Metal-free electrophilic phosphination of electron-rich arenes, arenols and aromatic thiols with diarylphosphine oxides†
Tao
Yuan
a,
Shenlin
Huang
b,
Chun
Cai
 a and
Guo-ping
Lu
a and
Guo-ping
Lu
 *a
*a
aChemical Engineering College, Nanjing University of Science &Technology, Xiaolingwei 200, Nanjing, 210094, China. E-mail: glu@njust.edu.cn
bCollege of Chemical Engineering, Jiangsu Key Lab of Biomass-Based Green Fuels and Chemicals, Nanjing Forestry University, Nanjing, 210037, P. R. China
First published on 27th November 2017
Abstract
A new protocol for achieving the phosphination of arenes, arenols and thiols has been disclosed. This chemistry, in which diaryl(((trifluoromethyl)sulfonyl)oxy)phosphines as a kind of electrophilic phosphination reagents are in situ generated from diarylphosphine oxides, provides an efficient and mild approach for the synthesis of aromatic organophosphorus compounds.
Aromatic organophosphorus compounds have wide biological activities and materials science applications, meanwhile they have been considered as versatile ligands in catalytic reactions or building blocks in polymer science.1 Therefore, much attention has been paid for the preparation of these compounds.2 There are four main strategies for the construction of these skeletons: (i) phosphorus-centered radical reactions triggered by oxidants or transition-metal catalysts;3 (ii) nucleophilic phosphinations in the presence of strong bases;4 (iii) the transition-metal catalyzed couplings of phosphines;5 and (iv) electrophilic phosphination reactions using halophosphines as the reactants.6
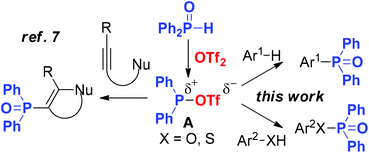
Nevertheless, most of these methods have some shortcomings, such as the use of toxic and costly metal catalysts, unstable reagents, stoichiometric oxidants or harsh conditions. As such, there is a need for new mild methods using stable phosphitylating agents to form these compounds. More recently, Miura's group has developed a metal-free electrophilic phosphination/cyclization of alkynes, in which an electrophilic phosphorus species A can be in situ generated from secondary phosphine oxides and trifluoromethane-sulfonic anhydride (Tf2O).7
These results led us to hypothesize that species A could be used as an electrophile for SEAr, allowing for the facile synthesis of phosphines and related compounds, a long standing goal of our research program.8 Herein we report such a methodology towards the construction of Caryl–P bonds from diarylphosphine oxides under metal-free conditions. Meanwhile, arenols and aryl mercaptans are also applied in the chemistry to form the O–P and S–P bonds at room temperature (Scheme 1).
 | ||
| Scheme 1 Work hypothesis: metal-free electrophilic phosphination of electron-rich arenes, arenols and arylthiols. | ||
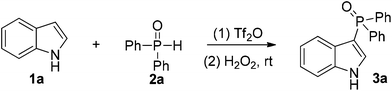
As a representative example, the reaction of indole 1a and diphenyl phosphine oxide 2a was selected as the model reaction to optimize the reaction conditions (Table 1). After screening of different solvents, MeCN provided the best yield of 3a (entries 1–7). The higher reaction temperature was not advantageous to the reaction (entry 8), presumably owing to the decomposition of the electrophilic phosphorus species A.9 It should be noted that the transformation was inhibited in the presence of bases (entries 9–13). Compared with other metal-free approaches,3a,b,6g this method is free of stoichiometric oxidants or toxic and unstable phosphines.
| Entry | Base | Solvent | T (°C) | Yieldb (%) |
|---|---|---|---|---|
| a Reaction conditions: (1) 1a 0.25 mmol, 2a 0.50 mmol, base 0.50 mmol, Tf2O 0.50 mmol, solvent 2 mL, 4 h; (2) H2O2 workup. b Isolated yields, the average value of two experiments. | ||||
| 1 | — | CH2Cl2 | 60 | 80 |
| 2 | — | Toluene | 60 | 30 |
| 3 | — | DMF | 60 | 0 |
| 4 | — | [Hmim]Br | 60 | 20 |
| 5 | — | [Hmim]Cl | 60 | 0 |
| 6 | — | CH3CN | 40 | 79 |
| 7 | — | CH3CN | 60 | 97 |
| 8 | — | CH3CN | 80 | 30 |
| 9 | 2,6-Lutidine | CH3CN | 60 | 90 |
| 10 | DMAP | CH3CN | 60 | 60 |
| 11 | Pyridine | CH3CN | 60 | 82 |
| 12 | DBU | CH3CN | 60 | 86 |
| 13 | K2CO3 | CH3CN | 60 | 45 |
With the optimized conditions in hand, various arenes were chosen to ascertain the scope of the protocol (Scheme 2). Heteroarenes including indole and 2,5-dimethyl-1H-pyrrole were applied in the phosphination successfully (3a, 3d). Only a poor yield of 3h (11%) was obtained using 2-aminonaphthalene as the starting material. The electron-donating groups on the arenes were beneficial to the electrophilic phosphination.
Substitution of a series of electron-rich arenes including 2-methoxynaphthalene, 1,3-dimethoxybenzene, m-xylene and 1-bromo-2,4-dimethoxybenzene was applied in the reaction successfully to afford the desired products (3b, 3c, 3f, 3g). A mixture of 3e and 3e′ was obtained in the reaction of methyl 3,5-dimethoxybenzoate.
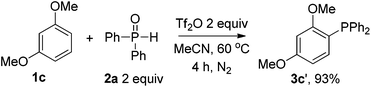
Nevertheless, the scope of disubstituted phosphine oxides was limited in the chemistry. Only 23% and 58% yields were afforded in the cases of 3i and 3j respectively. Diethyl phosphite failed to yield the desired product (3k). The reactions of other disubstituted phosphine oxides, such as phenyl phenylphosphinate and butyl(diphenyl)phosphine oxide and dicyclohexylphosphine oxide also didn't work under identical conditions. For ease of handling, the phosphine was isolated as phosphine oxide after treatment with H2O2 in all cases. The corresponding triarylphosphine (3c′) could also be derived without H2O2 treatment in workup procedures under an N2 atmosphere (Scheme 3). To our delight, the excellent para- or ortho-selectivity of the phosphination was observed in all cases.
To further extend the scope of the protocol, arenols could also react with diphenyl phosphine oxide in similar process at room temperature to yield a series of diphenyl aryl phosphinates 5 (Scheme 4). These results suggested that the nucleophilic phosphination of O in a phenolic hydroxyl group is better than C of arenes. The reactions proceed smoothly when naphthalen-2-ol or quinolin-6-ol were used as the substrates (5a, 5b). The transformations of various arenols containing electron-donating groups also worked in the protocol (5c–5e, 5g–5i). Although an excellent yield of 5f was obtained in the case of 2-bromophenol, no reaction took place using 4-nitrophenols as the starting material. It should be noted that no desired phosphoamide was found when aniline was used as the substrate (5e).
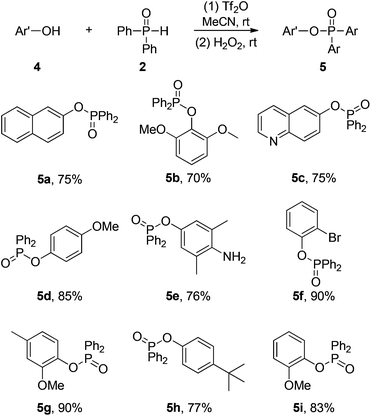 | ||
| Scheme 4 The electrophilic phosphination of arenols with diphenylphosphine oxide. Conditions: 4 0.25 mmol, 2 0.50 mmol, Tf2O 0.50 mmol, MeCN 2 mL, 4 h, rt; (2) H2O2 workup; isolated yields. | ||
Likewise, phosphonothioates which have promising bioactivities and pest-control applications10 could also be generated using aromatic thiols instead of arenols (Scheme 5). Compared with other approaches for the synthesis of these compounds,4b,11 the protocol is free of metal catalysts and stoichiometric oxidants. Although a series of aryl thiols could react with diarylphosphine oxides to produce the corresponding products with good to excellent yields (6a–6f), no reaction took place in the reaction of alkyl thiols with diphenyl phosphine oxides.
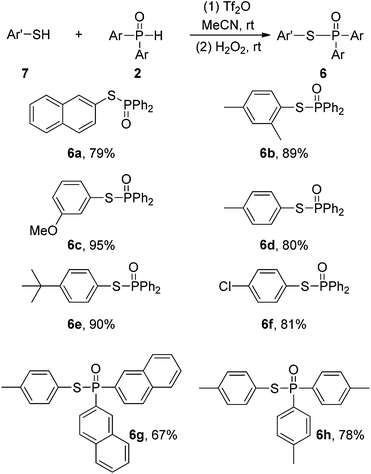 | ||
| Scheme 5 The electrophilic phosphination of aryl thiols with diarylphosphine oxides. Conditions: 7 0.25 mmol, 2 0.50 mmol, Tf2O 0.50 mmol, MeCN 2 mL, 4 h, rt; (2) H2O2 workup; isolated yields. | ||
The reaction of 1b and 2a still occurred in the presence of TEMPO indicating that no radical process was included in the reaction (Fig. S1 in the ESI†). Although the detailed mechanisms of these reactions remained to be elucidated, a tentative pathway for the C–H phosphination of arenes was proposed (Fig. S1 in the ESI†). Firstly, the electrophilic phosphorus species A was in situ generated from secondary phosphine oxides and trifluoromethane sulfonic anhydride (Tf2O).7,9 Then, o-substituted products (or p-substituted product) were formed by the reaction of A and arenes following the release of TfOH.8d,12
In summary, we have developed an efficient phosphination of arenes, arenols and arylthiols with diarylphosphine oxides. The research reveals a new route for the generation of triaryl phosphine oxides, phosphinates and phosphonothioates. The chemistry is operationally simple and allows for the incorporation of diverse arenes into phosphines using readily available reagents under mild and metal-free conditions. Triaryl phosphine can also be obtained without H2O2 treatment under an N2 atmosphere.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
We gratefully acknowledge the Natural Science Foundation of China (21402093) and the Chinese Postdoctoral Science Foundation (2016T90465, 2015M571761) for financial support.Notes and references
- (a) W. Tang and X. Zhang, Chem. Rev., 2003, 103, 3029 CrossRef CAS PubMed; (b) V. Spampinato, N. Tuccitto, S. Quici, V. Calabrese, G. Marletta, A. Torrisi and A. Licciardello, Langmuir, 2010, 26, 8400 CrossRef CAS PubMed; (c) C. Queffélec, M. Petit, P. Janvier, D. A. Knight and B. Bujoli, Chem. Rev., 2012, 112, 3777 CrossRef PubMed; (d) R. Martin and S. L. Buchwald, Acc. Chem. Res., 2008, 41, 1461 CrossRef CAS PubMed; (e) X. Chen, D. J. Kopecky, J. Mihalic, S. Jeffries, X. Min, J. Heath, J. Deignan, S. Lai, Z. Fu, C. Guimaraes, S. Shen, S. Li, S. Johnstone, S. Thibault, H. Xu, M. Cardozo, W. Shen, N. Walker, F. Kayser and Z. Wang, J. Med. Chem., 2012, 55, 3837 CrossRef CAS PubMed; (f) Q. Dang, Y. Liu, D. K. Cashion, S. R. Kasibhatla, T. Jiang, F. Taplin, J. D. Jacintho, H. Li, Z. Sun, Y. Fan, J. DaRe, F. Tian, W. Li, T. Gibson, R. Lemus, P. D. van Poelje, S. C. Potter and M. D. Erion, J. Med. Chem., 2011, 54, 153 CrossRef CAS PubMed.
- (a) J. L. Montchamp, Acc. Chem. Res., 2014, 47, 77 CrossRef CAS PubMed; (b) H. Fernández-Pérez, P. Etayo, A. Panossian and A. Vidal-Ferran, Chem. Rev., 2011, 111, 2119 CrossRef PubMed; (c) K. D. Berlin and G. B. Butler, Chem. Rev., 1960, 60, 243 CrossRef CAS.
- Selected reports on phosphorus-centered radical reactions: (a) X. L. Chen, X. Li, B. Qu, Y. C. Tang, W. P. Mai, D. H. Wei, W. Z. Bi, L. K. Duan, K. Sun, J. Y. Chen, D. D. Ke and Y. F. Zhao, J. Org. Chem., 2014, 79, 8407 CrossRef CAS PubMed; (b) J. Gong, L. Huang, Q. Deng, K. Jie, Y. Wang, S. Guo and H. Cai, Org. Chem. Front., 2017, 4, 1781–1784 RSC; (c) X.-Q. Pan, J.-P. Zou, W.-B. Yi and W. Zhang, Tetrahedron, 2015, 71, 7481 CrossRef CAS; (d) J. Ke, Y. Tang, H. Yi, Y. Li, Y. Cheng, C. Liu and A. Lei, Angew. Chem., Int. Ed., 2015, 54, 6604 CrossRef CAS PubMed; (e) L. Li, W. Huang, L. Chen, J. Dong, X. Ma and Y. Peng, Angew. Chem., Int. Ed., 2017, 56, 10539 CrossRef CAS PubMed; (f) M. Zhou, Y. Zhou and Q. Song, Chem. – Eur. J., 2015, 21, 10654 CrossRef CAS PubMed; (g) F. Su, W. Lin, P. Zhu, D. He, J. Lin, H.-J. Zhang and T.-B. Wen, Adv. Synth. Catal., 2017, 359, 947 CrossRef CAS; (h) W.-B. Sun, J.-F. Xue, G.-Y. Zhang, R.-S. Zeng, L.-T. An, P.-Z. Zhang and J.-P. Zou, Adv. Synth. Catal., 2016, 358, 1753 CrossRef CAS.
- Selected reports on nucleophilic phosphination: (a) S. Li, T. Chen, Y. Saga and L.-B. Han, RSC Adv., 2015, 5, 71544 RSC; (b) S. Song, Y. Zhang, A. Yeerlan, B. Zhu, J. Liu and N. Jiao, Angew. Chem., Int. Ed., 2017, 56, 2487 CrossRef CAS PubMed.
- Selected reports on the couplings of phosphines: (a) L. Wang, Y. Wu, Y. Liu, H. Yang, X. Liu, J. Wang, X. Li and J. Jiang, Org. Lett., 2017, 19, 782 CrossRef CAS PubMed; (b) A. L. Schwan, Chem. Soc. Rev., 2004, 33, 218 RSC; (c) J.-S. Zhang, T. Chen, J. Yang and L.-B. Han, Chem. Commun., 2015, 51, 7540 RSC; (d) Y. Wang, A. Hämäläinen, J. Tois and R. Franzén, Tetrahedron: Asymmetry, 2010, 21, 2376 CrossRef CAS; (e) J. Ang, T. Chen and L.-B. Han, J. Am. Chem. Soc., 2015, 137, 1782 CrossRef PubMed; (f) A.-X. Zhou, L.-L. Mao, G.-W. Wang and S.-D. Yang, Chem. Commun., 2014, 50, 8529 RSC; (g) C. Hou, Y. Ren, R. Lang, X. Hu, C. Xia and F. Li, Chem. Commun., 2012, 48, 5181 RSC; (h) L. Li, J. J. Wang and G. W. Wang, J. Org. Chem., 2016, 81, 5433 CrossRef CAS.
- Selected reports on Friedel–Crafts-type electrophilic phosphination: (a) J. A. Miles, M. T. Beeny and K. W. Ratts, J. Org. Chem., 1975, 40, 343 CrossRef CAS; (b) C. Romero-Nieto, A. Lopez-Andarias, C. Egler-Lucas, F. Gebert, J. P. Neus and O. Pilgram, Angew. Chem., Int. Ed., 2015, 54, 15872 CrossRef CAS PubMed; (c) Y. Unoh, T. Satoh, K. Hirano and M. Miura, ACS Catal., 2015, 5, 6634 CrossRef CAS; (d) Y. Unoh, Y. Yokoyama, T. Satoh, K. Hirano and M. Miura, Org. Lett., 2016, 18, 5436 CrossRef CAS PubMed; (e) A. Jayaraman and B. T. Sterenberg, Organometallics, 2016, 35, 2367 CrossRef CAS; (f) S. Hashimoto, S. Nakatsuka, M. Nakamura and T. Hatakeyama, Angew. Chem., Int. Ed., 2014, 53, 14074 CrossRef CAS PubMed; (g) M. Nielsen, C. B. Jacobsen and K. A. Jørgensen, Angew. Chem., Int. Ed., 2011, 50, 3211 CrossRef CAS PubMed.
- Y. Unoh, K. Hirano and M. Miura, J. Am. Chem. Soc., 2017, 139, 6106 CrossRef CAS PubMed.
- (a) Y.-M. Lin, G.-P. Lu, C. Cai and W.-B. Yi, Org. Lett., 2015, 17, 3310 CrossRef CAS PubMed; (b) Y.-M. Lin, G.-P. Lu, R.-K. Wang and W.-B. Yi, Org. Lett., 2017, 19, 1100 CrossRef CAS PubMed; (c) X. Zhang, G.-P. Lu and C. Cai, Green Chem., 2016, 18, 5580 RSC; (d) Y.-M. Lin, G.-P. Lu, G.-X. Wang and W.-B. Yi, Adv. Synth. Catal., 2016, 358, 4100 CrossRef CAS; (e) J. Gu and C. Cai, Org. Biomol. Chem., 2017, 15, 4226 RSC.
- (a) D. Chen, Q. Feng, Y. Yang, X. M. Cai, F. Wang and S. Huang, Chem. Sci., 2017, 8, 1601 RSC; (b) M. M. Khodaei and E. Nazari, J. Iran. Chem. Soc., 2012, 9, 507 CrossRef CAS.
- (a) G. G. Durgam, T. Virag, M. D. Walker, R. Tsukahara, S. Yasuda, K. Liliom, L. A. van Meeteren, W. H. Moolenaar, N. Wilke, W. Siess, G. Tigyi and D. D. Miller, J. Med. Chem., 2005, 48, 4919 CrossRef CAS PubMed; (b) T. Ruman, K. Długopolska, A. Jurkiewicz, D. Rut, T. Frączyk, J. Cieśla, A. Leś, Z. Szewczuk and W. Rode, Bioorg. Chem., 2010, 38, 74 CrossRef CAS PubMed; (c) N. N. Melnilkov, in Chemistry of Pesitcides, Springer-Verlag, New York, 1971 Search PubMed.
- (a) Y. Zhu, T. Chen, S. Li, S. Shimada and L. B. Han, J. Am. Chem. Soc., 2016, 138, 5825 CrossRef CAS PubMed; (b) M. Arisawa, T. Ono and M. Yamaguchi, Tetrahedron Lett., 2005, 46, 5669 CrossRef CAS; (c) P. Carta, N. Puljic, C. Robert, A.-L. Dhimane, L. Fensterbank, E. Lacôte and M. Malacria, Org. Lett., 2007, 9, 1061 CrossRef CAS PubMed; (d) J. Wang, X. Huang, Z. Ni, S. Wang, J. Wu and Y. Pan, Green Chem., 2015, 17, 314 RSC.
- (a) Z.-B. Xu, G.-P. Lu and C. Cai, Org. Biomol. Chem., 2017, 15, 2804 RSC; (b) R. B. Grossman, in The Art of Writing Reasonable Organic Reaction Mechanisms, Springer-Verlag Press, New York, 2nd edn, 2003 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: More experimental details, copies of NMR spectra of all products. See DOI: 10.1039/c7ob02620j |
| This journal is © The Royal Society of Chemistry 2018 |

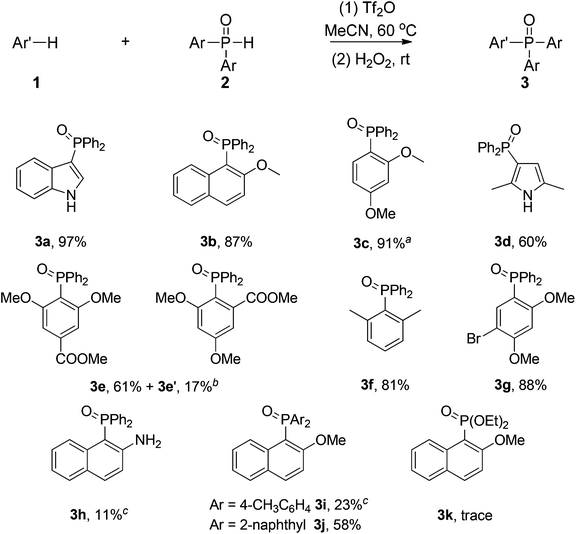
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.75 mmol of Tf2O was used.
0.75 mmol of Tf2O was used.