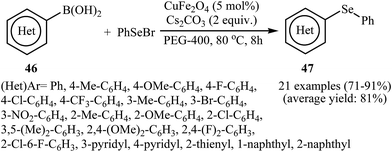Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Nanocatalysts for C–Se cross-coupling reactions
Khadijeh Didehban
 a,
Esmail Vessallya,
Akram Hosseinian
b,
Ladan Edjlali
*c and
Ebrahim Saedi Khosroshahic
a,
Esmail Vessallya,
Akram Hosseinian
b,
Ladan Edjlali
*c and
Ebrahim Saedi Khosroshahic
aDepartment of Chemistry, Payame Noor University, Tehran, Iran
bDepartment of Engineering Science, College of Engineering, University of Tehran, P.O. Box 11365-4563, Tehran, Iran
cDepartment of Chemistry, Tabriz Branch, Islamic Azad University, Tabriz, Iran. E-mail: l_edjlali@iaut.ac.ir
First published on 2nd January 2018
Abstract
This mini review is an attempt to highlight the most important contributions toward the applications of nanocatalysts in carbon–selenium cross-coupling reactions with the emphasis on the mechanistic aspects of the reactions. Literature has been surveyed from 2007 to 2017.
1. Introduction
Transition metal-catalyzed cross-coupling reactions have emerged as a powerful tool for the synthesis of a wide array of organic compounds with high atom and step economy.1–5 These reactions have a unique ability to form carbon–carbon and carbon–heteroatom (oxygen, nitrogen, sulfur, phosphorus, selenium, and tellurium) bonds under mild reaction conditions.6–9 In this context, extensive studies have been focused on the metal-catalyzed C–Se cross-coupling reactions for the synthesis of organo-selenium compounds,6 which are attractive synthetic targets for biochemists and synthetic chemists.10–13 Nanomaterial-based catalysts with high surface to volume ratio and reactive morphologies have been undergone tremendous growth during the past few decades.14–19 The use of nanoparticles as catalysts offers several advantages: (i) improving product yield and selectivity; (ii) shortening the reaction time; (iii) decreasing the catalyst loading; and (iv) recyclability of catalyst.16 Recently, the use of nanoparticles as eco-friendly catalysts in C–Se cross coupling reactions has attracted a lot of attention (Fig. 1). Therefore rapid recent advance in nanoparticles catalyzed C–Se cross coupling reactions prompts us to review the most important reports in this interesting field with the aim of stimulating new development in this exciting research area. As a continuation of our previous works,16 in this review we have classified these reactions based on the reactions type (e.g. C–Se coupling of alkynes with diorganyl diselenides and C–Se coupling of aryl/alkyl halides with Se0) with special emphasis on the mechanistic aspects of the reactions.2. C–Se coupling of aryl halides with diorganyl diselenides
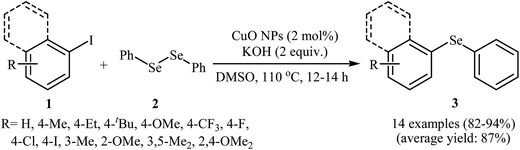
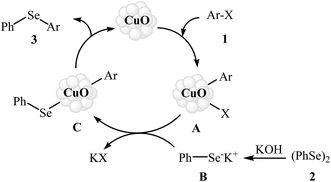
Aryl halides are among the most commonly employed nucleophilic partners in the C–Se cross-coupling reactions. Metal-catalyzed Se-arylation reactions of diorganyl diselenides with aryl halides has been the subject of a number of recent studies.20–23 However, the number of reported examples on nanosized metal catalyzed reactions between aryl halides and diorganyl diselenides are few and more studies are needed in this interesting research arena.In 2009, Rao and co-workers showed that nanocrystalline CuO was a viable catalyst for the Se-arylation of diaryl diselenides with aryl halides. Optimum yields attended the use of KOH as the base and DMSO as the solvent at 110 °C under an inert atmosphere. This optimized reaction condition was applied for the Se-arylation of a diverse set of aryl iodides 1 and diphenyl diselenides 2, and the reaction gave high to excellent yields of the expected diaryl selenides 3 (Scheme 1). However, in the cases of aryl bromides and chlorides the yields were rather poor. Noteworthy, other nano-metal oxides such as ZnO, NiO, Fe2O3, Bi2O3, and In2O3 were also found to promote this ligand-free reaction but in lower yields. According to the author proposed mechanistic pathway, the reaction may occur via oxidative addition followed by reductive elimination (Scheme 2).24
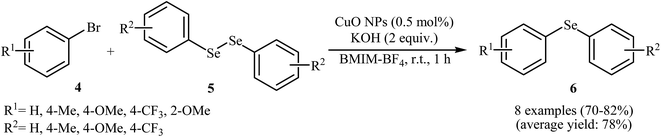
Subsequently, the group of Rodrigues further improved the efficiency of this reaction in terms of yield, reaction time and temperature by performing the process in BMIM-BF4 as a recyclable solvent. Thus, in the presence of 5 mol% of nano-CuO as catalyst and 2 equiv. of KOH as a base in BMIM-BF4 at room temperature, C–Se coupling of aryl bromides 4 with diaryl diselenides 5 furnished corresponding diaryl selenides 6 in good to high yields (Scheme 3). The authors disclosed that alkyl bromides were also efficient electrophilic partners for this reaction. It is noted that the solvent was recycled over four runs without significant loss of efficiency (78–82% yield over all runs).25
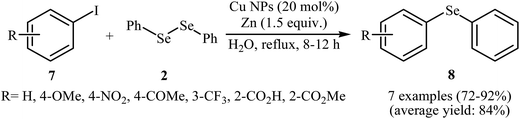
With the objective of designing a greener procedure to aryl selenides through aryl-selenylation of aryl halides, Saha and co-workers were able to demonstrate that a range of diaryl selenides 8 could be obtained from the reaction of aryl iodides 7 with diphenyl diselenides 2 in the most significant green solvent, water, employing copper(0) nanoparticle/Zn combination as cost-effective catalytic system under reflux conditions. Under optimized conditions [Cu NPs (20 mol%), Zn (1.5 equiv.), H2O, 8–12 h], the expected diaryl selenides 8 were obtained in good to excellent yields (Scheme 4). The authors nicely showed the application of this green and sustainable procedure for the high yielding syntheses of aryl vinyl selenides via treatment of vinyl bromides with corresponding diaryl diselenides. The author proposed a mechanistic pathway for the formation of diaryl selenides 8 is shown in Scheme 5.26 Very recently, this research team elegantly showed that iron(0) nanoparticles catalyzed reaction of aryl amines and diaryl diselenides resulted in the formation of corresponding diaryl selenides in good yields.27 It is noted that the same authors also was able to synthesis a series of functionalized selenophenes by CuO NPs catalyzed reaction of KSeCN with 1,3-dienyl bromides.28
 | ||
| Scheme 5 Mechanistic proposal for the reaction in Scheme 4. | ||
Nanosized copper ferrite (CuFe2O4 NPs) was also found to be an efficient catalyst for the carbon–selenium heterocoupling of aryl halides (Ar–I and Ar–Br) and diaryl diselenides in DMSO at 120 °C. Yields were good to almost quantitative (60–98% for 27 examples). The magnetic properties of CuFe2O4 NPs allow them to be separated from the reaction mixture and recovered with the simple use of an external magnet. The authors showed that this heterogeneous catalyst can be reused at least three times without significant loss in catalytic activity.29
3. C–Se coupling of aryl boronic acids with diorganyl diselenides
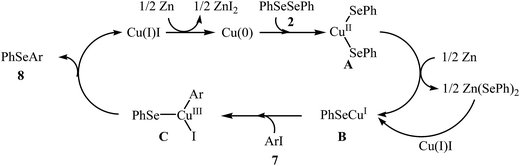
Nanostructured copper-based catalysts have recently attracted more and more attention because of their potential as viable alternatives to the expensive rare-earth metals used in many conventional commercial chemical processes.30,31 Interestingly, all works on the C–Se heterocoupling reactions between boronic acids and diorganyl diselenides catalyzed by nano-sized metal systems are based on the copper nanoparticles. In this section we have discussed the most important contributions toward the synthesis of aryl selenide derivatives from aryl boronic acids and diorganyl diselenides where copper or copper-based nanoparticles have been used as a catalyst.In 2009, Alves and co-workers reported for the first time the usefulness of Cu-based nanocatalysts for the heterocoupling reactions of boronic acids and diorganyl diselenides. Thus, in the presence of a catalytic amount of CuO nanoparticles (CuO NPs) as a recyclable catalyst in DMSO under air atmosphere, Se-arylation reactions of aryl and alkyl diselenides 9 with aryl boronic acids 10 furnished the corresponding selenides 11. High yields (75–98%) were achieved in reactions with both electron-rich and electron-poor boronic acids using only 3 mol% of the catalyst (Scheme 6). The scope of this ligand-free procedure was also extended to diphenyl ditelluride and resulted in 94% yield.32
 | ||
| Scheme 6 Nano-CuO catalyzed Se-arylation of aryl and alkyl diselenides 9 with aryl boronic acids 10. | ||
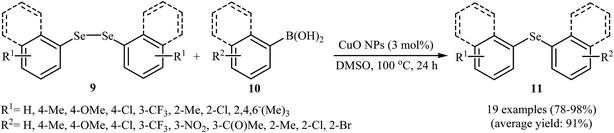
Shortly afterwards, the group of Nageswar found that the C–Se cross-coupling reactions of various boronic acids 12 and diorganyl diselenides 13 using magnetically separable CuFe2O4 nanocatalyst can lead to the synthesis of symmetrical and asymmetrical selenides 14 in high yields (Scheme 7). Ten organic solvents were examined and eco-friendly polyethylene glycol-400 (PEG-400) was found to be optimal for this reaction. Recyclability experiments showed that the catalytic efficiency decreased slightly during repeat runs and that this decrease correlated with a progressive increase in the size of nanoparticles due to their inherent agglomeration tendency (from 20 nm to 78 nm for the first and eighth runs, respectively).33 Similarly, copper nanoparticles [Cu3(BTC)2] (BTC = benzene-1,3,5-tricarboxylate) immobilized onto activated charcoal (AC) furnished the formation of C–Se bonds in a reaction involving boronic acids (aryl, vinyl, and alkenyl boronic acids) and diorganyl (phenyl and benzyl) diselenides. The reaction was run in DMSO and gave good to quantitative yields (61–100%).34
 | ||
| Scheme 7 Synthesis of selenides 14 through nano-CuFe2O4 catalyzed Se-arylation of diorganyl diselenides 13 with boronic acids 12 in PEG-400. | ||
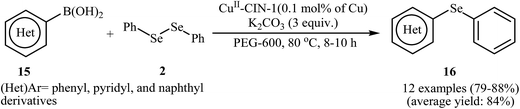
In 2014, Bhaumik and Islam along with their co-workers designed a novel reusable and non-leaching organo-copper catalyst (CuII-CIN-1), based on loading of bivalent Cu(II) over the surface of a nitrogen-rich porous covalent imine network material (CIN-1). This novel heterogeneous copper catalyst was successfully used for the synthesis of a series of organoselenides 16 via C–Se bond forming cross-coupling reaction between aryl boronic acids 15 and diphenyl diselenide 2 in PEG-600. This procedure furnished the desired selenides with use of a very low amount of Cu (0.1 mol%) in high yields (Scheme 8). The protocol showed excellent functional group tolerance, including alkoxy, bromo, cyano, nitro, formyl, and ester functionalities that would allow further elaboration of the products.35
Recently, the same group synthesized a novel heterogeneous copper catalyst Cu@PS-TSC by immobilizing Cu(OAc)2 onto the surface of a pyridine thiosemicarbazone functionalized polystyrene. The prepared catalyst was applied to the green and highly efficient synthesis of aryl selenides 18 through the reaction of aryl boronic acids 17 with diphenyl diselenide 2 in the presence of 3.0 equiv. of K2CO3 as a base in water (Scheme 9). The same catalysts have been also used to promote the phenyl tellurylation of aryl boronic acids with diphenylditelluride in PEG-600.36
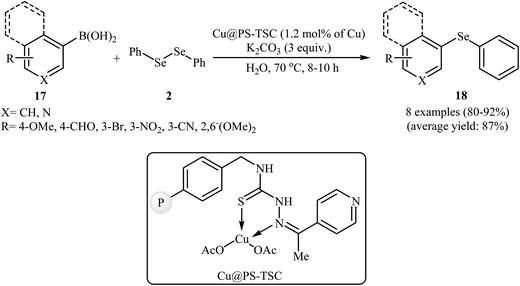 | ||
| Scheme 9 C–Se cross-coupling reaction between aryl boronic acids 17 with (PhSe)2 using Cu@PS-TSC as catalyst in water. | ||
4. C–Se coupling of inactive arene C–H bonds with diorganyl diselenides
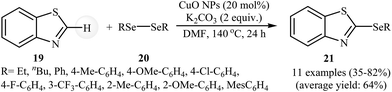
Transition metal-catalyzed direct C–H bond functionalization for the carbon–carbon and carbon–heteroatom bonds formation has emerged as a promising area in organic synthesis.37 In these sets of cross-coupling reactions, the inert C–H bonds can be treated as a functional group, similar to the traditionally used C-halide/pseudohalide bonds.9 In recent years, several progress has been made in the construction of new C–Se bonds according to this strategy.38The possibility of transition metal nanoparticles catalyzed selenylation of (hetero) arene C–H bonds with selenylating reagents was first realized by Rosario and co-workers, who synthesized a variety of 2-(organoselenyl)benzothiazole derivatives 21 from the reaction of unsubstituted benzothiazole 19 with diaryl diselenides 20 as nucleophilic partners in the presence of 20 mol% of CuO NPs and 1–2 equiv. of K2CO3 in DMF under an inert atmosphere. The reaction tolerated both aryl- and alkyl-diselenides and provided 2-(organoselenyl)benzothiazoles in moderate to high yields (Scheme 10). However, the reaction does not work well for benzyl diselenides. It is noted that the same reaction conditions were also successfully applied in the synthesis of 2-(organothio)benzothiazoles via coupling of benzothiazole with diorganyl disulfides.39
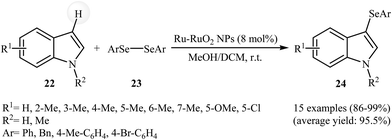
Very recently, Lin and co-workers reported the hydrothermal synthesis of ultrasmall Ru–RuO2 nanocrystals. The resulting material was employed as an efficient catalyst for the selenylation of heterocycles in a binary solvent MeOH/DCM under ambient conditions. Various indoles 22 and diaryl diselenides 23 could be used successfully in this protocol to afford the corresponding 3-(arylselanyl)indoles 24 in almost quantitative yields (Scheme 11). Extension to benzofuran and benzothiophene were attempted, but the corresponding 3-(phenylselanyl)benzofuran and 3-(phenylselanyl)benzothiophene were isolated in less than 5% yields. It is interesting to note that neither Ru powder nor RuO2 exhibited catalytic activity in the selenylation reaction. Thus, density functional theory (DFT) calculations were carried out to understanding the synergistic effect of metallic Ru and RuO2. The results revealed that a diaryl diselenide can initially be adsorbed on metallic Ru sites and cleaved into two ArSe* species, which then migrate to RuO2 sites and react with the nucleophilic partner to achieve the selenylation of heterocycles.40
5. C–Se coupling of alkynes with diorganyl diselenides
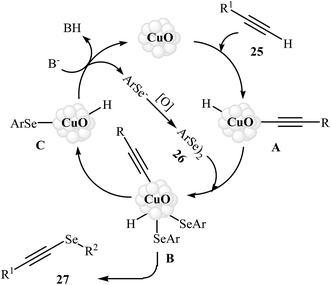
Alkynyl selenides as an important framework in organic synthesis41–44 have been synthesized, among various methods, by reaction of alkynyl halides with nucleophilic species of selenium generated from a metal;45 and selenodecarboxylation of arylpropiolic and cinnamic acid promoted by iodosobenzene diacetate.46 These various methods are of a limited application as in some of these procedures, the reaction conditions are harsh, while some procedures needs toxic metals or moisture sensitive organometallic reagents. These compounds can also be conveniently synthesized through the metal-catalyzed coupling of terminal alkynes with diselenides.47 Copper is the most popular metal for this reaction. In 2012, the group of Braga demonstrated for the first time the usefulness of nanoscale catalysts for the C–Se cross-coupling of terminal alkynes with diorganyl diselenides to obtain alkynyl selenides. Thus, in the presence of CuO nanopowder as a recyclable catalyst and K2CO3 as a base in DMSO at 80 °C, C–Se cross-coupling reaction between terminal alkynes 25 and diorganyl diselenides 26 furnished corresponding alkynyl selenides 27. The reaction tolerated both aromatic and aliphatic alkynes and selenides and gave final products in good to high yields (Scheme 12). Catalyst recycling for this system demonstrated very good yields of 71–79% over four cycles. In Scheme 13, a reasonable mechanism for this transformation is suggested. Terminal alkyne 25 reacts with the catalyst to give the [alkenyleCu] cluster A, which reacts with diorganyl diselenide 26 to produce the intermediate B. Subsequently, reductive elimination of this intermediate affords the observed alkynyl selenides 27 and the species C. The last step of the transformation involves the regeneration of the copper catalyst by reaction of C with base. It is noted that the authors also successfully extended this procedure to the synthesis of alkynyl telluride derivatives through the reaction of corresponding alkynes with diorganyl ditellurides.48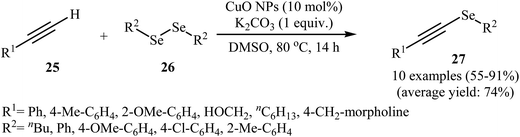 | ||
| Scheme 12 Synthesis of alkynyl selenides 27 via nano-CuO catalyzed C–Se cross coupling of terminal alkynes 25 with diorganyl diselenides 26. | ||
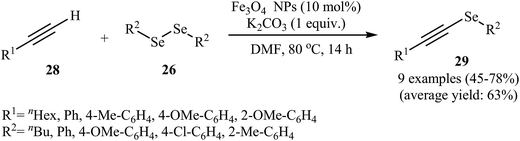
In another attempt, the same research team introduced magnetite ferroferric oxide (Fe3O4) nanoparticles as a novel catalyst for this transformation. The authors studied the reaction variables such as base, solvent, and temperature and found that performing the reaction in the presence of a stoichiometric amount of K2CO3 in DMF at 80 °C was the optimum reaction condition. Using optimum reaction condition the generality of the reaction was studied (Scheme 14). The results indicated that aromatic alkynes gave higher yields than aliphatic alkynes and the diorganyl diselenides reacted in the order of electron-poor aromatic diselenides > electron-rich aromatic diselenides > aliphatic diselenides. The broad substrate scope, high reactivity, the simplicity of catalyst separation (using external magnets), and excellent reusability (up to four reaction runs) of the catalyst were the merits of this protocol.49
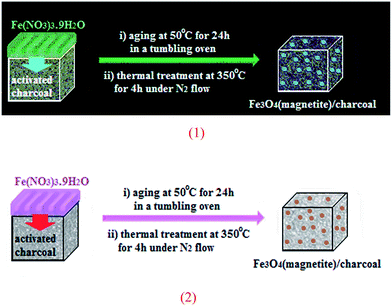
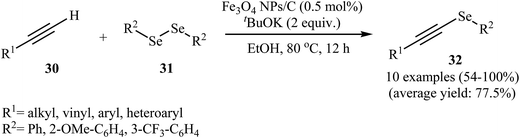
Using solid-state grinding method, Park et al. supported Fe3O4 nanoparticles on charcoal (Fig. 2). From high-resolution transmission electron microscopy (HRTEM) images, it is noted that the average size of the magnetite nanoparticles is around 13 nm. The porous-charcoal-based Fe3O4 catalyst was successfully used for C–Se cross-coupling reaction of terminal alkynes 30 with diorganyl diselenide 31. The reaction took place in refluxing ethanol in the presence of very low loading of catalyst (0.5 mol%) and generally provided corresponding alkynyl selenides 32 in good to quantitative yields (Scheme 15). Noteworthy, the catalyst could be reused for five reaction runs with only slight loss of the catalytic performance.50
6. C–Se coupling of aryl halides/boronic acids with selenourea
In 2010, Reddy and co-workers reported an elegant copper nanoparticles catalyzed C–Se cross-coupling reaction of aryl halides/boronic acids 33 with selenourea 34 into symmetrical diaryl selenides 35 employing CuO nanoparticles as the catalyst, KOH as the base, and DMSO as the solvent. The reaction was carried out under a nitrogen atmosphere at 80 °C and provided expected selenides in moderate to excellent yields (Scheme 16). The results established that the aryl halides reacted in the order of Ar–I > Ar–Br ≫ Ar–Cl. The results also showed that aryl iodides gave better results than aryl boronic acids. The authors assume that the mechanism of this process involves the initial formation of the intermediate A through the oxidative addition of aryl halide 33 to the CuO nanoparticles. The reaction of this intermediate with selenourea 34 gives the intermediate B, with undergoes reductive elimination to produce intermediate C. Subsequently, hydrolysis of this intermediate in the reaction mixture gives a benzeneselenate moiety and urea. Next, oxidative addition of aryl iodide 33 to CuO nanoparticles affords the intermediate A which undergoes reaction with benzeneselenate anion to give intermediate D. Finally, reductive elimination of this intermediate produces the observed diaryl selenides 35 (Scheme 17).51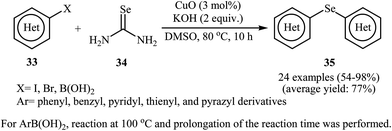 | ||
| Scheme 16 Nano-CuO catalyzed synthesis of symmetrical diaryl selenides 35 from aryl halides/boronic acids 33 and selenourea 34. | ||
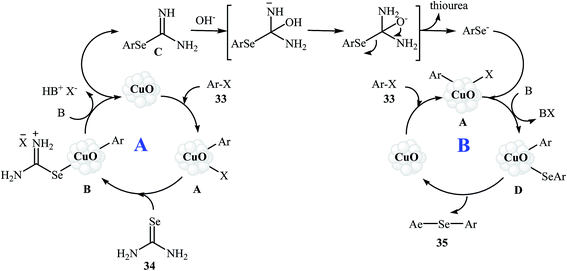 | ||
| Scheme 17 Mechanistic proposal for the reaction in Scheme 16. | ||
7. C–Se coupling of acyl chlorides with diorganyl diselenides
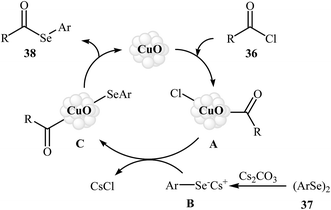
Nanoparticle catalyzed C–Se cross-coupling of acyl chlorides and diorganyl diselenides has been scarcely studied; in fact, only one example of such a reaction was reported in the literature. In this report, Braga's research team synthesized a series of synthetically important selenoesters 38 through treatment of acyl chlorides 36 with diaryl diselenides 37 using 5 mol% of inexpensive nanocrystalline CuO as the catalyst in BMIM-PF6 ionic liquid as a recyclable solvent. The reaction was run at 80 °C and generally provided the desired selenoesters 38 in moderate to high yields (Scheme 18). This heterogeneous catalyst was chemically stable and could be recovered and reused at least four times without a decrease in the catalytic activity and selectivity. Moreover, the ionic liquid was reusable and could be applied for four reaction runs with slight loss of efficiency. The author proposed mechanism for this reaction is given in Scheme 19.52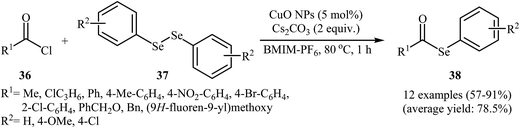 | ||
| Scheme 18 Nano-CuO catalyzed synthesis of selenoesters 38 from acyl chlorides 36 and diaryl diselenides 37. | ||
8. C–Se coupling of aryl/alkyl halides with Se0
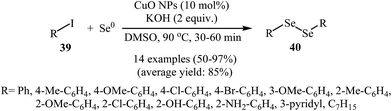
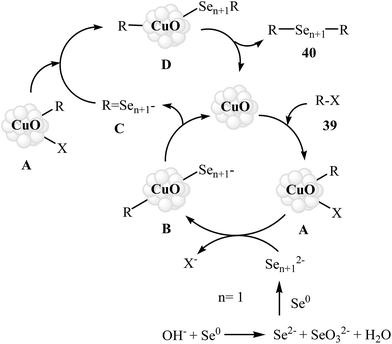
Organic diselenides are extremely important key intermediates in the organic synthesis because of their stability, ease of handling, and reactivity.53,54 Although a variety of methods have been developed for the synthesis of organic diselenide derivatives,55 they invariably limited by require strong reducing agents, by require highly toxic gas, or both. Therefore, the development of methods that benefit from environmentally friendly and sustainable starting material for the construction of titled compounds is highly desirable.In 2010, Rodrigues–Braga and co-workers reported a novel, simple, and honorable synthetic protocol for the C–Se cross-coupling reaction of alkyl, aryl, and heteroaryl iodides 39 with elemental selenium (Se0) using CuO NPs as the catalyst and KOH as the base in DMSO at 60 °C for 1 h to achieve corresponding symmetrical aryl and alkyl diselenides 40 in good to excellent yields (Scheme 20). This ligand-free procedure was also successfully extended to the synthesis of symmetrical diorganyl ditellurides via the reaction of organoiodides with elemental tellurium. On the basis of previous reports, the authors proposed that the reaction starts with the generation of complex A via the oxidative addition of organohalide 39 to CuO NPs, and meanwhile, formation of active dichalcogenolate anion from starting elemental chalcogen in the presence of superbasic DMSO–KOH system, and then the generation of the complex B via ligand exchange of intermediate A with the dichalogenolate anion. Subsequently, reductive elimination of intermediate B gives the initial coupling product C and regenerate the CuO NPs. In the next step, complex C by reaction with another complex A generates the complex D. Finally, the reductive elimination of D affords the observed products 40 with regeneration of the CuO nanoparticles (Scheme 21).56 Subsequently, the same group improved the efficiency of this reaction in terms of catalyst loading, reaction time and temperature by performing the process under microwave irradiation.57
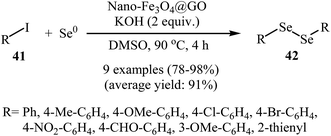
Inspired by these works, Kassaee and co-workers found that graphene oxide based nano-Fe3O4 (nano-Fe3O4@GO) can efficiently catalyze the cross-coupling of (hetero)aryl iodides 41 with Se0. Various organic solvents were examined and DMSO was found to be optimal for this reaction. Under optimized conditions [nano-Fe3O4@GO (7 mol%), KOH (2 equiv.), DMSO, 90 °C, 4 h], the corresponding symmetrical diorganyl diselenides 42 were obtained in high to almost quantitative yields (Scheme 22). Noteworthy, the catalyst was reusable and preserved its catalytic activity after recycling for four runs of reaction.54
9. Miscellaneous reactions
Due to the scarce availability and air instability of arylselenols (ArSeH), there are fewer reports on cross-coupling reactions of this type of reagent as compared to other selenium reagents. Consequently, nanoparticles catalyzed C–Se cross-coupling reactions employing arylselenols as electrophilic partners have been very limitedly studied. In 2007, a simple heterogeneous Ni-based nanocatalyst was successfully used by Ananikov, Orlov, and Beletskaya in regioselective hydroselenation of terminal alkynes 43 with benzeneselenol 44. The reaction was performed at room temperature under solvent-free conditions and provided syn-addition products 45 in good yields (Scheme 23). However, in the case of methyl propiolate corresponding trans-alkene was observed as the main product. The nanostructured Ni catalyst also showed high activity and excellent syn-selectivity for a range of internal alkynes. The author proposed mechanism for this reaction is depicted in Scheme 24.58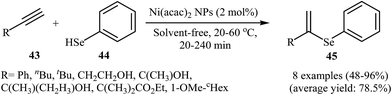 | ||
| Scheme 23 Regioselective hydroselenation of terminal alkynes 43 with benzeneselenol 44 reported by Ananikov. | ||
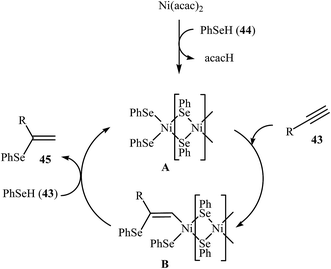 | ||
| Scheme 24 Mechanistic proposal for the reaction in Scheme 23. | ||
In 2012, the group of Nageswar described a nano-sized bimetallic-catalyzed C–Se cross-coupling of organoboranes 46 with phenylselenyl bromide (PhSeBr) into unsymmetrical diorganyl selenides 47 employing CuFe2O4 as the catalyst in PEG-400. The reaction is noteworthy in that both aryl and heteroaryl boronic acids are tolerated (Scheme 25). The results demonstrated that this heterogeneous catalyst can be easily separated and be used several times with the only slight loss of the catalytic activity.59
10. Conclusion
Organic selenides are of great importance in medicinal and organic chemistry, which not only found as skeleton units in biologically active molecules and therapeutic products, but also serves as building blocks in organic synthesis, and play a prominent role as organocatalysts for asymmetric synthesis. Thus a number of methods have been developed for their synthesis. Among various preparation methods of titled compounds, metal catalyzed C–Se cross-coupling reaction is considered one of the most interesting approaches due to the production of various type of selenides with high atom and step economy under environmentally friendly conditions. As illustrated, the use of nano-sized metal catalysts in C–Se cross-coupling reactions due to their high surface to volume ratio, allows for rapid transformations under mild conditions, with the benefits of high product yield and ease of catalyst separation. Despite the significant achievements during the past few years in this field, many challenges still remain to be overcome: (a) almost all of the nanoparticle catalyzed C–Se cross-coupling reactions are limited to the use of monometallic catalysts. Thus the exploration of bi-metallic, tri-metallic, and multi-metallic nanoparticles are highly desirable in term of the cost of preparation of the catalysts;60 (b) the number of reported examples in some reactions such as coupling of acyl chlorides with diorganyl diselenides and coupling of aryl halides/boronic acids with selenourea are narrow and there is an urgent need to study the scope and limitations of these reactions; and (c) other reactions such as coupling of diaryliodonium salts with various selenium surrogates should be explored. We conclude this review by hoping that it will stimulate researchers to further research in this interesting field.Conflicts of interest
There are no conflicts to declare.References
- N. Miyaura and A. Suzuki, Chem. Rev., 1995, 95, 2457–2483 CrossRef CAS.
- F.-S. Han, Chem. Soc. Rev., 2013, 42, 5270–5298 RSC.
- C. Valente, S. Çalimsiz, K. H. Hoi, D. Mallik, M. Sayah and M. G. Organ, Angew. Chem., Int. Ed., 2012, 51, 3314–3332 CrossRef CAS PubMed.
- C. M. So and F. Y. Kwong, Chem. Soc. Rev., 2011, 40, 4963–4972 RSC.
- M. Abdoli and H. Saeidian, J. Sulfur Chem., 2015, 36, 556–582 CrossRef CAS.
- I. P. Beletskaya and V. P. Ananikov, Chem. Rev., 2011, 111, 1596–1636 CrossRef CAS PubMed.
- F. M. Tappe, V. T. Trepohl and M. Oestreich, Synthesis, 2010, 3037–3062 CAS.
- C. F. Lee, Y. C. Liu and S. S. Badsara, Chem.–Asian J., 2014, 9, 706–722 CrossRef CAS PubMed.
- M. Abdoli, Z. Mirjafary, H. Saeidian and A. Kakanejadifard, RSC Adv., 2015, 5, 44371–44389 RSC.
- C. W. Nogueira, G. Zeni and J. B. Rocha, Chem. Rev., 2004, 104, 6255–6286 CrossRef CAS PubMed.
- S. Cao, F. A. Durrani and Y. M. Rustum, Clin. Cancer Res., 2004, 10, 2561–2569 CrossRef CAS PubMed.
- E. E. Alberto, V. d. Nascimento and A. L. Braga, J. Braz. Chem. Soc., 2010, 21, 2032–2041 CrossRef CAS.
- M. G. Szczepina, B. D. Johnston, Y. Yuan, B. Svensson and B. M. Pinto, J. Am. Chem. Soc., 2004, 126, 12458–12469 CrossRef CAS PubMed.
- V. Polshettiwar and R. S. Varma, Green Chem., 2010, 12, 743–754 RSC.
- C. W. Lim and I. S. Lee, Nano Today, 2010, 5, 412–434 CrossRef CAS.
- (a) E. Vessally, M. Babazadeh, A. Hosseinian, S. Arshadi and L. Edjlali, J. CO2 Util., 2017, 21, 491–502 CrossRef CAS; (b) E. Vessally, RSC Adv., 2016, 6, 18619–18631 RSC; (c) E. Vessally, A. Hosseinian, L. Edjlali, A. Bekhradnia and M. D. Esrafili, RSC Adv., 2016, 6, 71662–71675 RSC; (d) E. Vessally, L. Edjlali, A. Hosseinian, A. Bekhradnia and M. D. Esrafili, RSC Adv., 2016, 6, 49730–49746 RSC; (e) E. Vessally, A. Hosseinian, L. Edjlali, A. Bekhradnia and M. D. Esrafili, RSC Adv., 2016, 6, 99781–99793 RSC; (f) E. Vessally, S. Soleimani-Amiri, A. Hosseinian, L. Edjlali and A. Bekhradnia, RSC Adv., 2017, 7, 7079–7091 RSC; (g) E. Vessally, A. Hosseinian, L. Edjlali, A. Bekhradnia and M. D. Esrafili, Curr. Org. Synth., 2017, 14, 557–567 CrossRef CAS; (h) S. Arshadi, E. Vessally, L. Edjlali, R. Hosseinzadeh-Khanmiri and E. Ghorbani-Kalhor, Beilstein J. Org. Chem., 2017, 13, 625–638 CrossRef CAS PubMed; (i) S. Arshadi, E. Vessally, L. Edjlali, E. Ghorbani-Kalhor and R. Hosseinzadeh-Khanmiri, RSC Adv., 2017, 7, 13198–13211 RSC; (j) S. Arshadi, E. Vessally, M. Sobati, A. Hosseinian and A. Bekhradnia, J. CO2 Util., 2017, 19, 120–129 CrossRef CAS; (k) E. Vessally, R. Hosseinzadeh-Khanmiri, E. Ghorbani-Kalhor, M. Es'haghi and A. Bekhradnia, RSC Adv., 2017, 7, 19061–19072 RSC; (l) S. Soleimani-Amiri, E. Vessally, M. Babazadeh, A. Hosseinian and L. Edjlali, RSC Adv., 2017, 7, 19061–19072 RSC; (m) S. Arshadi, E. Vessally, A. Hosseinian, S. Soleimani-Amiri and L. Edjlali, J. CO2 Util., 2017, 21, 108–118 CrossRef CAS; (n) E. Vessally, S. Soleimani-Amiri, A. Hosseinian, L. Edjlali and M. Babazadeh, J. CO2 Util., 2017, 21, 342–352 CrossRef CAS; (o) E. Vessally, A. Hosseinian, L. Edjlali, E. Ghorbani-Kalhor and R. Hosseinzadeh-Khanmiri, J. Iran. Chem. Soc., 2017, 14, 2339–2353 CrossRef CAS; (p) E. Vessally, K. Didehban, M. Babazadeh, A. Hosseinian and L. Edjlali, J. CO2 Util., 2017, 21, 480–490 CrossRef CAS; (q) M. Babazadeh, S. Soleimani-Amiri, E. Vessally, A. Hosseinian and L. Edjlali, RSC Adv., 2017, 7, 43716–43736 RSC.
- A. Fihri, M. Bouhrara, B. Nekoueishahraki, J.-M. Basset and V. Polshettiwar, Chem. Soc. Rev., 2011, 40, 5181–5203 RSC.
- R. Narayanan, Molecules, 2010, 15, 2124–2138 CrossRef CAS PubMed.
- L. Shiri, A. Ghorbani-Choghamarani and M. Kazemi, Aust. J. Chem., 2016, 69, 585–600 CrossRef CAS.
- B. C. Ranu, T. Mandal and S. Samanta, Org. Lett., 2003, 5, 1439–1441 CrossRef CAS PubMed.
- N. Taniguchi and T. Onami, J. Org. Chem., 2004, 69, 915–920 CrossRef CAS PubMed.
- A. L. Braga, P. H. Schneider, M. W. Paixao and A. M. Deobald, Tetrahedron Lett., 2006, 47, 7195–7198 CrossRef CAS.
- S. Narayanaperumal, E. E. Alberto, K. Gul, O. E. Rodrigues and A. L. Braga, J. Org. Chem., 2010, 75, 3886–3889 CrossRef CAS PubMed.
- V. P. Reddy, A. V. Kumar, K. Swapna and K. R. Rao, Org. Lett., 2009, 11, 951–953 CrossRef CAS PubMed.
- D. Singh, E. E. Alberto, O. E. D. Rodrigues and A. L. Braga, Green Chem., 2009, 11, 1521–1524 RSC.
- A. Saha, D. Saha and B. C. Ranu, Org. Biomol. Chem., 2009, 7, 1652–1657 CAS.
- S. Panja, P. Maity, D. Kundu and B. C. Ranu, Tetrahedron Lett., 2017, 58, 3441–3445 CrossRef CAS.
- S. Panja, D. Kundu, R. Roy and B. C. Ranu, Org. Lett., 2014, 16, 4122–4125 CrossRef PubMed.
- K. Swapna, S. N. Murthy and Y. V. D. Nageswar, Eur. J. Org. Chem., 2011, 1940–1946 CrossRef CAS.
- M. B. Gawande, A. Goswami, F.-X. Felpin, T. Asefa, X. Huang, R. Silva, X. Zou, R. Zboril and R. S. Varma, Chem. Rev., 2016, 116, 3722–3811 CrossRef CAS PubMed.
- N. K. Ojha, G. V. Zyryanov, A. Majee, V. N. Charushin, O. N. Chupakhin and S. Santra, Coord. Chem. Rev., 2017, 353, 1–57 CrossRef CAS.
- D. Alves, C. G. Santos, M. W. Paixão, L. C. Soares, D. de Souza, O. E. Rodrigues and A. L. Braga, Tetrahedron Lett., 2009, 50, 6635–6638 CrossRef CAS.
- D. Kundu, N. Mukherjee and B. C. Ranu, RSC Adv., 2013, 3, 117–125 RSC.
- B. Mohan, C. Yoon, S. Jang and K. H. Park, ChemCatChem, 2015, 7, 405–412 CrossRef CAS.
- S. Roy, T. Chatterjee, B. Banerjee, N. Salam, A. Bhaumik and S. M. Islam, RSC Adv., 2014, 4, 46075–46083 RSC.
- S. Roy, T. Chatterjee and S. M. Islam, Tetrahedron Lett., 2015, 56, 779–783 CrossRef CAS.
- (a) C. S. Yeung and V. M. Dong, Chem. Rev., 2011, 111, 1215–1292 CrossRef CAS PubMed; (b) J. Yamaguchi, A. D. Yamaguchi and K. Itami, Angew. Chem., Int. Ed., 2012, 51, 8960–9009 CrossRef CAS PubMed; (c) P. B. Arockiam, C. Bruneau and P. H. Dixneuf, Chem. Rev., 2012, 112, 5879–5918 CrossRef CAS PubMed.
- (a) A. Mandal, H. Sahoo and M. Baidya, Org. Lett., 2016, 18, 3202–3205 CrossRef CAS PubMed; (b) S. Yu, B. Wan and X. Li, Org. Lett., 2014, 17, 58–61 CrossRef PubMed; (c) W. Jin, P. Zheng, W. T. Wong and G. L. Law, Asian J. Org. Chem., 2015, 4, 875–878 CrossRef CAS.
- A. R. Rosario, K. K. Casola, C. E. Oliveira and G. Zeni, Adv. Synth. Catal., 2013, 355, 2960–2966 CrossRef CAS.
- M. Lin, L. Kang, J. Gu, L. Dai, S. Tang, T. Zhang, Y. Wang, L. Li, X. Zheng and W. Zhu, Nano Res., 2017, 10, 922–932 CrossRef CAS.
- A. L. Braga, A. Reckziegel, C. C. Silveira and J. V. Comasseto, Synth. Commun., 1994, 24, 1165–1170 CrossRef CAS.
- M. Tingoli, M. Tiecco, L. Testaferri, A. Temperini, G. Pelizzi and A. Bacchi, Tetrahedron, 1995, 51, 4691–4700 CrossRef CAS.
- Y. Ma and C. Qian, Tetrahedron Lett., 2000, 41, 945–947 CrossRef CAS.
- A. H. Yu, R. H. Qiu, N. Y. Tan, L. F. Peng and X. H. Xu, Chin. Chem. Lett., 2011, 22, 687–690 CrossRef CAS.
- (a) A. L. Braga, A. Reckziegel, P. H. Menezes and H. A. Stefani, Tetrahedron Lett., 1993, 34, 393–394 CrossRef CAS; (b) J.-L. Zhang and Z.-C. Chen, Synth. Commun., 1997, 27, 3757–3762 CrossRef CAS.
- J. P. Das, U. K. Roy and S. Roy, Organometallics, 2005, 24, 6136–6140 CrossRef CAS.
- (a) E. Mohammadi and B. Movassagh, Tetrahedron Lett., 2014, 55, 1613–1615 CrossRef CAS; (b) B. Movassagh, A. Yousefi, B. Z. Momeni and S. Heydari, Synlett, 2014, 25, 1385–1390 CrossRef; (c) B. Movassagh and M. Navidi, Chin. Chem. Lett., 2012, 23, 1035–1038 CrossRef CAS; (d) D. Cook, A. Hill and D. á. James Wilson, J. Chem. Soc., Dalton Trans., 1998, 1171–1174 RSC.
- M. Godoi, E. W. Ricardo, T. E. Frizon, M. S. Rocha, D. Singh, M. W. Paixao and A. L. Braga, Tetrahedron, 2012, 68, 10426–10430 CrossRef CAS.
- M. Godoi, D. G. Liz, E. W. Ricardo, M. S. Rocha, J. B. Azeredo and A. L. Braga, Tetrahedron, 2014, 70, 3349–3354 CrossRef CAS.
- B. Mohan, J. C. Park and K. H. Park, ChemCatChem, 2016, 8, 2345–2350 CrossRef CAS.
- V. P. Reddy, A. V. Kumar and K. R. Rao, J. Org. Chem., 2010, 75, 8720–8723 CrossRef CAS PubMed.
- D. Singh, S. Narayanaperumal, K. Gul, M. Godoi, O. E. D. Rodrigues and A. L. Braga, Green Chem., 2010, 12, 957–960 RSC.
- (a) Y. Nishibayashi, J. D. Singh, S.-i. Fukuzawa and S. Uemura, J. Org. Chem., 1995, 60, 4114–4120 CrossRef CAS; (b) M. Tiecco, L. Testaferri, C. Santi, M. Tiecco, L. Testaferri, C. Santi, C. Tomassini, F. Marini, L. Bagnoli and A. Temperini, Tetrahedron: Asymmetry, 2000, 11, 4645–4650 CrossRef CAS; (c) M. Tiecco, L. Testaferri, C. Santi, C. Tomassini, F. Marini, L. Bagnoli and A. Temperini, Chem.–Eur. J., 2002, 8, 1118–1124 CrossRef CAS PubMed; (d) N. Ma, Y. Li, H. Xu, Z. Wang and X. Zhang, J. Am. Chem. Soc., 2009, 132, 442–443 CrossRef PubMed; (e) J. Ścianowski, A. J. Pacuła, M. Zielińska-Błajet and A. Wojtczak, New J. Chem., 2016, 40, 6697–6705 RSC.
- M. Z. Kassaee, E. Motamedi, B. Movassagh and S. Poursadeghi, Synthesis, 2013, 45, 2337–2342 CrossRef CAS.
- V. A. Potapov, Organic Diselenides, Ditellurides, Polyselenides and Polytellurides. Synthesis and Reactions, in Organic Selenium and Tellurium, ed. Z. Rappoport, J. F. Liebman and I. Marek, Wiley, New York, 2013 Search PubMed.
- D. Singh, A. M. Deobald, L. R. Camargo, G. Tabarelli, O. E. Rodrigues and A. L. Braga, Org. Lett., 2010, 12, 3288–3291 CrossRef CAS PubMed.
- G. V. Botteselle, M. Godoi, F. Z. Galetto, L. Bettanin, D. Singh, O. E. Rodrigues and A. L. Braga, J. Mol. Catal. A: Chem., 2012, 365, 186–193 CrossRef CAS.
- V. P. Ananikov, N. V. Orlov and I. P. Beletskaya, Organometallics, 2007, 26, 740–750 CrossRef CAS.
- K. H. V. Reddy, G. Satish, K. Ramesh, K. Karnakar and Y. Nageswar, Chem. Lett., 2012, 41, 585–587 CrossRef CAS.
- R. Narayanan, Molecules, 2010, 15, 2124–2138 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2018 |