Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Schiff base-functionalized mesoporous silicas (MCM-41, HMS) as Pb(II) adsorbents
Daniela F. Enachea,
Eugenia Vasilea,
Claudia Maria Simonescua,
Daniela Culitab,
Eugeniu Vasilea,
Ovidiu Opreaa,
Andreea Madalina Pandelea,
Anca Razvana,
Florina Dumitru *a and
Gheorghe Nechifora
*a and
Gheorghe Nechifora
aPolitehnica University Bucharest, 1 Polizu st., 011061, Bucharest, Romania. E-mail: florina.dumitru@upb.ro
b“Ilie Murgulescu” Institute of Physical Chemistry, Splaiul Independentei 202, Bucharest, Romania
First published on 2nd January 2018
Abstract
Three Schiff base-functionalized mesoporous silicas: MCM-41@salen, HMS-C12@salen and HMS-C16@salen have been synthesized by a post-synthetic grafting strategy and their sorption capacities toward Pb(II) from synthetic aqueous solutions have been assessed. FTIR spectra, TG/DSC analyses, X-ray powder diffraction, X-ray photoelectron spectroscopy (XPS), N2 adsorption isotherms and HRTEM micrographs were used to characterize the novel functionalized mesoporous silicas. The novel adsorbents have been tested for their capability in the remediation of synthetic aqueous systems containing Pb(II). The Langmuir maximum values of sorption capacities of these adsorbents toward Pb(II) are: 138.88 mg Pb(II) per g MCM-41@salen, 144.92 mg Pb(II) per g HMS-C12@salen, and 181.81 mg Pb(II) per g HMS-C16@salen, therefore, the functionalized silicas (MCM-41@salen, HMS-C12@salen, HMS-C16@salen) could be used as effective adsorbents of Pb(II) ions from wastewater.
1. Introduction
Modifications by post-grafting strategy of the silica surface of MCM-41/HMS materials have been widely studied and numerous organofunctionalized materials for selective and advanced separation processes have been obtained.1–6 Conventional routes of silica surface modification by chemical treatment methods are based on the reaction between the surface silanol groups and commercial silane coupling agents as precursors for immobilization of organic molecules. One of the most used silylation agents is 3-aminopropyl trimethoxysilane (APTES)7,8 and the amount of organic moieties obtained by grafting of APTES is usually 1–1.5 mmol gsolid−1.4,9Therefore, the post-grafting strategy represents a simple route for anchoring the functional chelating ligands into the pores and, consequently, affords the obtaining of a plethora of organo-functionalized mesoporous silicas that could be used as adsorbents of toxic materials.
Among these materials, those having grafted salicylaldoxime (salen) ligands represent efficient sorbents and they were extensively used for remediation of wastewater containing heavy metal ions. Soliman et al.10 obtained mono- and bis-salicylaldehyde Schiff base ligands grafted on silica surface in a three-step approach: silanization of silica surface with 3-chloropropyltrimethoxysilane, replacing the chlorine leaving group with amino-group from diethylenetriamine, followed by the condensation with salicylaldehyde. The as-prepared hybrid materials show high selectivity in extraction of Cu(II) (metal uptake capacity 0.957 mmol g−1) and Fe(III) (0.643 mmol g−1). In a similar approach, Kim et al.11 synthesized a chiral salen-mesoporous MCM-41 material that has been further involved in Mn(III) coordination, thus providing a catalyst for styrene epoxidation with high level of enantioselectivity. Sarkar et al.12 demonstrated that preconcentration of Cu(II), Zn(II), Co(II), Fe(III) and Ni(II) in water could be highly effective by using the silica gel modified with salicylaldoxime. Parvulescu et al.13,14 reported the preparation of n-propyl-salicylaldimine modified SBA-15/SBA-16 mesoporous silicas and the study of their efficiency in sorption of heavy metal ions, they found out that adsorption capacities decrease within the following series: Cu(II) > Co(II) ⋙ Mn(II). Functionalized SBA-15 mesoporous silica particles, bearing ethylenediaminepropyl-salicylaldimine and N-propylsalicylaldimine Schiff base ligands, were examined as adsorbents in solid phase extraction (SPE) of uranyl (UO22+) ions from water.15 The maximum adsorption capacities determined for these adsorbents were 105.3 and 54 mg uranyl per g adsorbent, respectively. Chen et al.16 prepared a Schiff base immobilized hybrid mesoporous silica membrane for the detection of Cu(II) by immobilizing 4-chloro-2-[(propylimino)methyl]-phenol (4-chloro-salen) onto the pore surface of mesoporous silica embedded in the pores of an anodic alumina membrane. The detection limit for Cu(II) was 0.8 μM. Gao et al.17 previously used the same ligand to obtain (via a self-assembly process) a mesoporous silica material SBA-15–APTES–4-chloro-2-[(propylimino)methyl]-phenol, as Zn(II)-sensitive fluorescent chemosensor.
Porous silica functionalized with N-propylsalicylaldimine was used for separation and preconcentration of Cu(II), Cr(III, VI), Cd(II), Pb(II) and Mn(II, VII) from natural waters.18
In this work, we describe the obtaining of ordered mesoporous silicas (MCM-41 and HMS type) with hemisalen Schiff base grafted onto the silica surface (Fig. 1).
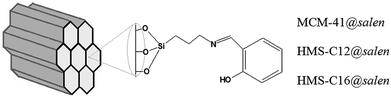 | ||
| Fig. 1 Schematic representation of mesoporous adsorbents: MCM-41@salen, HMS-C12@salen (C12 – dodecylamine), HMS-C16@salen (C16 – hexadecylamine). | ||
The functionalization of the MCM-41/HMS silicas has been carried out by heterogeneous grafting method: first, the silylating agent (3-aminopropyl)triethoxysilane (APTES) reacted with the surface hydroxyl groups to give the amino-functionalized silica (MCM-41@APTES, HMS-C12@APTES, HMS-C16@APTES) and then the salicylaldehyde was covalently attached (MCM-41@salen, HMS-C12@salen, HMS-C16@salen).
FTIR spectra, TG/DSC analyses, X-ray powder diffraction, X-ray photoelectron spectroscopy (XPS), N2 adsorption isotherms and HRTEM micrographs were used to characterize the novel functionalized materials.
The obtained mesoporous adsorbents have been assessed for the effectiveness in the remediation of synthetic aqueous systems containing Pb(II). The factors that influence the sorption behavior have been studied: heavy metal ion concentrations, pH of solution, contact time, as well as the adsorption isotherms.
2. Experimental
2.1. Techniques and materials
All the raw materials were commercially available and used as received.FTIR vibrational spectra were registered with a Bruker Tensor 27 spectrophotometer equipped with one ATR sampling unit, in the wavenumbers range of 500–4000 cm−1.
Thermal analysis was carried out with a Netzsch 449C STA Jupiter. Samples were placed in open alumina crucible and heated with 10 degrees per min from room temperature to 900 °C, under the flow of 20 mL min−1 dried air. An empty Al2O3 crucible was used as reference.
Powder X-ray diffraction (PXRD) patterns were registered on a Panalytical X'PERT PRO MPD diffractometer with graphite monochromatized CuKα radiation (λ = 1.54 Å). The samples were scanned in the Bragg angle, 2θ range of 2–10° and step size of 0.013°.
For TEM and HRTEM analysis we used TECNAI F30 G2 high-resolution transmission electron microscope operated at an accelerating voltage of 300 kV device. The samples have been prepared by dispersing the particles by ultrasonication in methanol and subsequently collected into a holey carbon-coated TEM support grid.
The N2 adsorption/desorption isotherms for pore size distribution and BET specific surface measurements were recorded on a Micromeritics ASAP 2020 analyzer. Before analysis the samples were outgassed at 120 °C for at least 6 hours under vacuum.
X-ray photoelectron spectroscopy (XPS) data were registered on a Thermo Scientific K-Alpha device, fully integrated, with an aluminium anode monochromatic source (1486.6 eV). The survey spectra were registered using a pass energy of 200 eV at bass pressure of 2 × 10−9 mbar.
The pH measurements were taken with Agilent 3200P pH Meter. Batch equilibrium experiments on the GFL 3031 Incubating Shaker at 150 rpm were performed. Atomic Absorption Spectrometry on contrAA® 300 Analytik Jena Atomic Absorption Spectrometer was applied to determine Pb(II) ions concentration in aqueous solutions. All the equilibrium experiments were carried out by contacting 0.025 g of adsorbent with 25 mL of synthetic aqueous solution of 100 mg L−1 Pb(II), at room temperature, for 24 hours and 150 rpm. After reaching the equilibrium, the Pb(II) concentrations in filtrate samples have been determined by AAS.
2.2. Preparation of functionalized mesoporous silicas
The reaction mixture was composed of 1.0CTAB![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9.21TEOS
9.21TEOS![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.55NaOH
2.55NaOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4857H2O (molar ratios). CTAB and NaOH formed the initial reaction mixture in water that was further heated at 80 °C for 30 min. TEOS was added at pH 12. After 2 h at 80 °C, white solid MCM-41 precipitated. The product was filtered off, washed with large volumes of water and methanol and dried in oven at 105 °C for 6 h. The surfactant (CTAB) has been removed by thermal treatment at 550 °C for 6 h.
4857H2O (molar ratios). CTAB and NaOH formed the initial reaction mixture in water that was further heated at 80 °C for 30 min. TEOS was added at pH 12. After 2 h at 80 °C, white solid MCM-41 precipitated. The product was filtered off, washed with large volumes of water and methanol and dried in oven at 105 °C for 6 h. The surfactant (CTAB) has been removed by thermal treatment at 550 °C for 6 h.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.27CnH2n+1NH2
0.27CnH2n+1NH2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9.09C2H5OH
9.09C2H5OH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 29.6H2O.
29.6H2O.The syntheses were accomplished at room temperature, the reaction mixtures were aged for 24 h and the obtained HMS solids were recovered by filtration, washed with deionized water and dried at 105 °C for 6 h. The primary amines (C12H25NH2, dodecylamine and C16H33NH2, hexadecylamine) have been removed by calcination in air at 630 °C for 4 h.
In each case, the resulting silica (MCM-41@APTES, HMS-C12@APTES or HMS-C16@APTES) was collected by centrifugation, then washed with toluene to remove the adsorbed APTES, filtered and dried at 80 °C, for 24 h.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30. After continuous stirring at room temperature for 5 h, the colorless reaction mixture (both liquid and solid phases) became yellow. The yellow solid was filtered, extensively washed with methanol and finally dried under reduced pressure.
30. After continuous stirring at room temperature for 5 h, the colorless reaction mixture (both liquid and solid phases) became yellow. The yellow solid was filtered, extensively washed with methanol and finally dried under reduced pressure.3. Results and discussion
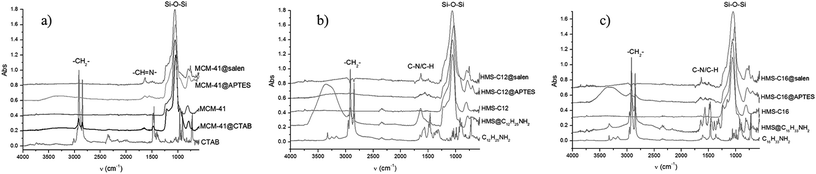
3.1. FTIR spectra
In the FTIR spectra (Fig. 2, Table 1) of functionalized silica materials, stretching vibrational bands specific to silica network and to organic moieties (i.e. the surfactant or the covalently bonded aminopropylsiloxane linker/salen ligands) are present. The strongest signals in FTIR spectra are assigned to Si–O–Si 1100 cm−1 corresponding with the silica network formed as a result of hydrolysis/condensation reactions between the silica precursors.| Compound/material | ν(N–H) | ν(CH2as/sym) | ν(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N) N) |
ν(C–C), ν(C–N) | δ(CH2)/δ(NH2) | δ(N+–CH3) | ν(Si–O–Si) |
|---|---|---|---|---|---|---|---|
| CTAB | — | 2913/2848 | — | 962/912 | 1461, 1475/— | 1488 | — |
| MCM-41@CTAB | — | 2923/2852 | — | 962/910 | 1477, 1467/— | 1490 | 1039 |
| MCM-41 | — | — | — | — | — | — | 1053 |
| MCM-41@APTES | 3351 (br) | 2926 | — | — | —/1643, 1551 | — | 1051 |
| MCM-41@salen | — | 2927 | 1648–1635 | 964/898 | 1459, 1498 | — | 1058 |
| C12H25NH2 | 3328 (s) | 2916/2847 | — | 1464, 1487/1649, 1567 | — | — | |
| HMS@C12H25NH2 | 3350 (br) | 2922/2853 | — | 960/888 | 1467/1641 | — | 1056 |
| HMS-C12 | — | — | — | — | — | — | 1058 |
| HMS-C12@APTES | — | 2942/2881 | — | 923 | 1489, 1457/1652, 1559 | 1032 | |
| HMS-C12@salen | — | 2922/2852 | 1633 | — | 1492, 1461 | — | 1065 |
| C16H33NH2 | 3331 (s) | 2917/2848 | — | 973/924 | 1471, 1451 | — | — |
| HMS@ C16H33NH2 | 3324 (s) | 2918/2850 | — | 955 | 1485, 1469/1642, 1565 | — | 1058 |
| HMS-C16 | — | — | — | — | — | — | 1060 |
| HMS-C16@APTES | 3330 (br) | 2947/2834 | — | — | 1452/1649, 1564 | — | 1055/1018 |
| HMS-C16@salen | — | 2920/2851 | 1635 | 1457/— | — | 1047 |
Peaks characteristic to the newly formed imine bonds (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N) appeared in MCM-41@salen, HMS-C12@salen, and HMS-C16@salen FTIR spectra at ∼1635 cm−1, indicative for the salen-type ligands grafted onto silica surface.
N) appeared in MCM-41@salen, HMS-C12@salen, and HMS-C16@salen FTIR spectra at ∼1635 cm−1, indicative for the salen-type ligands grafted onto silica surface.
3.2. XPS data
XPS scans (Fig. 3) carried out on MCM-41@salen, HMS-C12@salen, HMS-C16@salen revealed that they have as constitutive elements: C–C 1s, N–N 1s, O–O 1s, and Si–Si 2p with the corresponding binding energies presented in Table 2. The C–C 1s, N–N 1s are clearly resulted from the organic moieties grafted onto silica surface.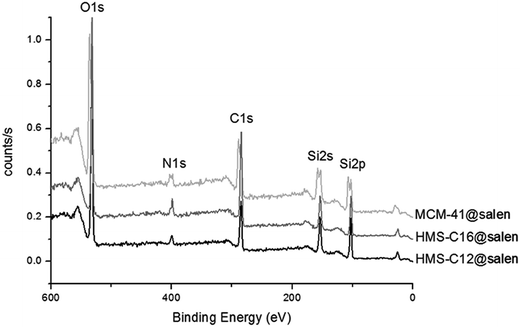 | ||
| Fig. 3 XPS spectra of MCM-41@salen, HMS-C12@salen, HMS-C16@salen (the spectra were normalized and shifted on the y axis). | ||
| Mesoporous silicas | Binding energy values (eV) | Found (% wt) | ||||||
|---|---|---|---|---|---|---|---|---|
| C–C 1s | N–N 1s | Si–Si 2p | O–O 1s | C | N | Si | O | |
| MCM-41@salen | 285.00 | 400.20 | 103.06 | 532.26 | 23.54 | 3.90 | 37.82 | 34.73 |
| HMS-C12@salen | 285.48 | 399.75 | 103.44 | 532.97 | 19.80 | 3.43 | 39.15 | 37.62 |
| HMS-C16@salen | 284.89 | 399.02 | 102.91 | 532.21 | 30.47 | 5.33 | 35.23 | 28.97 |
The atomic contents (converted in weight percents, Table 2) suggest that about 50% of the APTES moieties (for organic content: APTES = C3H8N and APTES + salicylaldehyde = C10H12N) have been reacted to form a bond between the amino group (APTES) and the carbonyl group (salicylaldehide). Supposing that all the nitrogen and carbon content comes from the APTES and APTES + salicylaldehyde, we found that the theoretical carbon-to-nitrogen (C![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) N) mass ratio is 5.57
N) mass ratio is 5.57![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 for 1
1 for 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 APTES
1 APTES![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) APTES + salicylaldehyde and this ratio agrees fairly well with the experimental ones (6.03
APTES + salicylaldehyde and this ratio agrees fairly well with the experimental ones (6.03![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 for MCM-41@salen, 5.77
1 for MCM-41@salen, 5.77![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 for HMS-C12@salen and 5.72
1 for HMS-C12@salen and 5.72![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 for HMS-C16@salen, respectively).
1 for HMS-C16@salen, respectively).
3.3. Thermal analysis
TG-DSC analyses (Fig. 4 and 5) also support the conclusions drawn from the FTIR and XPS spectra.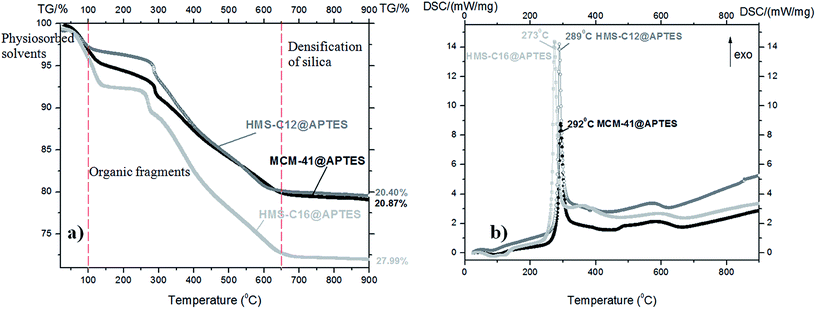 | ||
| Fig. 4 (a) TG curves and (b) DSC curves of the silanized mesoporous silicas: MCM-41@APTES, HMS-C12@APTES and HMS-C16@APTES. | ||
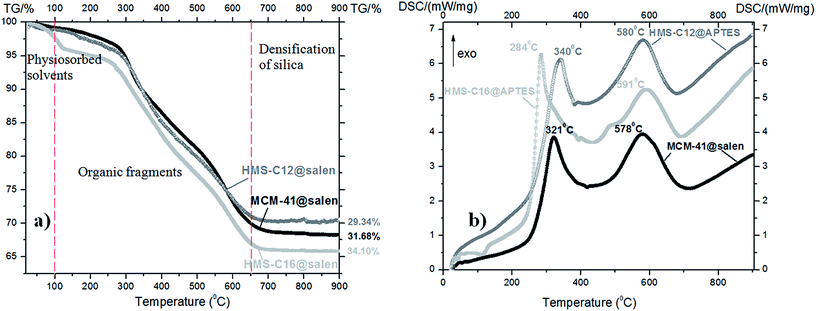 | ||
| Fig. 5 (a) TG curves and (b) DSC curves of the functionalized mesoporous silicas: MCM-41@salen, HMS-C12@salen and HMS-C16@salen. | ||
All the as-prepared mesoporous silicas have similar thermal behavior with three main mass loss peaks, illustrated by TG curves: volatilization of the adsorbed solvent (below 100 °C), decomposition of organic fragments grafted in the silica pores (100–650 °C), and densification of the silica matrix (above 650 °C up to 900 °C).
The DSC curves are very similar in shape for all three organofunctionalized silicas:
• they present one strong exothermic effect centered at 292 °C for MCM-41@APTES, at 289 °C for HMS-C12@APTES and at 273 °C for HMS-C16@APTES, respectively.
• two strong exothermic effects: first effect is centered at 321 °C for MCM-41@salen, at 340 °C for HMS-C12@salen and 284 °C for HMS-C16@salen, respectively, and the second effect occured at 578 °C (MCM-41@salen), at 580 °C (HMS-C12@salen), and at 591 °C (HMS-C16@salen), respectively. The former effect could be assigned to the decomposition of organic ligand grafted onto silicas and the latter to the removal of the residual combustion compounds originated from the decomposition of organic part.
The TG-DSC data are consistent with the results of as-prepared samples XPS measurements. The largest mass loss was found for HMS-C16@salen, similarly to the XPS results where the highest organic content was also found for HMS-C16@salen.
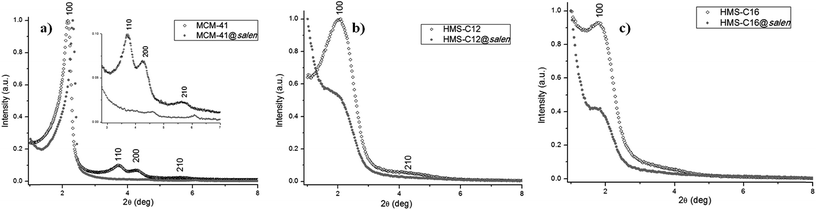
3.4. Powder XRD
The XRD patterns of all synthesized silicas show the hexagonal ordered porous structure (Fig. 6), with the following particularities:– MCM-41 (Fig. 6a, Table 3) exhibits one sharp (100) reflection at 2θ = 2.16° and additional Braggs peaks indexed as (110), (200), and (210) reflections characteristic for hexagonal symmetry (P6mm space group).21 In comparison with the XRD diagram of the parent MCM-41 subjected to post-synthetic organo-functionalization, MCM-41@salen exhibits the sharp reflection 2θ (100) at 2.30°, but only very weak peaks at 2θ = 4.60° (200) and 2θ = 6.09° (210), a common feature of MCM-41 silicas with organic ligands incorporated into the pore channels.
| Miller indices | MCM-41, a0 = 4.724 nm | MCM-41@salen, a0 = 4.424 nm | HMS-C12, a0 = 5.031 nm | HMS-C12@salen, a0 = 5.283 nm | HMS-C16, a0 = 5.783 nm | HMS-C16@salen, a0 = 5.899 nm | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| h | k | l | 2θ (°) | dhkl (nm) | 2θ (°) | dhkl (nm) | 2θ (°) | dhkl (nm) | 2θ (°) | dhkl (nm) | 2θ (°) | dhkl (nm) | 2θ (°) | dhkl (nm) |
| 1 | 0 | 0 | 2.16 | 4.09 | 2.30 | 3.83 | 2.03 | 4.36 | 1.93 | 4.57 | 1.76 | 5.01 | 1.73 | 5.11 |
| 1 | 1 | 0 | 3.71 | 2.38 | — | — | — | — | — | — | — | — | — | — |
| 2 | 0 | 0 | 4.26 | 2.08 | 4.60 | 1.92 | — | — | — | — | — | — | — | — |
| 2 | 1 | 0 | 5.82 | 1.52 | 6.09 | 1.45 | 4.42 | 2.00 | — | — | — | — | — | — |
– HMS-C12 (Fig. 6b, Table 3) and HMS-C16 (Fig. 6c, Table 3) exhibit a single low angle reflection (100) at 2θ = 2.03° (HMS-C12) and 2θ = 1.76° (HMS-C16) indicative of the average pore–pore correlation distance in their hexagonal lattices.20 For these silicas, it is assumed that the framework mesopores are wormholelike with some spatial orientation of the pores within the particles, i.e. local hexagonal symmetry. As the alkylamines used as surfactants are of C12 (HMS-C12) and C16 (HMS-C16) chain lengths, the position of the single (100) reflection is present at lower 2θ value for HMS-C16, indicating the increase of the average distance between the pore centres. Hexadecylamine, C16H33NH2, as structure-directing agent for HMS-C16, has a longer alkyl chain length and it afforded a silica material with larger framework mesopore sizes (HMS-C16: d100 = 5.01 nm vs. HMS-C12: d100 = 4.36 nm).
For HMS-C12@salen (Fig. 6b, Table 3) and HMS-C16@salen (Fig. 6c, Table 3), the post-synthetic grafting of organic ligands caused a significant decrease in the (100) peak intensity and the complete lack of the higher order Bragg reflections in their diffraction patterns (Fig. 6b and c), both features indicate the incorporation of organic moieties into the pore channels of the HMS-C12 and HMS-C16 materials, without the collapse of the pore structure. In conclusion, the pore channels in all HMS silicas prepared by us show uniform framework mesopore distribution with short range hexagonal order. The lack of longer range order is explained by the relatively weak hydrogen-bonding interactions that operate in the neutral (alkylamine:TEOS) assembly process.
The values of the corresponding unit cell parameter a0 were calculated using the formula: a0 = 2d100/31/2, where d100 represents the d-spacing value of the (100) diffraction peak in XRD patterns of the samples.
3.5. High-resolution transmission electron microscopy (HRTEM)
In addition to XRD patterns, HRTEM images (Fig. 7) further confirm the ordered structures of MCM-41@salen (Fig. 7A), HMS-C12@salen (Fig. 7B) and HMS-C16@salen (Fig. 7C).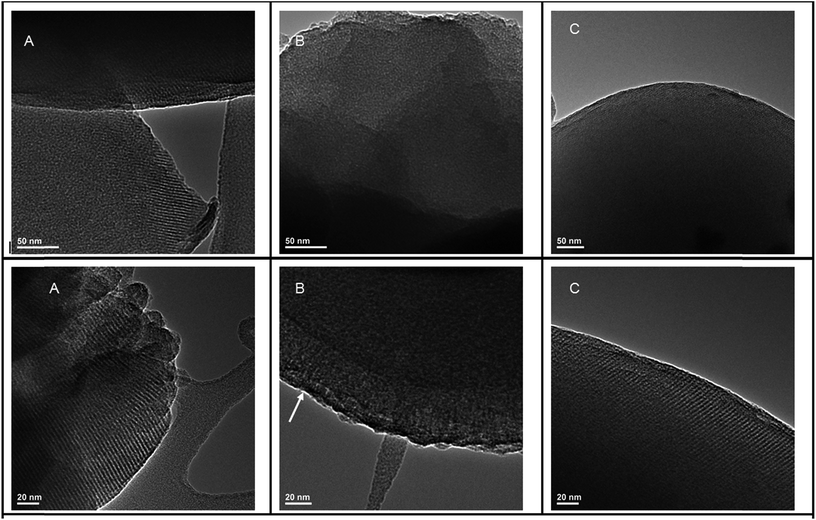 | ||
| Fig. 7 HRTEM images (scale bars 50 nm and 20 nm) for (A) MCM-41@salen; (B) HMS-C12@salen; (C) HMS-C16@salen. | ||
MCM-41@salen exhibits typical MCM-41 highly ordered mesoporous structure, whose hexagonal lattice has been preserved after the functionalization, in accord with the corresponding XRD data.
HRTEM images for HMS-C12@salen show uniform wormholelike channels (mesopores) distributed homogeneously throughout the bulk phase over a short range hexagonallike order (see arrow, Fig. 7B). HRTEM micrograph of HMS-C16@salen sample (Fig. 7C) revealed that the mesoporous framework is better-defined than that of HMS-C12@salen, the hexagonal order being comparable to the MCM-41@salen structure. It is known that for longer alkyl chains (number of carbon atoms > 12), the structure of the HMS template changes from rod-like micelles to lamellar micelles.20,22,23
3.6. Nitrogen adsorption–desorption isotherms
Using Brunauer–Emmett–Teller (BET) and Barrett–Joyner–Halenda (BJH) methods, the specific surface area and the average pore diameter of the samples have been calculated. The structural parameters of MCM-41, MCM-41@salen, HMS-C12, HMS-C12@salen, and HMS-C16, HMS-C16@salen samples (pore volume, pore diameter and BET surface area) were summarized in Table 4. The N2 sorption isotherms for the synthesized silicas: MCM-41, MCM-41@salen, HMS-C12, HMS-C12@salen, HMS-C16, and HMS-C16@salen (Fig. 8) present the features:| Sample | d100 (nm) XRD | a0 (nm) | Pore volume (cm3 g−1) | dpore (nm) | SBET (m2 g−1) |
|---|---|---|---|---|---|
| MCM-41 | 4.09 | 4.724 | 0.733 | 2.75 | 964 |
| MCM-41@salen | 3.83 | 4.424 | 0.029 | — | 27 |
| HMS-C12 | 4.36 | 5.030 | 0.759 | 3.14 | 694 |
| HMS-C12@salen | 4.57 | 5.283 | 0.098 | 3.67 | 134 |
| HMS-C16 | 5.01 | 5.783 | 0.949 | 4.06 | 653 |
| HMS-C16@salen | 5.11 | 5.899 | 0.053 | — | 8 |
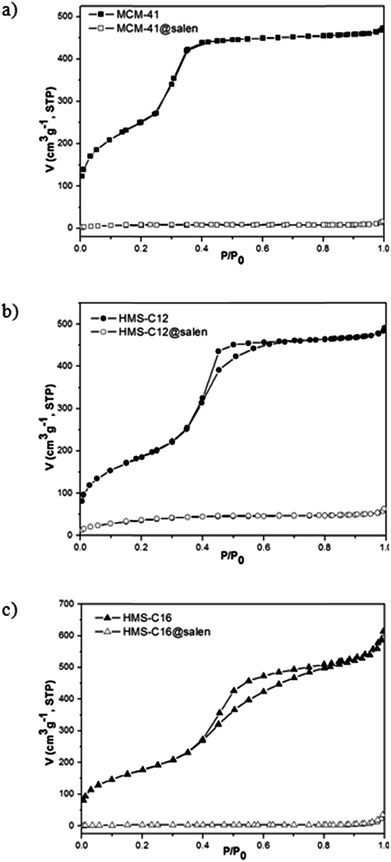 | ||
| Fig. 8 Nitrogen adsorption–desorption isotherms of: (a) MCM-41, MCM-41@salen, (b) HMS-C12, HMS-C12@salen, and (c) HMS-C16, HMS-C16@salen. | ||
• for all unmodified silicas (MCM-41, HMS-C12, HMS-C16), type IV isotherms characteristic for the mesoporous materials have been obtained.24,25 No hysteresis loop was observed for MCM-41, and this is a common feature for adsorbents having highly ordered mesopores, smaller than 4 nm width (Table 4).
• For HMS-C12 and HMS-C16, H2-type hysteresis loops are present and they are associated with slightly larger and less ordered mesoporous structures.
The pore volume and surface area of hemisalen functionalized silicas (MCM-41@salen, HMS-C12@salen, HMS-C16@salen) decrease dramatically in comparison with the parent MCM-41, HMS-C12, HMS-C16 silicas (Table 4). With the exception of HMS-C12@salen, which retains the framework mesoporosity and give a type IV isotherm (with capillary condensation step less visible), the other two silicas, MCM-41@salen and HMS-C16@salen, show type II isotherms and this fact could be attributed to pore blocking by salen ligands.
3.7. Pb(II) adsorption studies
Even at low concentration, Pb(II) damages tissues and organs such as kidneys, liver, reproductive and nervous system. Pb(II) has also indirect effects by inducing the production of reactive oxygen species (ROS) with negative consequences to cell macromolecules, proteins and polyunsaturated lipids.26 The application of conventional removal methods such as chemical precipitation,27 coagulation–flocculation,28 ion exchange,29 membrane filtration,30 reverse osmosis,31 electrochemical processes,32 is limited by their high costs and inefficiency, in the case of low levels of Pb(II). Adsorption is one of the most used conventional process to remediate wastewater due to its simplicity, ease and rapid application and manipulation, the potential use of many natural materials (low cost) and synthetic materials as adsorbents.33–39 Mesoporous materials (MCM-41/HMS) have high surface area, narrow pore size distribution, tunable texture and surface properties, and a relative chemical inertness. These properties recommend their applications in sorption, catalysis, separation, sensors, hosts and numerous other fields.40The MCM-41@salen, HMS-C12@salen, and HMS-C16@salen materials obtained by us have been used to uptake Pb(II) from synthetic aqueous systems and their sorption properties have been compared to those of parent MCM-41, HMS-C12, HMS-C16 mesoporous silicas.
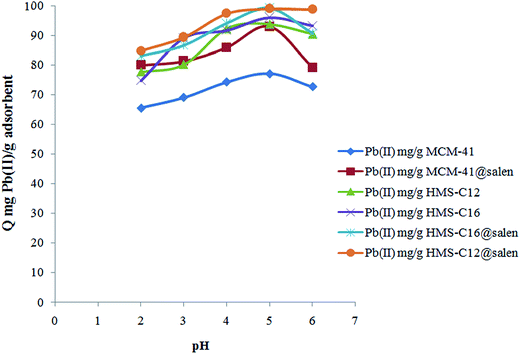
The influence of pH of solution in Pb(II) retention by MCM-41, MCM-41@salen, HMS-C12, HMS-C12@salen, HMS-C16 and HMS-C16@salen has been studied.
All the equilibrium tests were performed over the pH range of 2–6, by shaking 0.025 g of adsorbent with 25 mL of synthetic aqueous system of 100 mg L−1 Pb(II), at room temperature, for 24 hours and 150 rpm. The solutions were filtered and lead concentrations in the filtrate were determined by AAS, after reaching the equilibrium.
Fig. 9 presents the influence of pH of solution on sorption properties of our silica adsorbents, and their sorption capacity (Q) has been calculated as follows:
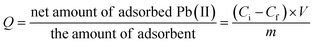 | (1) |
As can be observed (Fig. 9), the amount of Pb(II) retained by silicas increases with the increasing of the pH value. It reaches a maximum value at pH 5 and, then, it decreases slowly when pH solution changed from 5 to 6. At lower pH, a competition between Pb(II) ions and H+ from solution for the adsorbents sites may occur. The experiments were conducted at a maximum value of 6 for pH solution. Beyond this pH the formation of lead hydroxo-species (Pb(OH)+, Pb(OH)20, and Pb(OH)3−) occurs and the precipitation of the Pb(II)-species can compete with sorption.
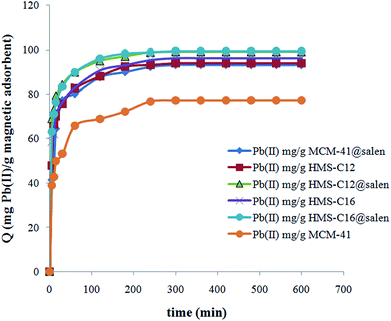
The equilibrium was reached after 300 minutes and the sorption process of Pb(II) onto silica adsorbents can be considered a fast process. The large number of the available active sites of adsorbents determines the adsorption to proceed rapidly in the early stages of the process. With a gradual decrease in the number of active sites, the adsorption process rate decreases.
The experimental values of sorption capacities are: 77.31 mg Pb(II) per g MCM-41, 93.28 mg Pb(II) per g MCM-41@salen, 94.07 mg Pb(II) per g HMS-C12, 99.27 mg Pb(II) per g HMS-C12@salen, 96.34 mg Pb(II) per g HMS-C16, and 99.58 mg Pb(II) per g HMS-C16@salen. As expected, the functionalization of MCM-41, HMS-C12 and HMS-C16 with salen-type ligands has as a result a higher sorption capacity of functionalized silicas. This result can be due to the fact that N and O atoms, from salen ligands grafted on the silica surface of MCM-41@salen, HMS-C12@salen, and HMS-C16@salen adsorbents, are Lewis donors complementary to borderline Lewis acceptors Pb(II) ions and, therefore, the organofunctionalized silicas are more efficient in Pb(II) adsorption.
Eqn (2) represents the linear expression of the Langmuir equation:42
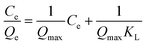 | (2) |
The Freundlich isotherm is frequently used to depict the sorption on heterogeneous surfaces, a process in which the concentration of adsorbate on the adsorbent surface increases with the adsorbate concentration and an infinite amount of adsorption can occur. The Freundlich model can be expressed as:
| Q = KFCe1/n | (3) |
The corresponding linear expression of Freundlich equation is:
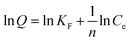 | (4) |
To study the thermodynamic equilibrium of Pb(II) sorption onto MCM-41, MCM-41@salen, HMS-C12, HMS-C12@salen, HMS-C16 and HMS-C16@salen, several experiments were performed with 5.28–100 mg L−1 Pb(II) solutions at room temperature and pH 5.
Langmuir and Freundlich isotherm models for Pb(II) sorption onto MCM-41, MCM-41@salen, HMS-C12, HMS-C12@salen, HMS-C16 and HMS-C16@salen using linear regression are shown in Fig. 11 and 12.
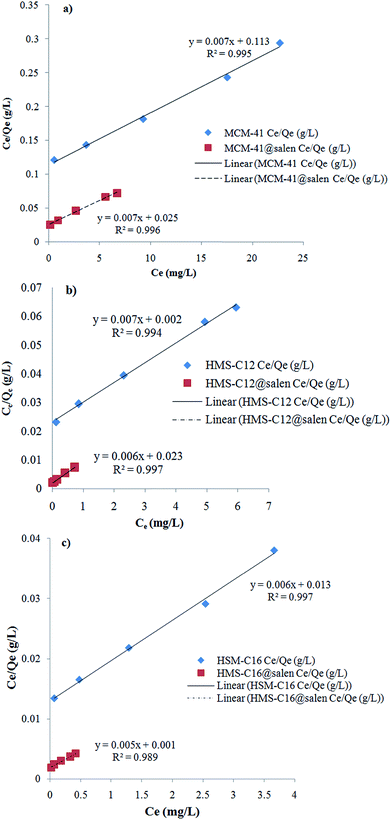 | ||
| Fig. 11 Langmuir linearized isotherms for Pb(II) sorption onto (a) MCM-41, MCM-41@salen; (b) HMS-C12, HMS-C12@salen, (c) HMS-C16, HMS-C16@salen. | ||
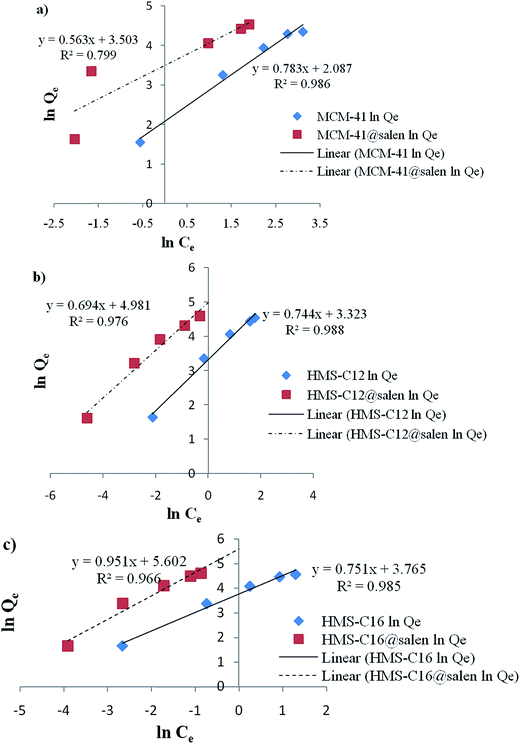 | ||
| Fig. 12 Freundlich linearized isotherms for Pb(II) sorption onto (a) MCM-41, MCM-41@salen; (b) HMS-C12, HMS-C12@salen, (c) HMS-C16, HMS-C16@salen. | ||
The Langmuir and Freundlich constants and the calculated coefficients for Pb(II) sorption onto MCM-41, MCM-41@salen, HMS-C12, HMS-C12@salen, HMS-C16 and HMS-C16@salen are listed in Table 5.
| Sample | Langmuir parameters | Freundlich parameters | ||||
|---|---|---|---|---|---|---|
| Qmax (mg g−1) | KL (L mg−1) | R2 | KF (mg g−1) | 1/n | R2 | |
| MCM-41 | 129.87 | 0.0677 | 0.9958 | 8.0679 | 0.7832 | 0.9860 |
| MCM-41@salen | 138.88 | 0.2845 | 0.9961 | 33.2249 | 0.5637 | 0.7995 |
| HMS-C12 | 131.57 | 3.8000 | 0.9946 | 27.7573 | 0.7440 | 0.9883 |
| HMS-C12@salen | 144.92 | 0.2974 | 0.9978 | 145.7073 | 0.6948 | 0.9760 |
| HMS-C16 | 149.25 | 0.5153 | 0.9970 | 43.1723 | 0.7517 | 0.9853 |
| HMS-C16@salen | 181.81 | 2.8947 | 0.9897 | 271.0220 | 0.9519 | 0.9660 |
It can be noticed that the correlation coefficients (R2) are closer to 1 for the Langmuir isotherm. Consequently, the Langmuir isotherm model is more appropriate to describe the sorption process for all the studied adsorbents rather than Freundlich isotherm model.
The maximum values of adsorption capacity (Langmuir model) of our unmodified (MCM-41, HMS-C12 and HMS-C16) and organofunctionalized (MCM-41@salen, HMS-C12@salen and HMS-C16@salen) silicas are similar to the adsorption capacities of different Pb(II) adsorbents reported in literature (Table 6).
| Adsorbent | Maximum adsorption capacity (mg g−1) | Reference |
|---|---|---|
| Chitosan-functionalized MCM-41 A | 90.91 | 43 |
| Large pore diameter MCM-41 | 174 | 44 |
| ALG-MCM-41 (ALG – alginate) | 140.84 | 45 |
| MCM-41 | 57.7 | 46 |
| Fe3O4@MCM-41-NH-oVan | 155.71 | 47 |
| Fe3O4@MCM-41-NH2 | 126.14 | 47 |
| Fe3O4@MCM-41 | 102.45 | 47 |
| MCM-41 | 129.87 | This work |
| MCM-41@salen | 138.88 | This work |
| HMS-C12 | 131.57 | This work |
| HMS-C12@salen | 144.92 | This work |
| HMS-C16 | 149.25 | This work |
| HMS-C16@salen | 181.81 | This work |
According to the pseudo-first-order model, the rate of adsorption on sorbent is proportional to the number of free active sites of sorbent.48 Lagergren eqn (5) is the mathematical expression of pseudo-first-order kinetic model:
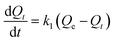 | (5) |
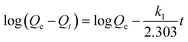 | (6) |
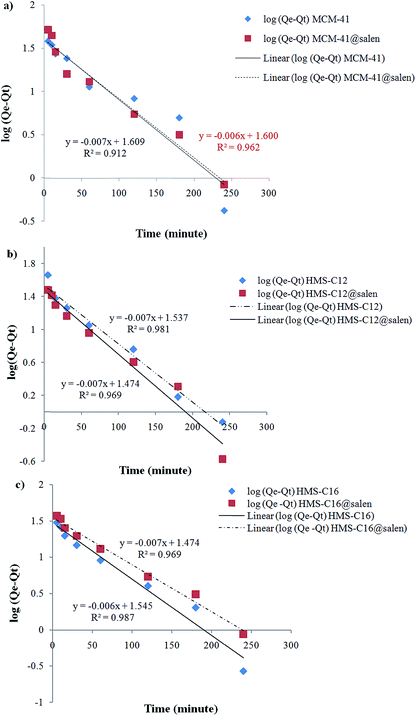 | ||
| Fig. 13 Pseudo-first order sorption kinetics of Pb(II) onto (a) MCM-41, MCM-41@salen; (b) HMS-C12, HMS-C12@salen; (c) HMS-C16 and HMS-C16@salen. | ||
The pseudo-second-order kinetic model is mathematically summarised as Ho eqn (7):
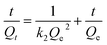 | (7) |
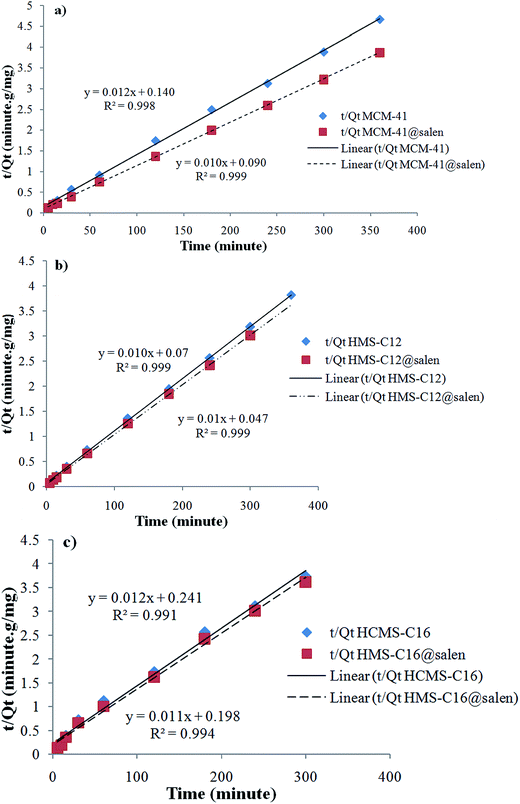 | ||
| Fig. 14 Pseudo-second order sorption kinetics of Pb(II) onto (a) MCM-41, MCM-41@salen; (b) HMS-C12, HMS-C12@salen; (c) HMS-C16 and HMS-C16@salen. | ||
The intraparticle diffusion model is depicted by the mathematical equation:
| Qt = kit0.5 | (8) |
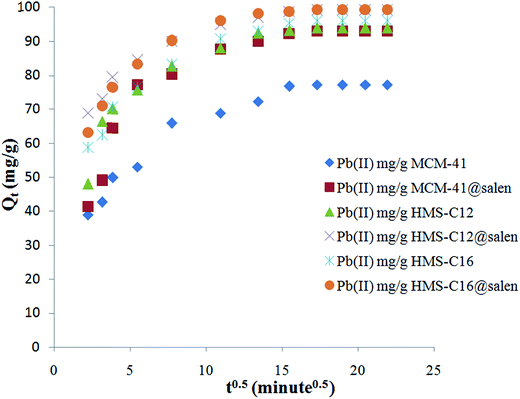 | ||
| Fig. 15 Intraparticle diffusion sorption kinetics of Pb(II) onto MCM-41, MCM-41@salen, HMS-C12, HMS-C12@salen, HMS-C16 and HMS-C16@salen. | ||
Since the plots for all six silicas are not linear and they do not pass through the origin, the intraparticle diffusion can not be considered the rate-limiting step for the Pb(II) sorption onto MCM-41, MCM-41@salen, HMS-C12, HMS-C12@salen, HMS-C16 and HMS-C16@salen samples. The plots are multilinear with two or three distinct regions. Thus, two or three different kinetic mechanisms are involved. According to literature data, it can be supposed that the initial curved region correlates with the external surface uptake, the second stage represents a gradual uptake reflecting intraparticle diffusion as the rate limiting step and the final plateau region shows the sorption equilibrium.50
Kinetic parameters show a good correlation with experimental kinetic values for pseudo-second order model (R2 > 0.991 for all six adsorbents – Table 7). Consequently, the chemisorption is considered to be the rate-limiting stage.49
| Sample | Pseudo-first order model | Pseudo-second order model | ||
|---|---|---|---|---|
| k1 (min−1) | R2 | k2 (g mg−1 min−1) | R2 | |
| MCM-41 | 1.6121 × 10−2 | 0.9124 | 1.1267 × 10−3 | 0.9989 |
| MCM-41@salen | 1.3818 × 10−2 | 0.9620 | 1.2222 × 10−3 | 0.9998 |
| HMS-C12 | 1.7963 × 10−2 | 0.9694 | 1.5451 × 10−3 | 0.9998 |
| HMS-C12@salen | 1.6351 × 10−2 | 0.9819 | 2.1277 × 10−3 | 0.9998 |
| HMS-C16 | 1.4969 × 10−2 | 0.9875 | 0.6072 × 10−3 | 0.9913 |
| HMS-C16@salen | 1.7963 × 10−2 | 0.9694 | 0.6896 × 10−3 | 0.9943 |
4. Conclusions
Organofunctionalized silicas, MCM-41@salen, HMS-C12@salen and HMS-C16@salen, obtained from parent mesoporous silicas: MCM-41, HMS-C12 and HMS-C16 (CTAB, dodecylamine and hexadecylamine, as templates) have been prepared and tested for the ability to adsorb Pb(II) from aqueous systems.Even though the framework mesoporosity partially decreased as a consequence of grafting the salen ligands onto the silica surface, the coordinative sites still remained accessible for binding heavy metal ions.
Kinetic studies have been performed to determine the kinetic model that describes the Pb(II) sorption onto MCM-41, MCM-41@salen, HMS-C12, HMS-C12@salen, HMS-C16 and HMS-C16@salen. The lead adsorption was well fitted by a pseudo second-order kinetic model (R2 > 0.991 for all six adsorbents) and a Langmuir isotherm model has been more appropriate to describe the sorption process for all the studied adsorbents rather than Freundlich isotherm model.
The values of Langmuir sorption capacity suggest that the obtained Schiff base-functionalized mesoporous silicas are relevant materials for the removal and recovery of Pb(II) and also could be used as effective adsorbents of Pb(II) from wastewaters.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
Financial support by the Ministry of National Education – Executive Unit for Financing Higher Education, Research and Development and Innovation (MEN – UEFISCDI), PNII – PCCA No. 92/2014 and Romanian Ministry of European Funds through the Financial Agreement POSDRU/159/1.5/S/132395 is gratefully acknowledged.References
- P. K. Jal, S. Patel and B. K. Mishra, Talanta, 2004, 62, 1005–1028 CrossRef CAS PubMed.
- A. Taguchi and F. Schüth, Microporous Mesoporous Mater., 2005, 77, 1–45 CrossRef CAS.
- K. Ariga, A. Vinu, J. P. Hill and T. Mori, Coord. Chem. Rev., 2007, 251, 2562–2591 CrossRef CAS.
- B. Samiey, C.-H. Cheng and J. Wu, Materials, 2014, 7, 673–726 CrossRef CAS PubMed.
- H. Yoshitake, J. Mater. Chem., 2010, 20, 4537–4550 RSC.
- H. Yoshitake, New J. Chem., 2005, 29, 1107–1117 RSC.
- A. Choplin and F. Quignard, Coord. Chem. Rev., 1998, 178–180, 1679–1702 CrossRef CAS.
- K. Parida, K. Gopal Mishra and S. K. Dash, Ind. Eng. Chem. Res., 2012, 51, 2235–2246 CrossRef CAS.
- T. Yokoi, Y. Kubota and T. Tatsumi, Appl. Catal., A, 2012, 421–422, 14–37 CrossRef CAS.
- E. M. Soliman, M. E. Mahmoud and S. A. Ahmed, Talanta, 2001, 54, 243–253 CrossRef CAS PubMed.
- G.-J. Kim and J.-H. Shin, Tetrahedron Lett., 1999, 40, 6827–6830 CrossRef CAS.
- A. R. Sarkar, P. K. Datta and M. Sarkar, Talanta, 1996, 43, 1857–1862 CrossRef CAS PubMed.
- V. Parvulescu, M. Mureseanu, A. Reiss, R. Ene and S.-H. Suh, Rev. Roum. Chim., 2010, 55, 1001–1008 CAS.
- M. Mureseanu, A. Reiss, I. Stefanescu, E. David, V. Parvulescu, G. Renard and V. Hulea, Chemosphere, 2008, 73, 1499–1504 CrossRef CAS PubMed.
- L. Dolatyari, M. R. Yaftian and S. Rostamnia, J. Environ. Manage., 2016, 169, 8–17 CrossRef CAS PubMed.
- X. Chen, A. Yamaguchi, M. Namekawa, T. Kamijo, N. Teramae and A. Tong, Anal. Chim. Acta, 2011, 696, 94–100 CrossRef CAS PubMed.
- L. Gao, Y. Wang, J. Wang, L. Huang, L. Shi, X. Fan, Z. Zou, T. Yu, M. Zhu and Z. Li, Inorg. Chem., 2006, 45, 6844–6850 CrossRef CAS PubMed.
- K. S. Abou-El-Sherbini, I. M. M. Kenawy, M. A. Hamed, R. M. Issa and R. Elmorsi, Talanta, 2002, 58, 289–300 CrossRef CAS PubMed.
- V. S.-Y. Lin, C.-Y. Lai, S. Jeftinija and D. M. Jeftinija, US Pat., 2009/0252811, 2009.
- P. T. Tanev and T. J. Pinnavaia, Chem. Mater., 1996, 8, 2068–2079 CrossRef CAS.
- V. Meynen, P. Cool and E. F. Vansant, Microporous Mesoporous Mater., 2009, 125, 170–223 CrossRef CAS.
- D. Banham, F. Feng, J. Burt, E. Alsrayheen and V. Birss, Carbon, 2010, 48, 1056–1063 CrossRef CAS.
- D. Banham, F. Feng, K. Pei, S. Ye and V. Birss, J. Mater. Chem. A, 2013, 1, 2812–2820 CAS.
- K. S. W. Sing, D. H. Everett, R. A. W. Haul, L. Moscou, R. A. Pierotti, J. Rouquerol and T. Siemieniewska, Pure Appl. Chem., 1985, 57(4), 603–619 CrossRef CAS.
- M. Thommes, K. Kaneko, A. V. Neimark, J. P. Olivier, F. Rodriguez-Reinoso, J. Rouquerol and K. S. W. Sing, Pure Appl. Chem., 2015, 87(9–10), 1051–1069 CAS.
- M. Cabral, A. Toure, G. Garçon, C. Diop, S. Bouhsina, D. Dewaele, F. Cazier, D. Courcot, A. Tall-Dia, P. Shirali, A. Diouf, M. Fall and A. Verdin, Environ. Pollut., 2015, 206, 247–255 CrossRef CAS PubMed.
- G. Németh, L. Mlinárik and Á. Török, J. Afr. Earth Sci., 2016, 122, 98–106 CrossRef.
- F. M. Pang, P. Kumar, T. T. Teng, A. K. M. Omar and K. L. Wasewar, J. Taiwan Inst. Chem. Eng., 2011, 42, 809–815 CrossRef CAS.
- D. S. Stefan and I. Meghea, C. R. Chim., 2014, 17, 496–502 CrossRef CAS.
- N. Abdullah, R. J. Gohari, N. Yusof, A. F. Ismail, J. Juhana, W. J. Lau and T. Matsuura, Chem. Eng. J., 2016, 289, 28–37 CrossRef CAS.
- K. Chon, J. Cho, S. J. Kim and A. Jang, Chemosphere, 2014, 117, 20–26 CrossRef CAS PubMed.
- S. Li, Y. Wei, Y. Kong, Y. Tao, C. Yao and R. Zhou, Synth. Met., 2015, 199, 45–50 CrossRef CAS.
- Y. Li, R. Zhang, X. Tian, C. Yang and Z. Zhou, Appl. Surf. Sci., 2016, 369, 11–18 CrossRef CAS.
- A. Behbahani, H. Eghbali, M. Ardjmand, M. M. M. Noufal, H. C. Williamson and O. Sayar, J. Environ. Chem. Eng., 2016, 4, 398–404 CrossRef CAS.
- M. E. Mahmoud, N. A. Fekry and M. M. A. El-Latif, Chem. Eng. J., 2016, 304, 679–691 CrossRef CAS.
- G. Zhou, C. Liu, L. Chu, Y. Tang and S. Luo, Bioresour. Technol., 2016, 219, 451–457 CrossRef CAS PubMed.
- Y. Zhu, D. Jiang, D. Sun, Y. Yan and C. Li, J. Environ. Chem. Eng., 2016, 4, 3570–3579 CrossRef CAS.
- I. Lakhdhar, D. Belosinschi, P. Mangin and B. Chabot, J. Environ. Chem. Eng., 2016, 4, 3159–3169 CrossRef CAS.
- G. Zhou, J. Luo, C. Liu, L. Chu, J. Ma, Y. Tang, Z. Zeng and S. Luo, Water Res., 2016, 89, 151–160 CrossRef CAS PubMed.
- M. R. El-Naggar, R. F. Aglan and M. S. Sayed, J. Environ. Chem. Eng., 2013, 1, 516–525 CrossRef CAS.
- F. Chen, M. Hong, W. You, C. Li and Y. Yu, Appl. Surf. Sci., 2015, 357, 856–865 CrossRef CAS.
- M. Rashid and F. K. Lutfullah, Journal of Water Process Engineering, 2014, 3, 53–61 CrossRef.
- Y. Guo, D. Liu, Y. Zhao, B. Gong, Y. Guo and W. Huang, J. Taiwan Inst. Chem. Eng., 2017, 71, 537–545 CrossRef CAS.
- S. A. Idris, C. M. Davidson, C. McManamon, M. A. Morris, P. Anderson and L. T. Gibson, J. Hazard. Mater., 2011, 185, 898–904 CrossRef CAS PubMed.
- H. Tavakoli, H. Sepehrian and R. Cheraghali, J. Taiwan Inst. Chem. Eng., 2013, 44, 343–348 CrossRef CAS.
- A. Heidari, H. Younesi and Z. Mehraban, Chem. Eng. J., 2009, 153, 70–79 CrossRef CAS.
- D. C. Culita, C. M. Simonescu, R.-E. Patescu, M. Dragne, N. Stanica and O. Oprea, J. Solid State Chem., 2016, 238, 211–220 CrossRef.
- T. Yousefi, S. Yavarpour, S. H. Mousavi, M. Torab-Mostaedi, R. Davarkhah and H. G. Mobtaker, Process Saf. Environ. Prot., 2015, 98, 211–220 CrossRef CAS.
- Y. S. Ho and G. McKay, Trans. Inst. Chem. Eng., 1998, 76, 332–340 CAS.
- R. S. Popuri, Y. Vijaya, V. M. Boddu and K. Abburi, Bioresour. Technol., 2009, 100, 194–199 CrossRef PubMed.
| This journal is © The Royal Society of Chemistry 2018 |