Lett., 2016, 18, 2491 CrossRef CAS PubMed; (b) M. S. Mayo, X. Yu, X. Feng, Y. Yamamoto and M. Bao, J. Org. Chem., 2015, 80, 3998 CrossRef CAS PubMed; (c) M. S. Mayo, X. Yu, X. Zhou, X. Feng, Y. Yamamoto and M. Bao, Org. Lett., 2014, 16, 764 CrossRef CAS PubMed; (d) F. Yang, T. Jin, M. Bao and Y. Yamamoto, Chem. Commun., 2014, 57, 4541 Search PubMed; (e) X. Yu, N. Huang, X. Feng, Y. Yamamoto and M. Bao, Synthesis, 2014, 46, 2422 CrossRef CAS; (f) L. Wang, X. Yu, X. Feng and M. Bao, J. Org. Chem., 2013, 78, 1693 CrossRef CAS PubMed; (g) L. Wang, X. Yu, X. Feng and M. Bao, Org. Lett., 2012, 14, 2418 CrossRef CAS PubMed; (h) F. Yang, T. Jin, M. Bao and Y. Yamamoto, Tetrahedron Lett., 2011, 52, 936 CrossRef CAS.
- M. Zhong, S. Sun, J. Cheng and Y. Shao, J. Org. Chem., 2016, 81, 10825 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. CCDC 1448399. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c7gc03175k |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2018 |