High-performance hybrid organic thermoelectric SWNTs/PEDOT:PSS thin-films for energy harvesting
Qinglin
Jiang†
,
Xiaoqi
Lan†
,
Congcong
Liu
*,
Hui
Shi
,
Zhengyou
Zhu
,
Feng
Zhao
,
Jingkun
Xu
 * and
Fengxing
Jiang
* and
Fengxing
Jiang
 *
*
Department of Physics, Jiangxi Science and Technology Normal University, Nanchang 330013, China. E-mail: lcc.0705@163.com; xujingkun1971@yeah.net; xujingkun@tsinghua.org.cn; f.x.jiang@live.cn
First published on 12th January 2018
Abstract
Hybrid organic thermoelectric (HOTE) materials have been considered as a promising alternative for future energy harvesting. Herein, a HOTE thin film was proposed and fabricated by a simple method. Highly conductive poly(3,4-ethylenedioxythiophene):poly(styrene sulfonate) (PEDOT:PSS) developed by vacuum filtration has significantly contributed to the optimization of single-walled carbon nanotubes (SWNTs)/PEDOT:PSS (SN–PP) thermoelectric composite films. PEDOT:PSS was coated on the surface of SWNTs, effectively creating electrical connecting junctions between SWNTs. The SN–PP composite films achieved high electrical conductivity and Seebeck coefficient as well as a relatively low thermal conductivity simultaneously. A notable ZT value of 0.12 was obtained at 60 wt% SWNTs, which is generally higher than those reported in previous studies. Our approach may provide quite a useful strategy to prepare HOTE materials with high performance.
Introduction
Hybrid organic thermoelectric (HOTE) materials have gained increasing attention for the development of new green energy transfer due to their light weight, rich resources, structural variations, and intrinsically low thermal conductivity.1–4 Owing to these features, compared to efficient inorganic thermoelectric (TE) materials, HOTE materials have been considered as a promising alternative for future energy harvesting. The performance evaluation of TE materials is generally based on the dimensionless figure of merit, ZT = σS2T/κ, where σ, S, T, and κ are the electrical conductivity, Seebeck coefficient or thermopower, absolute temperature, and thermal conductivity, respectively. In order to achieve a high TE performance, a large power factor (σS2) is necessary in connection with a low κ. As we know, for the σS2, an opposite dependence between σ and S limits its development. To solve this issue, lots of works focused on the fabrication of nanocomposites with a high σ or S value in an individual material.5–8 Although an optimized σS2 value was achieved, both σ and S of the composite materials could not simultaneously reach the highest value of the component, especially for organic/inorganic nanocomposites. A proper method for fabricating composite materials with high TE performance is significant. Fabricating organic/inorganic composites is a promising choice due to the strong π–π interaction between carbon materials such as graphene (GE),9–12 carbon nanotubes (CNTs),13,14 and conducting polymers (CPs).15–17Single-walled CNTs (SWNTs) are renowned for their extremely stable 1D nanostructure, and excellent electric and mechanical properties.18 As a TE material, the carbon nanotube has been regarded as a potential candidate due to its low resistivity and large thermopower.13,19 Importantly, the quantum confinement effect of charge carriers in individual SWNTs, together with the size effect of heat carriers, makes it a promising TE material.20 Nevertheless, the ZT of bulk SWNTs is as low as 10−3 owing to their large thermal conductivity (∼35 W m−1 K−1).21,22 The manufacturing and processing technology of SWNTs is a big challenge due to its extraordinarily high stiffness.23 A common processing methodology used is functionalization with hydrophilic groups such as hydroxyl and carboxyl or composite formation with nonconductive polymers such as polyvinyl alcohol (PVA), poly(methyl methacrylate) (PMMA), poly(vinylidene fluoride) (PVDF) and so on.24 As TE materials, they easily damage the structure, resulting in the property depreciation of electron transfer. Therefore, it is very important to develop a feasible method for the fabrication of composite materials to optimize the TE performance, given the intrinsic properties of SWNTs.
CPs are good candidates as organic electronic materials when combined with CNTs. Compared to nonconductive polymers, CPs can serve as an effective interface and link individual CNTs by π–π interactions to realize good carrier transport. Moreover, Yao et al.21 thought that CPs adsorbed on the hexagonal lattice of CNTs easily resulted in a good order of chain packing, which could reduce conjugated defects and lower the carrier hopping barrier. Secondly, CPs have the direct benefit of reducing the thermal conductivity of CNTs due to their intrinsically low thermal conductivity values (∼0.17 W m−1 K−1).17 Additionally, introduction of CPs can improve the manufacturability of bulk CNTs. Recently, intensive efforts have been devoted to improve the TE performance of CNTs by combining CPs with an enhanced ZT value.
Among CPs, poly(3,4-ethylenedioxythiophene):poly(styrene sulfonate) (PEDOT:PSS) has been proved to be one of the most promising organic TE materials, owing to its high ratio of σ/κ.16,25,26 Presently, most research studies focus on the improvement of σ with an additive such as organic solvents,17,27 ionic liquids,28,29 and salts,30,31 as well as solvent treatment by dipping the PEDOT:PSS film in dimethyl sulfoxide (DMSO),16,32 ethylene glycol (EG),33,34 or acids.35,36 The enhanced σ of PEDOT:PSS was mainly derived from the conformational change of PEDOT chains or the removal of nonconductive PSS.16,31 Interestingly, with the increase of σ, S slightly increased rather than decreased for treated PEDOT:PSS films. Significant improvement of σ has been achieved; however, a steady S in the PEDOT:PSS film limits the further increase of σS2, resulting in a low ZT value eventually. If trying to improve S by tuning the doping level of PEDOT:PSS, it would cause significant deterioration in σ due to the opposite dependence between them.32,37 Recently, many attempts have been made to enhance the ZT value of PEDOT:PSS. One of the most fascinating materials is nanocomposites combining the advantages of two or more components.1,38 Actually, SWNTs/PEDOT:PSS (SN–PP) composites have successfully improved the TE performance of an individual material based on direct mixing,39in situ polymerization,40 and layer-by-layer assembly.41 In spite of an enhanced ZT value (∼0.02) at room temperature, it is far from meeting the requirement of application in TE energy conversion. It is strongly expected that there is much room for the improvement of σ of SN–PP composites due to the high σ of PEDOT:PSS films (∼1500 S cm−1).42 Here in this work, we have developed an effective processing method for the construction of SN–PP composite films and achieved a breakthrough ZT of 0.12, which is among the highest values of organic-based TE materials.
Experimental
Chemicals
PEDOT:PSS aqueous solution (PH1000; Baytron), SWCNTs dispersion (0.1 wt%, Nanjing XFNANO Materials Tech Co. Ltd), dimethyl sulfoxide (DMSO; analytical grade; Beijing Chemical Works), and the PVDF membrane (pore size: 0.22 μm; J&K Scientific Co. Ltd) were used directly as received.Preparation
A 200 μL of pristine PEDOT:PSS aqueous solution was added into 10 mL of DMSO with ultrasonic dispersion for 1 h. Then, a series of SWCNT dispersions with a designed ratio were added into the above solution, and the resulting mixture was sonicated for 1 h. Finally, SN–PP composite films were prepared by vacuum filtration of the mixture dispersions onto a porous PVDF membrane. The as-prepared films were then dried in a vacuum oven at 60 °C for 24 h.Measurements
The electrical conductivity and Seebeck coefficient were measured using a four-point probe apparatus with a Keithley 2700 multimeter (Cleveland, OH) and a regulated DC power supply (MCH-303D-II, China) in conjunction with Labview (National Instruments, Austin, TX). The electrical conductivity was measured by using a current–voltage (I–V) sweeping measurement technique with a silver paint. For the Seebeck coefficient measurement, temperature gradients along the long edge of the sample were measured by using two T-type thermocouples. The temperature and electrical potential differences were given by ΔT = Thot − Tcold and ΔV = Vcold − Vhot. The Seebeck coefficient was calculated by S = −(ΔV/ΔT). The temperature difference ΔT of about 5 K was used in measurements. The in-plane thermal conductivity of the SN–PP composite film was measured based on the 3ω-method by using the LINSEIS TFA equipment.Regarding other characteristics, the surface morphology was probed by scanning electron microscopy (SEM, ZEISS-SIGMA) and transmission electron microscopy (TEM, JEM-2100F). The surface composition changes of the samples were analyzed by X-ray photoelectron spectroscopy (XPS, Thermo ESCALAB 250Xi, Thermo Scientific, Waltham, MA, USA).
Results and discussion
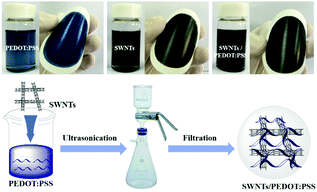
Herein, we designed and fabricated a hybrid organic SN–PP thin-film by directly mixing pristine PEDOT:PSS and SWNTs via a vacuum filtration method. This will achieved efficient mixing and high-quality film formation of SN–PP as well as high σ by depleting partially nonconductive PSS. Inspired by our previous work,42 as shown in Scheme 1, 200 μL of pristine PEDOT:PSS aqueous solution was added into 10 mL of DMSO with SWNTs and the resulting mixture was sonicated for 1 h. SN–PP thin-films were obtained by direct vacuum filtration with a porous PVDF membrane. This process contained three key aspects including SWNTs solubility, PEDOT:PSS treatment, and thin-film formation. As we know, the poor solubility in any solvent limits the use of SWNTs.24 However, SWNTs can be dispersed in some polar solvents to some extent by sonication with transient stability.43 DMSO is an ideal organic solvent for the dispersion of SWNTs.44 Secondly, DMSO treatment can lead to a wide variation in the charge transport properties of PEDOT:PSS by changing the degree of phase segregation of conductive PEDOT and nonconductive PSS or depleting a part of PSS.42 Moreover, PSS favours of stability of SWNTs in liquid.45 Thirdly, the hybrid SN–PP can easily form a high-quality flexible thin-film for electronic application. Additionally, PEDOT:PSS can effectively attach on SWNTs and bridged tube–tube junctions resulting in a better electron transfer.39,40 The integration of these features gives rise to a desired SN–PP thin-film (Scheme 1).As we know, pure SWNTs films with random distribution lead to less-branched networks owing to their poor dispersion in water. Although most research studies to solve this issue are focused on using hydrophilic functional groups (–OH, –COOH, –NH2, –CN, etc.) or different kinds of surfactants, these approaches result in poor electron transport properties of the SWNTs film due to the structural damage or non-conductive additives, since the electron transport properties depend on the individual SWNTs and their effective connectivity with each other.46 The pure PEDOT:PSS film presents a uniform and smooth surface with good mechanical flexibility and foldability (Scheme 1). More importantly, it can be transferred from the PVDF filter to other substrates for further processing. One can see that the surface becomes rough with the increase of SWNTs content in PEDOT:PSS. However, the as-obtained SN–PP thin-film (60 wt% SWNTs) shows excellent flexibility and light weight, and it can be optionally rolled up, bent, twisted, and even folded without cracking (Scheme 1). Note that, with the decrease of PEDOT:PSS, the space among separated SWNTs becomes much clearer. SN–PP composite films exhibit poor flexibility and transferability, especially those with more than 60 wt% SWNTs. For further measuring the flexibility of the thin-films, durability of their electrical conductivity was measured (Fig. 1). As the bending times increased, the electrical conductivity of thin films decreased. After 1000 bending times, the electrical conductivity of the PEDOT:PSS film decreased only 6%, while that of the SN–PP film with 60 wt% SWNTs decreased almost 12%. Note that the PEDOT:PSS film exhibited better flexibility than the SN–PP thin film. It is suggested that PEDOT:PSS plays an important role in connecting SWNTs resulting in a flexible composite thin-film.
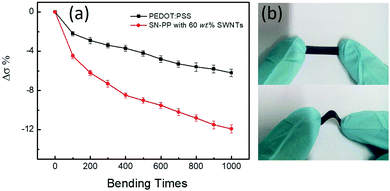 | ||
| Fig. 1 (a) Reliability of the flexible PEDOT:PSS and SN–PP thin film with 60 wt% SWNTs, (b) demonstration of the bending reliability test. | ||
Fig. 2 presents the morphologies of SN–PP composite films with different SWNTs contents. Initially, the surface of pure PEDOT:PSS was uniform and smooth (Fig. 2a). However, it changed obviously after the addition of 10 wt% SWNTs, which was comprised of individual SWCNTs and their bundles randomly intertwined together to form a good SWNTs network (Fig. 2b). Upon increasing the content to 60 wt%, the SWCNTs network coated with a PEDOT:PSS layer became more clear. The crystalline SWNTs bundles acted as the backbone of the composite, and a thin amorphous PEDOT:PSS layer was coated on the surface of the SWNTs (Fig. 2e). This observation further proves that the PEDOT:PSS chains wrapped around the outer walls of the SWNTs, which can be attributed to the π–π interactions between PEDOT:PSS and SWNTs.39,47
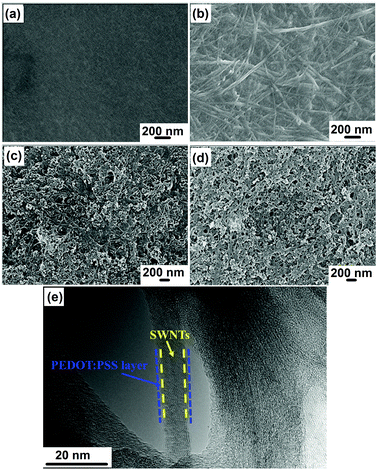 | ||
| Fig. 2 SEM images of HOTE SN–PP thin-films with different SWNTs contents: (a) 0 wt%, (b) 10 wt%, (c) 60 wt%, and (d) 90 wt%. (e) TEM image of SN–PP thin films with 60 wt% SWNTs. | ||
XPS analysis was conducted to study chemical composition changes in the as-prepared films (Fig. 3). The S2p peak between 166.5 and 171.0 eV corresponds to the sulfur atoms in PSS, while the doublet peaks between 163.0 and 166.5 eV are due to the sulfur atoms from the thiophene group in PEDOT (Fig. 3a).3,42 It is obvious that the S2p XPS intensity ratio of PEDOT to PSS increased for the PEDOT:PSS (red line in Fig. 3a) and SN–PP composite (blue line in Fig. 3a) films prepared by vacuum filtration compared to that of the drop-cast PEDOT:PSS film (prepared by dropping the pristine PEDOT:PSS aqueous solution on a glass substrate). This indicates that the contents of PSS in solid films have changed significantly. In other words, some PSS has been removed from the formed solid film, which is in agreement with previous reports.4,16,48 By contrast, there is no significant change in the S2p peak intensity ratio of PEDOT to PSS between the PEDOT:PSS film and the PEDOT:PSS/SWNTs composite film. It is worth mentioning that the content of non-conductive PSS has a negative effect on the electrical conductivity of the PEDOT:PSS film. Most reports have achieved a significantly enhanced electrical conductivity (>103 S cm−1) for PEDOT:PSS by removing part of PSS. Additionally, the removal of PSS also led to a conformational change of PEDOT chains in the PEDOT-rich solid film of PEDOT:PSS from a coil to linear conformation.17,49,50 It is known that the removal of PSS can effectively increase the electron transport of the PEDOT:PSS film by improving the interchain and interdomain charge hopping between PEDOT grains.51 Furthermore, the electron binding energy of C1s in a C–C bond is typically at 284–285 eV (Fig. 3b). It should be noted that a shift toward a higher binding energy has been observed in the SN–PP composite film, which can be attributed to the sign of the partial charge exchange between PEDOT:PSS and SWNTs due to the π–π interactions.52
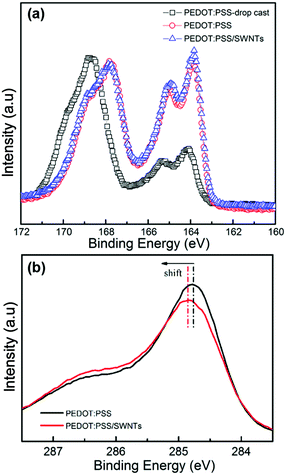 | ||
| Fig. 3 XPS analysis of drop-cast PEDOT:PSS, PEDOT:PSS, and SN–PP thin-films with 60 wt% SWNTs: (a) S2p spectra and (b) C1s spectra. | ||
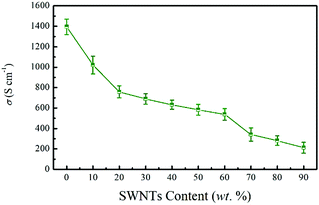
Fig. 4 presents the electrical conductivity of the SN–PP composite films as a function of SWNTs content. As can be seen, the PEDOT:PSS film showed quite a high electrical conductivity of 1395 S cm−1. This notable value could be attributed to the significant aggregation of PEDOT-rich nanoparticles caused by the partial dissolution of PSS in the organic solvents.42 After adding 10 wt% SWNTs, the electrical conductivity was reduced to 1021 S cm−1 and it continuously decreased with the increase of SWNTs contents in PEDOT:PSS, which is attributed to the barrier of the surfactants on the surface of SWNTs and less conductive PEDOT grains to connect the SWNTs. It is worth noting that this high electrical conductivity was much higher than that of traditional nanotube-filled polymer composites (ranging from 10−3 to 10 S cm−1).53,54 The electrical conductivity of pristine SWNTs is 125 S cm−1. In this work, the highly conductive PEDOT:PSS is believed to create less electrically resistive junctions between SWNTs. Therefore, increasing the amount of PEDOT:PSS within the composite will stabilize more SWNTs and create a large number of electrically bridged junctions. This behavior enables a high electrical conductivity in the composites.
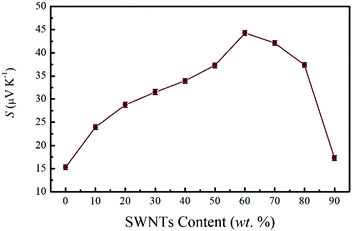
On the other hand, the Seebeck coefficient of these composite films is positive as seen in Fig. 5, indicating the dominant contribution of hole carriers. It is obvious that the PEDOT:PSS film displays a Seebeck coefficient of 15.3 μV K−1, which is similar to that of reported pristine PEDOT:PSS (14.1 μV K−1),17 suggesting the little effect of the dilution–filtration process on the Seebeck coefficient of PEDOT:PSS. The Seebeck coefficient of pristine SWNTs is 52 μV K−1. With the increase of SWNTs content, as the electrical conductivity decreased, the Seebeck coefficient appeared to be significantly enhanced. The highest Seebeck coefficient of 44.3 μV K−1 has been achieved for the SN–PP film with 60 wt% SWNTs which is more than three times higher than that of pristine PEDOT:PSS (14.1 μV K−1).17 When carriers transport within the SWNTs network under a temperature gradient, a hopping mechanism for a heterogeneous model may be appropriate to explain the enhanced Seebeck coefficient.6,50 On going from one SWNTs/PEDOT:PSS nanowire to another, the carrier would pass through a SWNTs/PEDOT:PSS interface and a PEDOT:PSS layer. Hence, lots of nanometer-sized barriers in the form of interfaces would exist on the way. When a large number of carriers hop together mainly in one direction, a process called energy filtering may arise.55–57 In other words, appropriate potential barriers at crystallite boundaries preferentially allow the carriers with higher energy to pass and increase the mean carrier energy in the flow. Eventually, the significantly enhanced Seebeck coefficient was achieved in the SN–PP composite films. By the way, the value displays a slight decrease at high SWNTs content (from 70 to 90 wt%) due to the self-aggregation of partial SWNTs as observed in the SEM image (Fig. 2d).
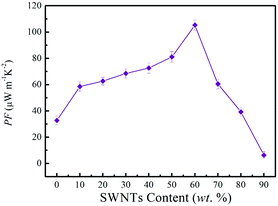
The power factor of SN–PP thin-films with different SWNTs contents was evaluated based on the electrical conductivity and Seebeck coefficient, as shown in Fig. 6. With the increase of SWNTs content, the power factor of thin-films showed an increasing trend and an optimized power factor of 105 μW m−1 K−2 was obtained at 60 wt% SWNTs.
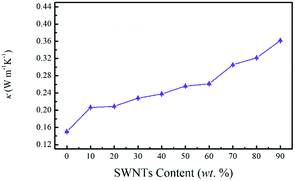
Despite their high Seebeck coefficient, carbon nanotubes have been considered to be irrelevant for TE applications due to their high intrinsic thermal conductivity (1000 W m−1 K−1).40,41 As for SWNTs based composites, a significant increase in the overall thermal conductivity of the composite film was expected as the SWNTs content increased. In this work, the in-plane thermal conductivity of the SN–PP composite film was measured based on the 3ω-method by using the LINSEIS TFA equipment (Fig. 7). Surprisingly, our SN–PP composite film retained a low polymer-like thermal conductivity, which was insensitive to the SWNTs content, slightly increasing from 0.15 to 0.36 W m−1 K−1 as the content was increased from 0 to 90 wt%. Such low thermal conductivity was four orders of magnitude smaller than that of SWNTs, offering great potential in the enhancement of the ZT value. This could be attributed to the fact that many of the tube–tube junctions are connected by PEDOT:PSS, resulting in less favorable paths for thermal energy transport. PEDOT:PSS has different vibrational spectra from that of SWNTs, impeding phonon transport across SWNTs–(PEDOT:PSS)–SWNTs interfaces.39 Therefore, the higher loading of PEDOT:PSS interferes with the phonon transport. Furthermore, particle-like PEDOT:PSS in the composite may act as a scattering centre for phonons,40 leading to a relatively low thermal conductivity.
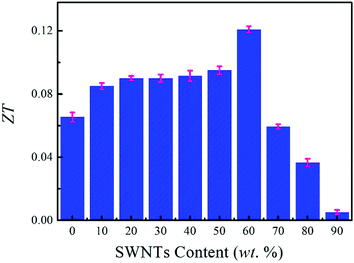
We evaluated the ZT value based on the electrical conductivity, Seebeck coefficient, and thermal conductivity, as shown in Fig. 8. With the increase of SWNTs content, the ZT value of composite films shows an increasing trend and an optimized ZT value of 0.12 is obtained at 60 wt% SWNTs, which is generally higher than those reported in previous studies (Table 1).21,39–41,58 We expect that some TE composites with high performance can be achieved using a method similar to that suggested here. For instance, by replacing SWNTs with other materials such as graphene, bismuth telluride (Bi2Te3), and molybdenum disulfide (MoS2) that possess a high Seebeck coefficient, the TE performance may be further improved because of the square dependence of the ZT value on the Seebeck coefficient.
| Materials | Methods | PF (μW m−1 K−2) | ZT | Ref. |
|---|---|---|---|---|
| Polypyrrole/MWNTs | In situ polymerization | 2.2 | — | 58 |
| Polyaniline/SWNTs | In situ polymerization | 20 | 0.02 | 21 |
| PEDOT:PSS/MWNTs | Template-directed | 0.23 | — | 40 |
| PEDOT:PSS/SWNTs | Two-step spin casting | 21.1 | — | 41 |
| PEDOT:PSS/SWNTs | Direct mixing | 24 | 0.02 | 39 |
| PEDOT:PSS/SWNTs | Dilution filtration | 83.9 | — | 57 |
| PEDOT:PSS/SWNTs | Vacuum filtration | 105 | 0.12 | This work |
Conclusions
A highly conductive PEDOT:PSS layer prepared by a vacuum filtration method was coated on the surface of SWNTs, effectively creating electrical connecting junctions between SWNTs. The as-prepared SN–PP composite films show high electrical conductivity and Seebeck coefficient, and a relatively low thermal conductivity, giving rise to a maximum ZT value of 0.12 at 60 wt% SWNTs. These results indicated that highly conductive PEDOT:PSS prepared by vacuum filtration may be generally favourable for the fabrication of high performance HOTE composite materials.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (51402134, 51463008, 51572117, and 21563013), the Ganpo Outstanding Talents 555 projects, the Jiangxi Provincial Department of Science and Technology (20161BAB216129 and 20151BAB217024), and the Jiangxi Provincial Department of Education (GJJ150809).Notes and references
- N. Toshima, K. Oshima, H. Anno, T. Nishinaka, S. Ichikawa, A. Iwata and Y. Shiraishi, Adv. Mater., 2015, 27, 2246 CrossRef CAS PubMed.
- L. Liang, G. Chen and C. Guo, Mater. Chem. Front., 2017, 1, 380 RSC.
- F. Jiang, J. Xiong, W. Zhou, C. Liu, L. Wang, F. Zhao, H. Liu and J. Xu, J. Mater. Chem. A, 2016, 4, 5265 CAS.
- J. Xiong, F. Jiang, H. Shi, J. Xu, C. Liu, W. Zhou, Q. Jiang, Z. Zhu and Y. Hu, ACS Appl. Mater. Interfaces, 2015, 5, 14917 Search PubMed.
- J. Wang, K. Cai, J. Yin and S. Shen, Synth. Met., 2017, 224, 27 CrossRef CAS.
- D. Suh, S. Lee, H. Mun, S. Park, K. Lee, S. Kim, J. Choi and S. Baik, Nano Energy, 2015, 13, 67 CrossRef CAS.
- Y. Zheng, Y. Luo, C. Du, B. Zhu, Q. Liang, H. Hng, K. Hippalgaonkar, J. Xu and Q. Yan, Mater. Chem. Front., 2017, 1, 2457 RSC.
- G. Chen, W. Xu and D. Zhu, J. Mater. Chem. C, 2017, 5, 4350 RSC.
- S. Cho, S. Kang, W. Kim, E. Lee, S. Woo, K. Kong, I. Kim, H. Kim, T. Zhang, J. Stroscio, Y. Kim and H. Lyeo, Nat. Mater., 2013, 12, 913 CrossRef CAS PubMed.
- P. Dollfus, V. Nguyen and J. Martin, J. Phys.: Condens. Matter, 2015, 27, 133204 CrossRef PubMed.
- A. Dey, A. Maity, M. Khan, A. Sikder and S. Chattopadhyay, RSC Adv., 2016, 6, 22453 RSC.
- L. Wang, Q. Yao, W. Shi, S. Qu and L. Chen, Mater. Chem. Front., 2017, 1, 741 RSC.
- Y. Nakai, K. Honda, K. Yanagi, H. Kataura, T. Kato, T. Yamamoto and Y. Maniwa, Appl. Phys. Express, 2014, 7, 025103 CrossRef.
- W. Zhao, S. Fan, N. Xiao, D. Liu, Y. Tay, C. Yu, D. Sim, H. Hng, Q. Zhang, F. Boey, J. Ma, X. Zhao, H. Zhang and Q. Yan, Energy Environ. Sci., 2012, 5, 5364 CAS.
- O. Bubnova, Z. U. Khan, H. Wang, S. Braun, D. Evans, M. Fabretto, P. Hojati-Talemi, D. Dagnelund, J. Arlin, Y. Geerts, S. Desbief, D. Breiby, J. Andreasen, R. Lazzaroni, W. Chen, I. Zozoulenko, M. Fahlman, P. Murphy, M. Berggren and X. Crispin, Nat. Mater., 2014, 13, 190 CrossRef CAS PubMed.
- G. Kim, L. Shao, K. Zhang and K. Pipe, Nat. Mater., 2013, 12, 719 CrossRef CAS PubMed.
- F. Jiang, J. Xu, B. Lu, Y. Xie, R. Huang and L. Li, Chin. Phys. Lett., 2008, 25, 2202 CrossRef CAS.
- L. Yang, S. Wang, Q. Zeng, Z. Zhang and L. Peng, Small, 2013, 9, 1225 CrossRef CAS PubMed.
- G. Wu, Z. Zhang, Y. Li, C. Gao, X. Wang and G. Chen, ACS Nano, 2017, 11, 5746 CrossRef CAS PubMed.
- J. Small, L. Shi and P. Kim, Solid State Commun., 2003, 127, 181 CrossRef CAS.
- Q. Yao, L. Chen, W. Zhang, S. Liufu and X. Chen, ACS Nano, 2010, 4, 2445 CrossRef CAS PubMed.
- L. Liang, G. Chen and C. Guo, Compos. Sci. Technol., 2016, 129, 130 CrossRef CAS.
- M. Bryning, D. Milkie, M. Islam, L. Hough, J. Kikkawa and A. Yodh, Adv. Mater., 2007, 19, 661 CrossRef CAS.
- D. Tasis, N. Tagmatarchis, A. Bianco and M. Prato, Chem. Rev., 2006, 106, 1105 CrossRef CAS PubMed.
- H. Shi, C. Liu, Q. Jiang and J. Xu, Adv. Electron. Mater., 2015, 1, 1500017 CrossRef.
- Q. Jiang, C. Liu, J. Xu, B. Lu, H. Song, H. Shi, Y. Yao and L. Zhang, J. Polym. Sci., Part B: Polym. Phys., 2014, 52, 737 CrossRef CAS.
- C. Liu, F. Jiang, M. Huang, R. Yue, B. Lu, J. Xu and G. Liu, J. Electron. Mater., 2011, 40, 648 CrossRef CAS.
- C. Liu, J. Xu, B. Lu, R. Yue and F. Kong, J. Electron. Mater., 2012, 41, 639 CrossRef CAS.
- C. Badre, L. Marquant, A. Alsayed and L. Hough, Adv. Funct. Mater., 2012, 22, 2723 CrossRef CAS.
- Z. Zhu, H. Song, J. Xu, C. Liu, Q. Jiang and H. Shi, J. Mater. Sci.: Mater. Electron., 2015, 26, 429 CrossRef CAS.
- Y. Xia and J. Ouyang, Macromolecules, 2009, 42, 4141 CrossRef CAS.
- S. Lee, H. Park, S. Kim, W. Son, I. Cheong and J. Kim, J. Mater. Chem. A, 2014, 2, 7288 CAS.
- T. Takano, H. Masunaga, A. Fujiwara, H. Okuzaki and T. Sasaki, Macromolecules, 2012, 45, 3859 CrossRef CAS.
- X. Crispin, F. Jakobsson, A. Crispin, P. Grim, P. Andersson, A. Volodin, C. Haesendonck, M. Auweraer, W. Salaneck and M. Berggren, Chem. Mater., 2006, 18, 4354 CrossRef CAS.
- Y. Xia, K. Sun and J. Ouyang, Adv. Mater., 2012, 24, 2436 CrossRef CAS PubMed.
- Z. Li, G. Ma, F. Jiang, Y. Zhou, K. Li, X. Min, K. Huo and Y. Zhou, Angew. Chem., Int. Ed., 2016, 55, 979 CrossRef CAS PubMed.
- M. Culebras, C. Gómez and A. Cantarero, J. Mater. Chem. A, 2014, 2, 10109 CAS.
- C. Cho, B. Stevens, J. Hsu, R. Bureau, D. Hagen, O. Regev, C. Yu and J. Grunlan, Adv. Mater., 2015, 27, 2996 CrossRef CAS PubMed.
- D. Kim, Y. Kim, K. Choi, J. Grunlan and C. Yu, ACS Nano, 2010, 4, 513 CrossRef CAS PubMed.
- Z. Zhang, G. Chen, H. Wang and X. Li, Chem. – Asian J., 2015, 10, 149 CrossRef CAS PubMed.
- H. Song, C. Liu, J. Xu, Q. Jiang and H. Shi, RSC Adv., 2013, 3, 22065 RSC.
- J. Xiong, F. Jiang, W. Zhou, C. Liu and J. Xu, RSC Adv., 2015, 5, 60708 RSC.
- T. Fujigaya and N. Nakashima, Sci. Technol. Adv. Mater., 2015, 16, 024802 CrossRef PubMed.
- L. Hu, D. Hecht and G. Grüner, Chem. Rev., 2010, 110, 5790 CrossRef CAS PubMed.
- Z. Zhang and X. Xu, Chem. Eng. J., 2014, 256, 85 CrossRef CAS.
- M. Itkis, A. Pekker, X. Tian, E. Bekyarova and R. Haddon, Acc. Chem. Res., 2015, 48, 2270 CrossRef CAS PubMed.
- L. Wang, Q. Yao, W. Shi, S. Qu and L. Chen, Mater. Chem. Front., 2017, 1, 741 RSC.
- X. Wang, F. Meng, H. Tang, Z. Gao, S. Li, F. Jiang and J. Xu, J. Mater. Sci., 2017, 52, 9806 CrossRef CAS.
- J. Ouyang, Q. Xu, C. Chu, Y. Yang, G. Li and J. Shinar, Polymer, 2004, 45, 8443 CrossRef CAS.
- Z. Zhu, C. Liu, F. Jiang, J. Xu and E. Liu, Synth. Met., 2017, 225, 31 CrossRef CAS.
- C. Sangeeth, M. Jaiswal and R. Menon, J. Phys.: Condens. Matter, 2009, 21, 072101 CrossRef PubMed.
- D. Yoo, J. Kim, S. Lee, W. Cho, H. Choi, F. Kim and J. Kim, J. Mater. Chem. A, 2015, 3, 6526 CAS.
- D. Zhu, Y. Bin and M. Matsuo, J. Polym. Sci., Part B: Polym. Phys., 2007, 45, 1037 CrossRef CAS.
- C. Hewitt, A. Kaiser, S. Roth, M. Craps, R. Czerw and D. Carroll, Nano Lett., 2012, 12, 1307 CrossRef CAS PubMed.
- G. Snyder and E. Toberer, Nat. Mater., 2008, 7, 105 CrossRef CAS PubMed.
- C. Meng, C. Liu and S. Fan, Adv. Mater., 2010, 22, 535 CrossRef CAS PubMed.
- H. Song, Y. Qiu, Y. Wang, K. Cai, D. Li, Y. Deng and J. He, Compos. Sci. Technol., 2017, 153, 71 CrossRef CAS.
- H. Song, K. Cai, J. Wang and S. Shen, Synth. Met., 2016, 211, 58 CrossRef CAS.
Footnote |
| † These authors contributed equally to this work. |
| This journal is © the Partner Organisations 2018 |