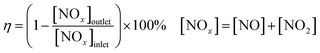Open Access Article
Open Access ArticleNovel CeMoxOy-clay hybrid catalysts with layered structure for selective catalytic reduction of NOx by NH3†
Boyang Xuabc,
Youlin Liu *abc,
Yuesong Shen*abc and
Shemin Zhuabc
*abc,
Yuesong Shen*abc and
Shemin Zhuabc
aCollege of Materials Science and Engineering, Nanjing Tech University, Nanjing 210009, China. E-mail: youlinliu@njtech.edu.cn; sys-njut@163.com
bJiangsu Collaborative Innovation Center for Advanced Inorganic Function Composites, Nanjing Tech University, Nanjing 210009, China
cJiangsu National Synergetic Innovation Center for Advanced Materials, Nanjing Tech University, Nanjing 210009, China
First published on 11th January 2018
Abstract
A facile method is to prepare novel CeMoxOy-clay hybrid catalysts with layered structures by using organic cation modified clay as support. During the preparation process, cerium cations and molybdate anions are easily adsorbed and impregnated into the interlamellar space of the organoclay, and after calcination they undergo transformation to highly dispersed CeMoxOy nanoparticles within the interlamellar space of the clay. As expected, the prepared CeMo0.15Ox-OC-T catalysts with layered structures had high selective catalytic reduction (SCR) activity such as high NOx conversion of >90% in the wide temperature range of 220–420 °C. Meanwhile, they also exhibit high stability and tolerance to water vapor (5 vol%) and SO2 (200 ppm), demonstrating that these novel catalysts could serve as a good alternative for NH3-SCR in practical application.
Introduction
Nowadays, great challenges for the effective removal of nitrogen oxides from outgases remains. The selective catalytic reduction of NOx with NH3 (NH3-SCR) has been proven to be an effective approach for NO reduction from stationary sources. However, the commercially available V2O5–WO3 (MO3)/TiO2 catalysts has several inevitable drawbacks such as easy sublimation and loss of V2O5 in the application process as well as biological toxicity, and narrow operation temperature window.1 Therefore, it is desirable to develop a highly efficient, stable and environmental-friendly catalyst.Recently, cerium-based catalysts had becoming promising candidate due to its nontoxic, high surface area and remarkable oxygen storage capacity in SCR reaction as support,2,3 promoter,4 or main active component.5–7 However, pure CeO2 possesses poor SCR activity,8 it is necessary to adjust their surface acidity,9,10 redox property11,12 and structure stability13 of CeO2-based catalysts to improve the SCR performance.
Layered clays such as montmorillonite (MMT), are one type of clay minerals in where negatively changed aluminosilicate layers having an excess of negative charge that interacted strongly by electrostatic forces with charge balancing cations, typically alkali metal ions, located in interlamellar space. In spite that layered clay are constituted by layers with large surface area, the interlamellar space is generally not accessible to any substrate due to the strong electrostatic interaction.14 It is found that pillaring interlayered clays (PILC) in which inorganic metal polycations were introduced and intercalated in the interlamellar space of the swelling clay and attracted much attention in application of NH3-SCR.15 These different pillared clays catalysts such as vanadium supported WO3–TiO2-pillared clays,16 Cu/Co modified Al2O3/TiO2-pillared MMT,17,18 Fe modified TiO2-pillared MMT,19,20 Mn–Ce supported TiO2-pillared clays,21 Mn–Ce supported Al2O3–CrO2-pillared clays,22 exhibited more improved SCR activities because these intercalation (pillaring) could provide a possibility of tailoring porous structure, redox and acidic properties of clay. However, these catalysts either toxic, or had still low catalytic activity, or complicated preparation process, limiting their practical application for NH3-SCR.
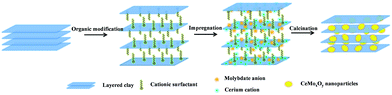
Here, we first presented a facile and cost-effective approach to prepare the novel CeMoxOy-clay hybrid catalyst with layered structure, designed by using organic cations modified clay (denoted as OC) as support and CeMo mixed oxides as active component. As shown in Scheme 1, organic cations (such as the quaternary ammonium surfactants) modified the clay such as Na-based MMT (denoted as NaC) with layered structure by ion exchange, where organic cations could increase the basal space of clay. Then molybdate anion could be adsorbed into the layered structure by the organic cations, meanwhile, cerium ion also could uniformly insert the interlamellar space of clay. After calcination, the intercalated ions were converted into the corresponding metal oxides, well dispersed CeMoxOy nanoparticles with a particle size of 10–20 nm are hosted in the clay matrix (denoted as CeMoxOy-OC-T, T: calcination temperature). The layered structure of novel catalyst has more responsibilities to high SCR activity. In addition, the organic cations also could decrease alkali metal content (Na, K) of clay by ion exchange, further improving SCR activity.
Experimental
Chemicals and materials
All reagents used were of at least analytical grade without further purification. Cerium nitrate, ammonium molybdate, oxalic acid, octadecyltrimethyl ammonium bromide, cetyltrimethyl ammonium bromide, polyoxyethylene were obtained from Aladdin Reagent Co. Na-based MMT with its cation exchange capacity (CEC) of 0.82 mmol g−1 was supplied by FengHong Company (Zhejiang, China).Synthetic procedures
Organoclay (OC) was prepared by using Na-based MMT (NaC) into organic modifiers solution by ion exchange. The organic modifier solution contained octadecyltrimethyl ammonium cations (70 wt%) and cetyltrimethyl ammonium cations (30 wt%). 10 g Na-based MMT was added into 150 mL of organic modifier solution, and turbid solution was stirred for 24 h, and then the solid was filtered, washed and dried at 80 °C for 24 h, and this process of ion exchange was repeated three times, and the cation exchange capacity (CEC) of the obtained organoclay was 1.35 mmol g−1. 1.6 g ammonium molybdate and 25.4 g Ce(NO3)4 were dissolved into the 150 mL of oxalic acid solution (1.5 wt%). After stirring 30 min, and the above organoclay was added into the above solution and stirred at 20 °C for 24 h to obtain the suspicion. Then the suspicion was dried at 80 °C for 6 h, and then added 0.1 g polyoxyethylene to the suspicion, finally the mixture was molded and dried at 100 °C for 5 h. The different catalysts were obtained at different calcination temperature (such as 450, 500, 550, 600, 650 °C) for 4 h (denoted as CeMoxOy-OC-T T: calcination temperature), respectively. For comparison, the catalyst with NaC as support was also prepared with the above similar conditions (denoted as CeMoxOy-NaC-T).Catalyst characterization
Scanning electron microscopy (SEM: Nanosem 430) was used to analyse the morphology of samples. Transmission electron microscopy (TEM) was performed on Jeol-2100f. All samples subjected to TEM measurements were ultrasonically dispersed in ethanol at 30 min and dropped on copper grids.The crystal structure of catalysts were determined by the powder X-ray reflection diffraction (XRD) performed on Rigaku DMAX-RB with a radiation of Cu Kα (λ = 1.5406 Å). The 2θ scans cover the range 5–80° with a step size of 0.02° and a scan rate of 5° min−1.
Nitrogen adsorption and desorption isotherms were measured on TristarII3020 at 77 K. Specific surface area was calculated by BET method, pore-size distribution was calculated from the adsorption branch using BHJ method, and total pores volume was obtained at P/P0 of 0.97.
Temperature-programmed reduction by hydrogen (H2-TPR) were carried out on Micromeritics AutochemII2920 chemisorption analyzer. Prior to each experiment, samples were pre-treated in helium stream at 300 °C for 2 h and then cooled to 50 °C. After switching to 10 vol% H2 in helium balance with the gas rate of 50 mL min−1, TPR experiment started at a ramping rate of 10 °C min−1 up to 800 °C.
Temperature-programmed desorption of ammonia (NH3-TPD) data were collected using Micromeritics AutochemII2920 chemisorption analyzer. Prior to each experiment, samples were pre-treated in helium stream at 300 °C for 2 h and then cooled to 50 °C for ammonia adsorption. NH3 in helium balance (50 mL min−1) was introduced for 1 h to achieve adsorption equilibrium. Then the samples were purged with helium for 1 h to remove the weakly adsorbed ammonia. Finally ammonia was desorbed using helium at a flow rate of 50 mL min−1 from 50 °C to 700 °C with a ramping rate of 10 °C min−1.
Catalyst performance test
The catalytic activities of various catalysts for NH3-SCR of NO were carried out in a fixed-bed quartz reactor (i.d. = 45 mm) containing 10 mL of catalyst. The feed gas mixture consists of 850 ppm NO, 850 ppm of NH3, 10 vol% of O2. A total flow rate of 830 mL min−1 was maintained for all experiments with N2 as balance gas, and the corresponding space velocity was 5000 h−1. The concentration of NO, NO2 in the inlet and outlet was measured by a flue gas analyzer (HORIBA METRON, S48 32/HMT). All measured data were collected after 1 h when the SCR of NO by NH3 reached a steady state at each temperature. NOx conversion (η) was obtained by following equation:Poisoning and regeneration of catalysts
The poisoning of water vapor (and/or 200 ppm SO2) was investigated by introducing 5 vol% H2O (and/or 200 ppm SO2) to the inlet gas (850 ppm NO, 850 ppm NH3, 10% O2, balance N2).Results and discussion
The X-ray diffraction patterns of the CeMoxOy-OC-550 catalysts with different Mo/Ce molar ratios (0.05, 0.10, 0.15, and 0.20) were first presented in Fig. 1a. For CeMoxOy-OC-550 catalysts, there are mixed phases including diffraction peaks characteristic of cubic CeO2 structure (JCPDS 65-5923) as well as OC (Fig. 1a). When Mo was added to CeO2-OC-550, the diffraction peaks intensities of CeO2 decreased and it is found that no new diffraction peaks appeared, suggesting molybdenum oxide had been well dispersed in CeO2-OC-550 catalyst. Fig. 1b showed XRD patterns of CeMo0.15Ox-OC-T catalysts with different calcination temperature (450–650 °C), it can be seen that the diffraction peaks intensities of cerium oxide increased with the increase of calcination temperature. This result implied higher calcination temperature was beneficial to increasing particles size by aggregation and growth.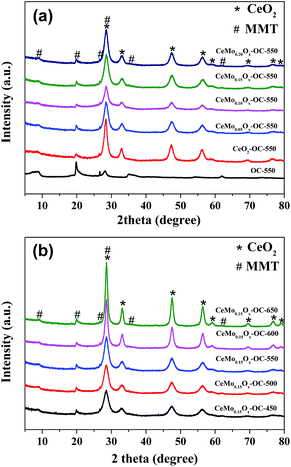 | ||
| Fig. 1 XRD patterns of (a) CeMoxOy-OC-550 catalysts with different Mo/Ce ratios, (b) CeMo0.15Ox-OC-T catalysts with different calcination temperature. | ||
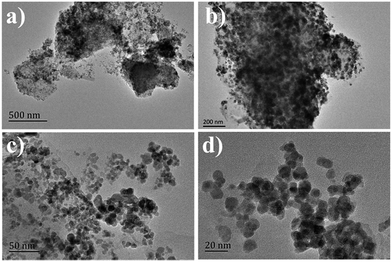
The morphology of the CeMo0.15Ox-OC-550 catalyst was examined by SEM and TEM. Fig. S1† showed that CeMo0.15Ox-OC-550 catalyst still maintained the layered structure of clay. Fig. 2 showed that CeMoxOy nanoparticles were in the range of 10–20 nm (Fig. 2c and d), and uniformly dispersed within layers of clay (Fig. 2a, b and S2†), which might be responsible for high SCR activity.
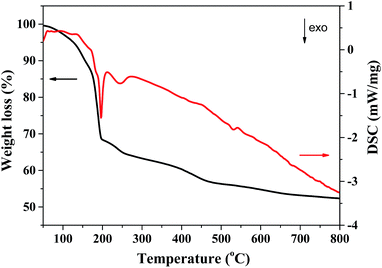
The simultaneous TG-DSC analysis (Fig. 3) of the as-prepared CeMo0.15Ox-OC precursor showed a continuous weight loss of 43.7% from 40 to 500 °C. The weight loss of 31.5% from 40 to 200 °C accompanied with an exothermic peak at 196 °C could be attributed to desorption of the adsorbed water and intercalated water as well as the part decomposition of the nitrates. The weight loss of 12.2% from 200 to 500 °C companied with an exothermic peak around 245 °C could be attributed to the decomposition of organic species and the combustion of carbon species. After 500 °C, the weight of the precursor has little change, which indicated that the organic species in the sample could be removed after calcined at 500 °C in air.
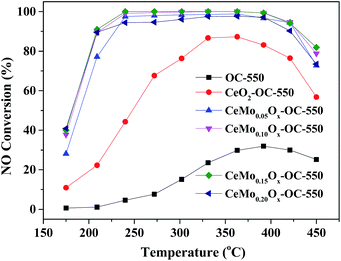
The catalytic activity of different catalysts was investigated as shown in Fig. 4. For CeO2-based SCR catalysts, it is found that Mo content had a significant effect on catalytic activity.23 The influence of Mo/Ce molar ratios on the NOx conversion of CeMoxOy-OC-550 catalysts were evaluated at different operating temperature between 175 and 450 °C as shown in Fig. 4, it is shown that Mo/Ce molar ratios had obvious effect on catalytic activity. It is found that the OC-550 as a catalyst exhibited very little catalytic activity for SCR with the maximum NOx conversion of only 32% at 390 °C, however, when CeO2 was added into OC-550, the SCR activity of CeO2-OC-550 catalyst has been greatly improved with its maximum NOx conversion of 87% at 360 °C. While the addition of a small amount of Mo (Mo/Ce = 0.05) to CeO2-OC-550, NOx conversion had significantly increased, indicating Mo played a synergetic role in promoting SCR activity of CeO2-OC-550 catalyst. The NOx conversion further increased with the increase of Mo/Ce molar ratios from 0.05 to 0.15, the CeMo0.15Ox-OC-550 catalyst exhibited the best SCR activity and widest temperature window with the NOx conversions of nearby 100% between 240 and 390 °C. When Mo/Ce molar ratio further increased to 0.20, NOx conversion began to decrease in operation temperature range.
The surface acidity of catalysts with different Mo/Ce ratios was investigated by NH3-TPD analysis as shown in Fig. S2a.† It is found that curves of NH3-TPD for all these catalysts exhibited two desorption peaks at 110 °C and 420 °C, corresponding to weak acid position and strong acid position, respectively. There were negligible changes in the acid strength of these catalysts, indicating that different Mo/Ce ratios had little influence on acid strength. However, the addition of Mo increased the overall amount of acid of catalysts, while amount of acid sites were not changed linearly with the increase of molybdenum content in these catalysts. The surface redox property of catalyst is another important factor for NH3-SCR. Fig. S2b† presented that all curves of H2-TPR had one reduction peak for CeMoxOy-OC-550 catalysts. For the CeO2-OC-550 catalyst, the reduction peak at about 500 °C could be assigned to the reduction of surface Ce4+ (to Ce3+).24 Comparing with the CeO2-OC-550 catalyst, the position of reduction peaks had insignificantly changed when the different amount of Mo were added. This result implied Mo was not directly related with the reduction of CeO2. In addition, the reduction temperature of molybdenum oxides (MoO3) into MoO2 was also in the range of 450–560 °C.25 In addition, the surface chemical states of CeO2-OC-550 catalyst and CeMo0.15Ox-OC-550 catalyst were performed using XPS (Fig. S3†). Fig. S3† showed that the XPS spectra of Ce 3d for both catalysts could be deconvoluted into eight different fitted peaks with two peaks for Ce3+ and the remaining six peaks for Ce4+.26 It is found that when Mo was added into CeO2-OC-550, the Ce3+/(Ce3++Ce4+) ratio of CeMo0.15Ox-OC-550 catalyst increased from 26% to 39%. The increase of the Ce3+/(Ce3+ + Ce4+) ratio could lead to enhancing SCR activity. This result agreed with previous reports that the value of Ce3+ ratio had an important role in improving catalytic activity for NH3-SCR.27,28
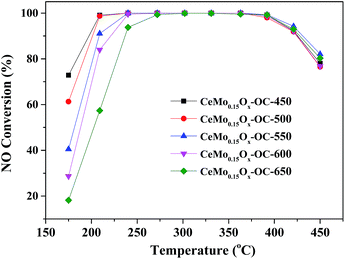
In practical application, the catalyst for NH3-SCR should have good thermal stability. Calcination temperature is another important parameter that affects the structure phase of catalyst, and has obvious influence on various characteristic of catalysts such as crystal size, specific surface area and pore structure.29 Therefore, the CeMo0.15Ox-OC-T catalyst were calcined at different temperature (450, 500, 550, 600, 650 °C) for 4 h to test thermal stability and the corresponding catalytic results are shown in Fig. 5. All of these catalysts as the increase of calcination temperature from 450 to 650 °C, all these catalyst exhibited high SCR activities, whereas the NOx conversion had a decline trend at low operating temperature (175–240 °C). It is noted that the NOx conversion showed little change at high operating temperature (240–450 °C), indicating that the CeMo0.15Ox-OC-T catalyst exhibited a much superior stable for the high-temperature NH3-SCR performance. Fig. S4† presented that the different calcination temperature exhibited little influence on both strength and amount of weak acid, reduction property on these catalysts. Researchers had suggested that the weak acid was responsible to high SCR activity.23 These results illustrated that there were similar weak acid and reduction property with different calcination temperature. Combined the result of XRD patterns, the particle size of catalysts might be responsible to the low-temperature SCR activity.
To understand the influence of organic modification to Na-based MMT (NaC) on SCR activity, we compared CeMo0.15Ox-OC-550 catalyst with CeMo0.15Ox-NaC-550 catalyst. As shown in Fig. 6a, it is clear that the CeMo0.15Ox-NaC-550 catalyst exhibited very low NOx conversion of only 20–30% in the range of 220–420 °C. However, when NaC were modified by the organic anions, the prepared CeMo0.15Ox-OC-550 catalyst exhibited obviously improved SCR activity with NOx conversion of >90% in the range of 220–420 °C. This result further revealed that the organic modification played a crucial role in the catalytic performance of NH3-SCR.
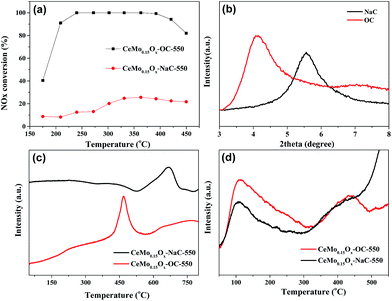 | ||
| Fig. 6 The NOx conversion (a), XRD (b), H2-TPR (c), NH3-TPD (d) of CeMo0.15Ox-OC-550 and CeMo0.15Ox-NaC-550 catalysts. | ||
Fig. 6b showed that the XRD pattern of the starting clay (NaC) exhibited a peak at 2 theta about 5.6° assigned to the basal spacing d001 = 1.58 nm, it is worthwhile to note that this value represented the distance between the two layers with the one layer thickness (about 0.96 nm). After being modified by organic cations, the d-spacing of the organoclay has obviously increased to 2.14 nm, indicating the organic species were intercalated into the interlayers of clay. This increased d-spacing is favorable for the adsorption, impregnation and dispersion of active ions in the interlamellar space of clay. Besides, CeMo0.15Ox-OC-550 illustrated higher specific surface area and larger pore volume than those of CeMo0.15Ox-NaC-550 in Table 1. This high surface area could promote exposure of active phase and utilization efficiency of active sites and large pore volume would favour diffusion of reactants and product, leading to high SCR activity. Table 1 also showed that alkali metal content (Na, K) obviously decreased after organic modification, this result were favorable to the high SCR activity, because high alkali metal could obviously reduce the catalytic activity of NH3-SCR.30–32
| Sample | BET surface area (m2 g−1) | Pore volume (cm3 g−1) | Na content (wt%) | K content (wt%) |
|---|---|---|---|---|
| OC | 61 | 0.11 | 0.10 | 0.39 |
| CeMo0.15Ox-OC-550 | 83 | 0.16 | 0.03 | 0.18 |
| CeMo0.15Ox-NaC-550 | 38 | 0.07 | 1.18 | 0.55 |
Fig. 6c showed that the H2-TPR profile of CeMo0.15Ox-NaC-550 and CeMo0.15Ox-OC-550 catalysts. For CeMo0.15Ox-NaC-550 catalyst, there is a high reduction peak at 670 °C, which could be assigned to the reduction of the surface Ce4+ (to Ce3+). Noticeably, CeMo0.15Ox-OC-550 catalyst had the reduction peak at 467 °C, which is much lower than that of CeMo0.15Ox-NaC-550 catalyst. This lower reduction peak could be ascribed to the reduction of more well dispersed surface CeO2 and MoO3 species, which were favorable for SCR activity. Fig. 6d showed that CeMo0.15Ox-NaC-550 catalyst had two desorption processes of ammonia, corresponding to the weak acid and strong acid, respectively. However, in the case of CeMo0.15Ox-OC-550 catalyst, the two ammonia desorption processes had little changed, suggesting that organic modification had negligible influence on acid strength for clay, while amount of weak acid had improved obviously, which could be attributed to more well uniformly dispersed CeMoxOy nanoparticles. This result also revealed that weak acid playing an important role in SCR activity, which was agree with previously results for the CeO2-based SCR catalysts.27,33
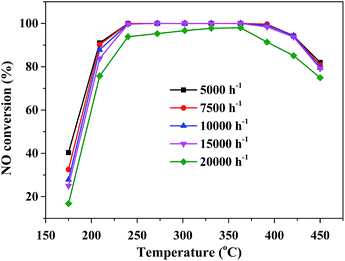
The SCR activity of catalyst with the different GHSV was also be shown in Fig. 7. It is clear that the increase of GHSV from 5000 to 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 h−1 resulted in the slight decrease of SCR activity at low temperature especially between 175 and 210 °C, while there was little effect on the high-temperature SCR performance. And CeMo0.15Ox-OC-550 catalyst showed rather high NOx conversion exceeding 90% within a broad operation temperature window from 220 to 420 °C. And GHSV further increased to 20
000 h−1 resulted in the slight decrease of SCR activity at low temperature especially between 175 and 210 °C, while there was little effect on the high-temperature SCR performance. And CeMo0.15Ox-OC-550 catalyst showed rather high NOx conversion exceeding 90% within a broad operation temperature window from 220 to 420 °C. And GHSV further increased to 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, the SCR activity slightly decreased.
000, the SCR activity slightly decreased.
It is the typical features that there are water vapor and SO2 in the real operating conditions for flue gas of power plant. Water vapor is one of the main components in flue gases and often leads to catalyst deactivation. Fig. 8 showed that the influence of 5 vol% H2O vapor on the NOx conversion for CeMo0.15Ox-OC catalyst at 330 °C. It is shown that the NOx conversion of CeMo0.15Ox-OC catalyst was 100% for 24 h. After adding 5 vol% H2O at 0.5 h, the NOx conversion started to decrease from 100% to 98.5%, and still remained stable after 18 h running in the presence of 5 vol% H2O. When water vapor was cut off, the NOx conversion quickly recovered to 100% and kept stable for another 5 h, probably due to the reversible deactivation by weak comparative adsorption on the active sites where H2O vapor competed with NH3 or NO.27 These results suggested that this prepared catalyst had high tolerance to water vapor.
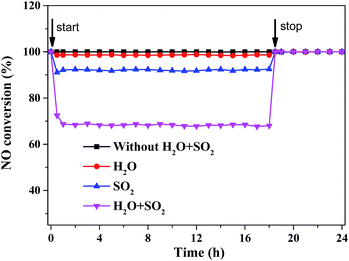 | ||
| Fig. 8 NOx conversion of CeMo0.15Ox-OC-550 catalyst with different conditions in the presence of 5 vol% H2O, 200 ppm SO2 and 5 vol% H2O + 200 ppm SO2, respectively, at 5000 h−1, 330 °C. | ||
As there is still certain amount of residual SO2 in flue gases, the poisoning effect of SO2 on this catalyst was investigated (Fig. 8). When 200 ppm of SO2 was added to the flue gases at 0.5 h, the NOx conversion of CeMo0.15Ox-OC-550 catalyst started to decrease from 100% to 91.7%, and then still kept stable for 91.7% within another 18 h, demonstrating that CeMo0.15Ox-OC-550 catalyst had high resistance to SO2. It is probably that the CeMo0.15Ox nanoparticle has high dispersed interlayers of clay structure, which could enhance the stability of SO2 poisoning. When SO2 was cut off at 18.5 h, the NOx conversion for CeMo0.15Ox-OC-550 has quickly recovered to 100%. This result suggested that SO2 also led to reversible deactivation on the active sites of CeMo0.15Ox-OC-550 catalyst by weak comparative adsorption with NH3 or NO. This data again demonstrated our catalyst possessed high resistance to SO2. In addition, after adding 5 vol% H2O and 200 ppm SO2 to the system simultaneously, the NOx conversion further decreased about 68%, suggesting the synergistic deactivating effect of H2O and SO2 had inhibited the catalytic performance of CeMo0.15Ox-OC-550. It is believed that water vapor and SO2 could inhibit the adsorption and activation of NH3 & NO on the active sites, leading to an obvious decline of the NOx conversion.23 Noticeably, the NOx conversion had almost been unchanged in the next 18 h. Once water vapor and SO2 were removed, the NOx conversion for CeMo0.15Ox-OC-550 also recovered quickly to 100% and stable for another 5 h, these result indicated that water vapor and SO2 had insignificant effect for catalytic active structure of this catalyst, demonstrating that our catalyst possessed good resistance to the co-existence of water vapor and SO2.
Conclusions
In summary, a facile method was presented to prepare CeMoxOy-based clay hybrid catalyst with layered structure. With organic cations modified clay as support, after a simple adsorption and impregnation of cerium cations and molybdate anions, and calcination at high temperature, well-dispersed CeMoxOy nanoparticles in the layered matrix of clay were prepared and exhibited high and stable SCR performance, presenting the promising potential as candidate for NH3-SCR. This excellent SCR activity could be attributed to novel layered structure, well-dispersed CeMoxOy nanoparticles and lower alkali metal content.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work is financially supported by the Natural Science Foundation of Jiangsu Province of China (No. BK20160982).References
- C. Tang, H. Zhang and L. Dong, Catal. Sci. Technol., 2016, 6, 1248 CAS.
- K. Cheng, J. Liu, T. Zhang, J. Li, Z. Zhao, Y. Wei, G. Jiang and A. Duan, J. Environ. Sci., 2014, 26, 2106 CrossRef PubMed.
- M. Sakai, Y. Nagai, Y. Aoki and N. Takahashi, Appl. Catal., A, 2016, 510, 57 CrossRef CAS.
- X. Wang, S. N. Zhao, Y. B. Zhang, Z. Wang, J. Feng, S. Y. Song and H. J. Zhang, Chem. Sci., 2016, 7, 1109 RSC.
- Y. Geng, W. Shan, S. Xiong, Y. Liao, S. Yang and F. Liu, Catal. Sci. Technol., 2016, 6, 3149 CAS.
- Y. Shen, S. Zhu, T. Qiu and S. Shen, Catal. Commun., 2009, 11, 20 CrossRef CAS.
- L. Chen, J. Li, M. Ge and R. Zhu, Catal. Today, 2010, 153, 77 CrossRef CAS.
- S. Yang, Y. Guo, H. Chang, L. Ma, Y. Peng, Z. Qu, N. Yan, C. Wang and J. Li, Appl. Catal., B, 2013, 136–137, 19 CrossRef CAS.
- Y. Shen, RSC Adv., 2012, 2, 5957 RSC.
- B. Han, Y. Shen, S. Zhu, Y. Liu and S. Shen, J. Rare Earths, 2016, 34, 1010 CrossRef CAS.
- M. Salazar, S. Hoffmann, L. Tillmann, V. Singer, R. Becker and W. Grünert, Appl. Catal., B, 2017, 218, 793 CrossRef CAS.
- H. Wang, Z. Qu, H. Xie, N. Maeda, L. Miao and Z. Wang, J. Catal., 2016, 338, 56 CrossRef CAS.
- S. Gillot, G. Tricot, H. Vezin, J.-P. Dacquin, C. Dujardin and P. Granger, Appl. Catal., B, 2017, 218, 338 CrossRef CAS.
- S. Navalon, M. Alvaro and H. Garcia, Appl. Catal., B, 2010, 99, 1 CrossRef CAS.
- T. Grzybek, Catal. Today, 2007, 119, 125 CrossRef CAS.
- W. Ferjani, L. K. Boudali, G. Delahay and C. Petitto, Chem. Lett., 2016, 45, 872 CrossRef CAS.
- L. Chmielarz, P. Kuśtrowski, M. Zbroja, B. Gil-Knap, J. Datka and R. Dziembaj, Appl. Catal., B, 2004, 53, 47 CrossRef CAS.
- J. L. Valverde, A. de Lucas, F. Dorado, A. Romero and P. B. García, J. Mol. Catal. A: Chem., 2005, 230, 23 CrossRef CAS.
- L. S. Cheng, R. T. Yang and N. Chen, J. Catal., 1996, 164, 70 CrossRef CAS.
- R. Q. Long and R. T. Yang, J. Catal., 1999, 186, 254 CrossRef CAS.
- Y. Wang, B. Shen, C. He, S. Yue and F. Wang, Environ. Sci. Technol., 2015, 49, 9355 CrossRef CAS PubMed.
- D. Chen, C. Cen, J. Feng, C. Yao, W. Li, S. Tian and Y. Xiong, J. Chem. Technol. Biotechnol., 2016, 91, 2842 CrossRef CAS.
- S. Ding, F. Liu, X. Shi, K. Liu, Z. Lian, L. Xie and H. He, ACS Appl. Mater. Interfaces, 2015, 7, 9497 CAS.
- S. Zhan, H. Zhang, Y. Zhang, Q. Shi, Y. Li and X. Li, Appl. Catal., B, 2017, 203, 199 CrossRef CAS PubMed.
- V. O. O. Gonçalves, C. Ciotonea, S. Arrii-Clacens, N. Guignard, C. Roudaut, J. Rousseau, J.-M. Clacens, S. Royer and F. Richard, Appl. Catal., B, 2017, 214, 57 CrossRef.
- C. Zhu, T. Ding, W. Gao, K. Ma, Y. Tian and X. Li, Int. J. Hydrogen Energy, 2017, 42, 17457 CrossRef CAS.
- W. Yan, Y. Shen, S. Zhu, Q. Jin, Y. Liu and X. Li, Catal. Lett., 2016, 146, 1221 CrossRef CAS.
- D. W. Kwon, K. B. Nam and S. C. Hong, Appl. Catal., A, 2015, 497, 160 CrossRef CAS.
- B. Guan, H. Lin, L. Zhu, B. Tian and Z. Huang, Chem. Eng. J., 2012, 181, 307 CrossRef.
- Y. Shen and S. Zhu, Catal. Sci. Technol., 2012, 2, 1806 CAS.
- S. Boxiong, Y. Yan, C. Jianhong and Z. Xiaopeng, Microporous Mesoporous Mater., 2013, 180, 262 CrossRef.
- Y. Peng, J. Li, X. Huang, X. Li, W. Su, X. Sun, D. Wang and J. Hao, Environ. Sci. Technol., 2014, 48, 4515 CrossRef CAS PubMed.
- Y. Shen, Y. Su and Y. Ma, RSC Adv., 2015, 5, 7597 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7ra12153a |
| This journal is © The Royal Society of Chemistry 2018 |