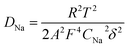Open Access Article
Open Access ArticleEffect of Ti-doping on the electrochemical performance of sodium vanadium(III) phosphate
Bao Zhanga,
Tao Zenga,
Yi Liub and
Jia-feng Zhang *a
*a
aSchool of Metallurgy and Environment, Central South University, Changsha, 410083, PR China. E-mail: yjyzjf@csu.edu.cn
bTianjin Lishen Battery Joint-Stock Co., Ltd, Tianjin, 300384, PR China
First published on 1st February 2018
Abstract
Na3V2−xTix(PO4)3 (x = 0.00, 0.05, 0.10, and 0.15) was successfully synthesized by a conventional solid-state route. The XRD results show that Ti is incorporated in the lattice of Na3V2(PO4)3 and the tetragonal structure has not been changed after doping. Among all the composites, the Na3V1.9Ti0.1(PO4)3 composite delivers the highest discharge capacity of 114.87 mA h g−1 at 0.1C and possesses a capacity retention of 96.23% after 20 cycles at 0.1C, demonstrating the better rate performance and cycle stability in the potential range of 2.0–3.8 V. Electrochemical impedance spectroscopy (EIS) results reveal that the Na3V1.9Ti0.1(PO4)3 composite has a lower charge transfer resistance and a higher Na-ion diffusion coefficient compared to other composites. The results indicate that Ti-doping in Na3V2(PO4)3 can effectively enhance the electrochemical performance of this tetragonal compound, especially at a high charge/discharge rate.
1 Introduction
Since the combustion of fossil fuels results in air pollution and increases the greenhouse effect, much attention has been focused on the exploitation of new energy sources. Development of new alternative energy storage systems with low cost, high safety and environmentally friendly electrode materials are critical to meet our rising energy demands and, in particular, to facilitate the adoption of renewable energy generation. Large-scale electric energy storage is a key enabler for the use of renewable energy.1 Lithium ion batteries with high energy and power densities have been considered as the best power sources for portable equipment today.2–4 LiFePO4, FePO4, LiMnPO4, Li(Fe,Mn)PO4, LiCoPO4, LiNiPO4, Li3V2(PO4)3, and LiCo1/2Ni1/2PO4, LiCoO2, LiNi1/3Co1/3Mn1/3O2 have been researched as cathode materials for Li-ion batteries.5–16 However, there is serious concern about the availability of lithium owing to the amount of lithium reserves in the earth, especially for large-scale applications.17,18 On this occasion, the eco-friendly sodium-ion batteries have become the focus in the field of energy and environment due to the abundance of sodium.17,19–21In recent years, many electrode materials such as NaxCoO2,22 Na0.44MnO2,23 NaV6O15,24 Na1−xNi0.5Mn0.5O2,25 NaNi1/3Mn1/3Co1/3O2,26 NaFePO4,27 NaFeSO4F,28 NaV1−xCrxPO4F,29 Na2FePO4F,30 Na3V2(PO4)2F3,31 Na2Ti3O7,32,33 and Na2C8H4O4 (ref. 34) have been extensively investigated for sodium ion batteries. Recently, NASICON (for Na+ superionic conductor)-type electrode materials that can generate large interstitial spaces, through which sodium ions can diffuse, have aroused much interest of many researchers.35,36
Na3V2(PO4)3 (NVP), which is a polyanionic material, has been greatly exploited37 for its several advantages: (1) good ionic mobility, (2) a high theoretical specific capacity (117.6 mA h g−1), (3) two very flat and stable redox electric potentials (3.4 and 1.6 V vs. Na+/Na) based on the V4+/V3+ and V3+/V2+ redox couples, respectively. However, the stability of the structure of NVP also needs to be improved. It has been reported that transition metal doping is a useful way to improve the electrochemical performance of Li3V2(PO4)3.38 Several cations, such as Fe3+,39 Cr3+,40 Mg2+,41 Co2+,42 and Ti4+,43 can be doped at the V site in the LVP, resulting in improved utilization of materials, high rate capabilities, electric conductivity, and cyclic stability. In the present work, we investigate aliovalent substitution of VIII with TiIV in NVP. So far, no studies have been done to dope titanium in NVP. We examine the effect of low levels of Ti doping on the electrochemical performance of NVP. The addition of titanium did not influence the structure of NVP, but the particle size was reduced and the electrical conductivity was improved, resulting in high capacity and rate capability.
2 Experiment
2.1 Synthesis
The Na3V2−xTix(PO4)3 (x = 0.00, 0.05, 0.10, 0.15) composites were synthesized by a conventional solid-state route. NaH2PO4·2H2O and V2O5 were used as the starting materials and ethanedioic acid was used as the reductant. In a typical synthesis process, V2O5, NaH2PO4·2H2O, nano-size TiO2, and H2C2O4 (in the appropriate amount) were mixed and ball-milled with alcohol media in a container of a planetary ball-mill at 300 rpm for 5 h. After ball-milling, the obtained mixture was dried at 75 °C, then calcinated at 400 °C for 4 h and heated up to 750 °C and held there for 10 h in an atmosphere-controlled furnace under argon. The heating rate was 5 °C min−1. The Na3V2−xTix(PO4)3 (x = 0.00, 0.05, 0.10, 0.15) composites was obtained.2.2 Material characterization
Thermogravimetric (TG/DSC) analysis of the mixture was measured on a SDT Q600 TG-DTA apparatus at temperature between 25 and 900 °C at a heating rate of 5 °C min−1 under argon flow. The powder X-ray diffraction (XRD, Rint-2000, Rigaku, Japan) measurement using Cu Kα radiation was employed to identify the crystalline phases of the synthesized composites. XRD Rietveld refinement was analyzed by software (MAUD). The morphologies and chemical compositions of the composites were observed by scanning electron microscopy (SEM, JEOL, JSM-5600LV, Japan) and a energy dispersive X-ray (EDX) detector.2.3 Electrochemical performance tests
The cathodes used for the electrochemical tests consisted of 80 wt% active material, 10 wt% acetylene black, and 10 wt% polyvinylidene fluoride (PVDF), which was used as the binder. N-Methyl-2-pyrrolidone (NMP) was used as a solvent and an aluminum foil as a current collector (∼2.0 mg cm−2). It was dried in a vacuum oven at 120 °C for 4 h. Electrodes of the desired size were then punched out from the dried slurry. Electrochemical cells were assembled in a glove box filled with high-purity argon; pure sodium foil was used as the counter electrode. The electrolyte solution was 1 M NaClO4 (EC:DEC) (1% VC) soaked in glass fiber separators (GF/D-Whatman). The test cells were galvanostatically cycled between 2.0 and 3.5 V. Na+/Na at various C-rates based on the theoretical capacity of 118 mA h g−1. The cyclic voltammetry (CV) profiles were recorded with a LK2005A electrochemical workstation (Lanlike Co., China). Electrochemistry impedance spectroscopy (EIS) was measured with an electrochemical workstation (CHI 660D) by applying an AC voltage of 5 mV in the frequency range from 100 kHz to 0.04 Hz.3 Results and discussion
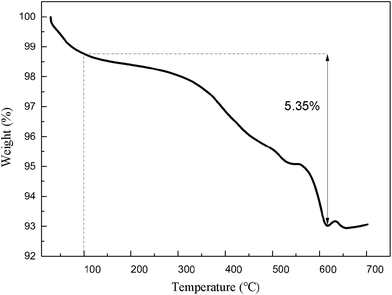
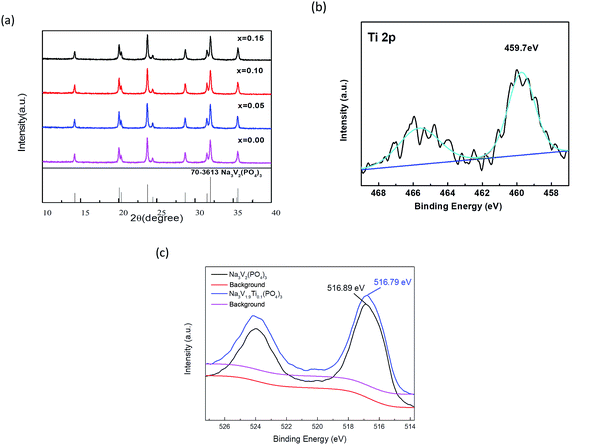
Fig. 1 shows the TGA curve of Na3V1.9Ti0.1(PO4)3. The TGA test was taken in the oxygen. It was observed that the carbon content in the Na3V1.9Ti0.1(PO4)3 was 5.35%.XRD patterns of Na3V2−xTix(PO4)3 (x = 0.00, 0.05, 0.10, 0.15) with various amounts of Ti doping are shown in Fig. 2a. All the peaks are indexed to a single phase of tetragonal crystal type Na3V2(PO4)3, which is consistent with the references. It is clear that no impurity peaks such as Ti-doped composites are detected, indicating that the as-prepared composites are pure phase and the structure is unchanged after Ti doping. These sharp peaks in the patterns suggest good crystallinity of the powders.
The XPS spectra of Ti 2p (Na3V1.9Ti0.1(PO4)3) was shown in Fig. 2b. The binding energy of Ti in the picture to be 459.7 eV, matched well with some previous work,44 which indicate the valence of Ti in Na3V1.9Ti0.1(PO4)3 is +4. The XPS spectra of V 2p (Na3V2(PO4)3 and Na3V1.9Ti0.1(PO4)3) was shown in Fig. 2c. It is obviously that the binding energy of V in Ti-doping sample (Na3V1.9Ti0.1(PO4)3) is 516.79 eV and the binding energy of V in Na3V2(PO4)3 is 516.89 eV, which means that the doping of titanium reduce the valence of V. The result means that Ti is doped in the V site.45
Fig. 3 show the refined XRD data for Na3V2−xTix(PO4)3 (x = 0.00, 0.05, 0.10, 0.15). The calculated pattern matched well with the PDF standard card. The refined lattice parameters and atomic coordination are listed in Table 1. The Rw of Na3V2−xTix(PO4)3 (x = 0.00, 0.05, 0.10, 0.15) were 9.9%, 11.7%, 10.2% and 11.4%, respectively. The decrease of cell volume can be attributed to the insertion of Ti, which is consistent with the previous result.44 As can be seen in Table 1 that the Na3V1.9Ti0.1(PO4)3 had the smallest volumes. This indicates that the Na3V1.9Ti0.1(PO4)3 composites had smallest particle size.
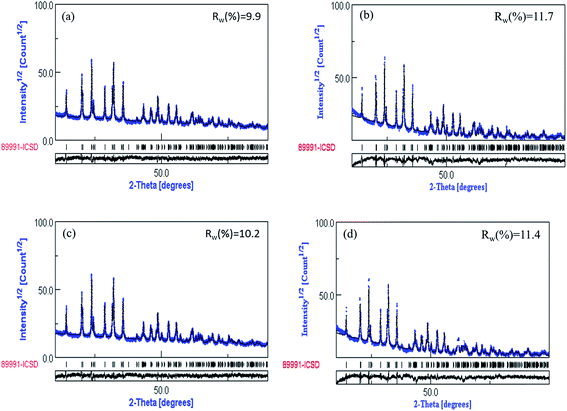 | ||
| Fig. 3 Refinement XRD profiles for Na3V2−xTix(PO4)3. (a) x = 0.00, (b) x = 0.05, (c) x = 0.10, (d) x = 0.15. | ||
| a (Å) | c (Å) | Vol. (Å3) | Rw (%) | |
|---|---|---|---|---|
| Na3V2(PO4)3 | 8.72436 | 21.80602 | 1659.8 | 9.9 |
| Na3V1.95Ti0.05(PO4)3 | 8.72275 | 21.80401 | 1659.0 | 11.7 |
| Na3V1.9Ti0.1(PO4)3 | 8.71929 | 21.80088 | 1657.4 | 10.2 |
| Na3V1.85Ti0.15(PO4)3 | 8.72137 | 21.80135 | 1658.3 | 11.4 |
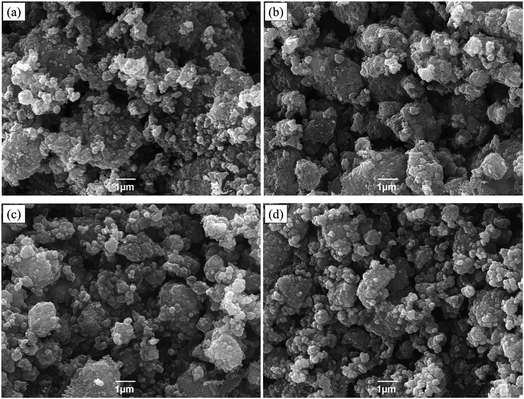
Fig. 4 shows the SEM images of Na3V2−xTix(PO4)3 (x = 0.00, 0.05, 0.10, 0.15). It's observed that the primary particles are in nano-scale and agglomerated to form secondary particles, showing similar irregular granular shapes. The results indicate a more homogeneous distribution of Ti-doping composites than the pristine one.
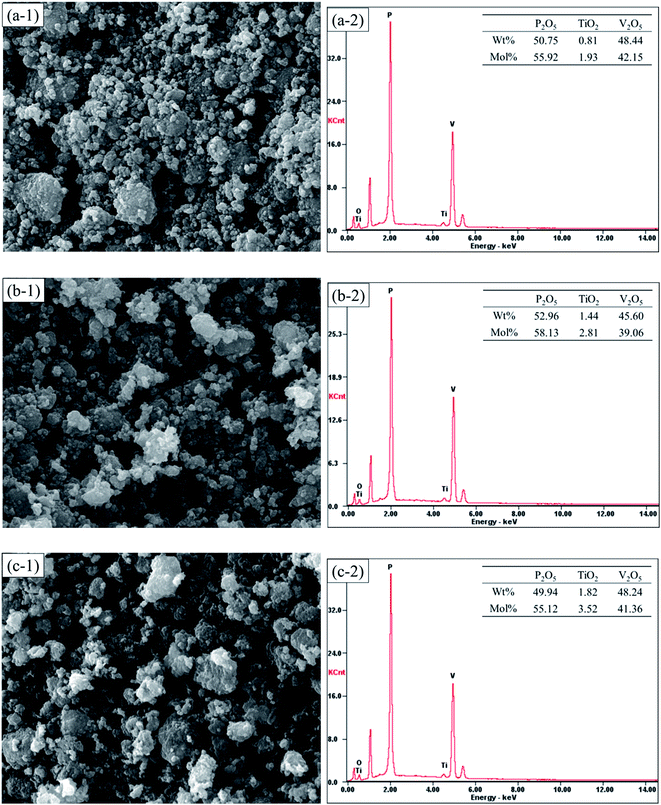
Fig. 5 shows the SEM images of Na3V2−xTix(PO4)3 (x = 0.00, 0.05, 0.10, 0.15). EDX was used to investigate the existed component elements in the composites. It can be seen in Fig. 5 that the element Ti is existed in Na3V2−xTix(PO4)3 (x = 0.00, 0.05, 0.10, 0.15), and the contents of Ti gradually increases with the rise of Ti concentrations, indicating that the Na3V2−xTix(PO4)3 (x = 0.00, 0.05, 0.10, 0.15) composites was successfully synthesized.
Fig. 6 shows the initial charge–discharge voltage profiles of Na3V2−xTix(PO4)3 (x = 0.00, 0.05, 0.10, 0.15) at a current rate of 0.1C in the potential range of 2.0–3.8 V. The curves indicate that two Na ions were extracted from/inserted into Na3V2(PO4)3 per formula unit during the charging/discharging processes, which lasted for 10 h.46 It can be seen from Fig. 6 that the charge curves for all cathodes exhibited a plateau at approximately 3.4 V and so did the corresponding discharge curves; these were attributed to the two-phase transitions occurring during the electrochemical reaction, which was agreed well with the CV curves as showed in Fig. 7. The initial discharge capacity of Na3V2−xTix(PO4)3 composites (x = 0.00, x = 0.05, x = 0.10, x = 0.15) are 102.58 mA h g−1, 107.63 mA h g−1, 114.87 mA h g−1 and 111.95 mA h g−1, respectively. The coulombic efficiency of the initial cycle is about 94.9%, 96.7%, 98.9% and 97.8%, respectively. As can be seen from Fig. 6 that the capacities of Ti-doped Na3V2−xTix(PO4)3 (x = 0.00, 0.05, 0.10, 0.15) are higher than pristine Na3V2(PO4)3. In addition, the voltage difference of Ti-doped Na3V2−xTix(PO4)3 is smaller than that of pristine Na3V2(PO4)3, resulting from the lower electrode polarization. It is clearly seen from Table 3 that Na3V1.9Ti0.1(PO4)3 exhibits the lowest polarization potential of 0.03 V with the best electrochemical performance and the highest initial discharge capacity, indicating that Ti-doping does not block the tunnels of Na ions and probably increases the charge and discharge capacities and charge–discharge efficiency.
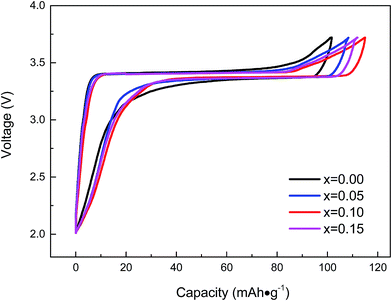 | ||
| Fig. 6 Initial charge–discharge voltage profiles of Na3V2−xTix(PO4)3 (x = 0.00, 0.05, 0.10, 0.15) at 0.1C. | ||
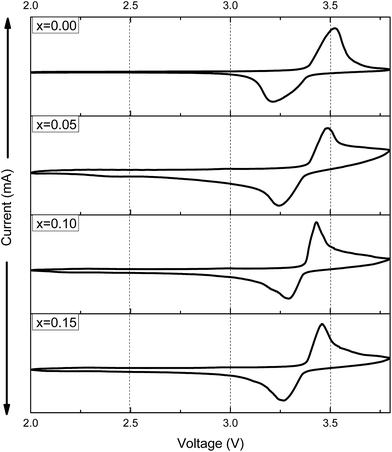
CV measurement was carried out at a scan rate of 0.1 mV s−1 to provide more information about the electrochemical properties, the second voltammograms (CV) of Na3V2−xTix(PO4)3 (x = 0.00, 0.05, 0.10, 0.15) samples in the voltage range of 2.0–3.8 V are presented in Fig. 7. It can be observed that the CV curves are very similar. There are one oxidation peak and one reduction peak, corresponding to the relative Na+ ion extraction and reinsertion. However, the Na+ ion extraction and insertion processes for Ti doped samples are more stable, which is ascribed to relatively sharper peaks. Moreover, the Na3V1.9Ti0.1(PO4)3 sample present the smallest potential differences between anodic and cathodic peaks, which is consistent with the result in charge–discharge voltage profiles, indicating the good reversibility of the Na+ ion extraction/re-insertion and lower ohmic resistance in the electrode.
As shown in Fig. 8, five current densities, i.e. 0.1C, 0.5C, 1C, 2C and 5C, are employed to evaluate the rate capability of Na3V2−xTix(PO4)3 (x = 0.00, 0.05, 0.10, 0.15) (charging current density is 0.1C). It can be observed that the profiles reveal a large plateau at 3.4 V for discharge and the rate performance of the composites is highly affected by the Ti-doped content. Increasing Ti-doping amount first led to the enhancement of rate capability. It can be seen in Fig. 8 that Ti-doping samples show better rate performance than pristine Na3V2(PO4)3. Moreover, Na3V1.9Ti0.1(PO4)3 shows the best rate performance, delivering a capacity of 114.87 mA h g−1, 113.75 mA h g−1, 111.07 mA h g−1, 106.67 mA h g−1, and 98.63 mA h g−1. Moreover, the discharge voltage plateau of Na3V1.9Ti0.1(PO4)3 is still higher than 3.3 V at 5C rate, indicating that Na3V1.9Ti0.1(PO4)3 has a lower polarization. The results prove that Ti-doping can enhance the electrochemical performance.
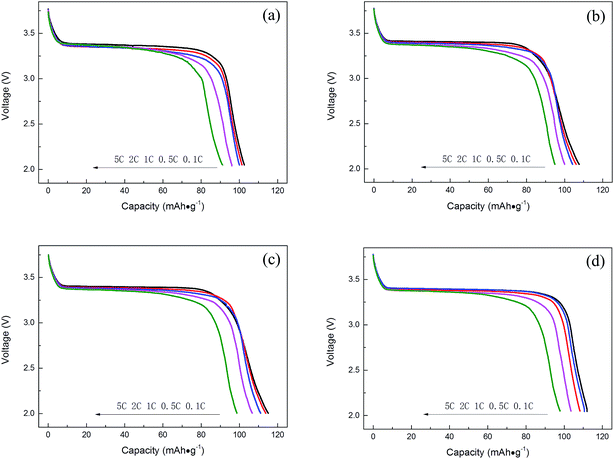 | ||
| Fig. 8 The discharge curves of Na3V2−xTix(PO4)3 (x = 0.00, 0.05, 0.10, 0.15) at various rates. (a) x = 0.00, (b) x = 0.05, (c) x = 0.10, (d) x = 0.15. | ||
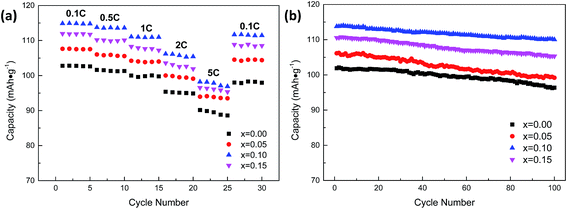
All the Na3V2−xTix(PO4)3 (x = 0.00, 0.05, 0.10, 0.15) samples were cycled at five current densities (0.1, 0.5, 1, 2, and 5C) between 2.0 and 3.8 V, and the results are shown in Fig. 9a. Each current density was tested for 5 cycles. It can be observed that the Ti-doping samples show a better rate performance than pristine Na3V2(PO4)3. Even so, a further increase of the titanium content was not as advantageous as for the former samples. Moreover, the rate performance of Na3V1.9Ti0.1(PO4)3 demonstrates the best among all samples, and it keeps a 97.02% at all rates, higher than that of the others. The sample with suitable Ti-doping content (x = 0.10) shows the best rate performance compared with other samples. The results proved that Ti-doping can improve the rate performance of Na3V2(PO4)3.
Fig. 9b shows the cycling performance of Na3V2−xTix(PO4)3 (x = 0.00, 0.05, 0.10, 0.15) electrodes at 0.5C. As can be seen from Fig. 9b that the Ti-doping samples, expect Na3V2−xTix(PO4)3 (x = 0.05), show a better cycling performance than pristine Na3V2(PO4)3. The discharge capacity of Na3V2−xTix(PO4)3 composites (x = 0.00, x = 0.05, x = 0.10, x = 0.15) are 101.83 mA h g−1, 106.02 mA h g−1, 113.75 mA h g−1 and 110.59 mA h g−1, respectively, and 94.59%, 93.44%, 96.70% and 95.25% remained after 100 cycles in the range of 2.0–3.8 V at 0.5C. The results indicate that suitable Ti-doping content (x = 0.10, x = 0.15) can enhance the cycling performance of Na3V2(PO4)3.
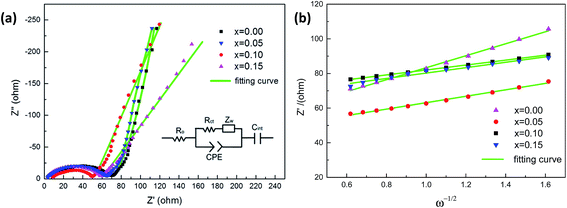
Fig. 10a presents the AC impedance spectra of Na3V2−xTix(PO4)3 (x = 0.00, 0.05, 0.10, 0.15). The spectras show an intercept at a high frequency, followed by a depressed semicircle in the high-middle frequency region, and a straight line in the low frequency region. An equivalent circuit was conducted to refine the spectra. In the circuit, Ro represents the ohmic resistance of the electrolyte and electrode, as the intercept impedance on the Z-axis. CPE represents the double layer capacitance. Rct is the charge transfer resistance, Zw represents the diffusion-controlled Warburg impedance, and Cint indicates the capacitance caused by the cumulation or loss of Na+ in the crystal of electrode material. The parameters obtained from the equivalent circuit fitting are shown in Table 2. It is found that the Rct of Na3V2−xTix(PO4)3 composites with x = 0.00, x = 0.05, x = 0.10, x = 0.15 are 61.01 Ω, 59.35 Ω, 44.75 Ω and 53.87 Ω, respectively, which indicates that Ti-doping can increase the charge transfer speed of the electrochemical reaction. Moreover, the minimum Rct of Na3V1.9Ti0.1(PO4)3 results in its best electrochemical performance among all the samples.
| Samples | Ro (Ω cm−2) | Rct (Ω cm−2) | DNa (10−16) |
|---|---|---|---|
| x = 0.00 | 7.36 | 61.01 | 5.98 |
| x = 0.05 | 2.44 | 59.35 | 11.49 |
| x = 0.10 | 7.41 | 44.75 | 14.53 |
| x = 0.15 | 2.20 | 53.87 | 13.27 |
| Samples | x = 0.00 | x = 0.05 | x = 0.10 | x = 0.15 |
|---|---|---|---|---|
| Overpotential (V) | 0.11 | 0.06 | 0.03 | 0.05 |
The diffusion coefficient of lithium ions (DNa) are calculated by equations. The results are also summarized in Table 2.
4 Conclusion
The Na3V2−xTix(PO4)3 (x = 0.00, 0.05, 0.10, 0.15) was successfully synthesized by a conventional solid state route. Based on the XRD refinement results, Ti has incorporated into the lattice of Na3V2(PO4)3. The amount of Ti doping has an important impact on electrochemical performance. In the electrochemical test, Na3V1.9Ti0.1(PO4)3 exhibits the best specific capacity of 114.87 mA h g−1 at 0.1C in the potential range of 2.0–3.8 V and possessing the capacity retention of 96.70% after 100 cycles at 0.5C. The Ti-added Na3V2(PO4)3 composites also show higher rate capability and cycle performance compared with the pristine Na3V2(PO4)3. The results of CV and EIS revealed that the doping titanium at an appropriate amount could increase the diffusion coefficient of sodium ions and result in the improvement of the reversibility of the materials. The results indicate that the Ti doping is an effective approach to achieve excellent electrochemical performance for the sodium ion pyrophosphate.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We gratefully acknowledge the financial support for this work of the National Natural Science Foundation of China (General Program) under grant number 51272290 and 51402365.References
- T. Ramireddy, M. M. Rahman and N. Sharma, et al., Carbon coated Na7Fe7(PO4)6F3: A novel intercalation cathode for sodium-ion batteries, J. Power Sources, 2014, 271, 497–503 CrossRef CAS.
- C. Sun, S. Rajasekhara and J. B. Goodenough, et al., Monodisperse Porous LiFePO4 Microspheres for a High Power Li-Ion Battery Cathode, J. Am. Chem. Soc., 2011, 133(7), 2132–2135 CrossRef CAS PubMed.
- Y. Janssen, D. S. Middlemiss and S. Bo, et al., Structural Modulation in the High Capacity Battery Cathode Material LiFeBO3, J. Am. Chem. Soc., 2012, 134(30), 12516–12527 CrossRef CAS PubMed.
- M. Konarova and I. Taniguchi, Synthesis of carbon-coated LiFePO4 nanoparticles with high rate performance in lithium secondary batteries, J. Power Sources, 2010, 195(11), 3661–3667 CrossRef CAS.
- E. Kobayashi, L. S. Plashnitsa and T. Doi, et al., Electrochemical properties of Li symmetric solid-state cell with NASICON-type solid electrolyte and electrodes, Electrochem. Commun., 2010, 12(7), 894–896 CrossRef CAS.
- A. Pan, D. Choi and J. Zhang, et al., High-rate cathodes based on Li3V2(PO4)3 nanobelts prepared via surfactant-assisted fabrication, J. Power Sources, 2011, 196(7), 3646–3649 CrossRef CAS.
- T. Jiang, W. Pan and J. Wang, et al., Carbon coated Li3V2(PO4)3 cathode material prepared by a PVA assisted sol–gel method, Electrochim. Acta, 2010, 55(12), 3864–3869 CrossRef CAS.
- M. Minakshi, P. Singh and N. Sharma, et al., Lithium Extraction–Insertion from/into LiCoPO4 in Aqueous Batteries, Ind. Eng. Chem. Res., 2011, 50(4), 1899–1905 CrossRef CAS.
- M. Minakshi, Lithium intercalation into amorphous FePO4 cathode in aqueous solutions, Electrochim. Acta, 2010, 55(28), 9174–9178 CrossRef CAS.
- F. Sauvage, L. Laffont and J. M. Tarascon, et al., Factors affecting the electrochemical reactivity vs. lithium of carbon-free LiFePO4 thin films, J. Power Sources, 2008, 175(1), 495–501 CrossRef CAS.
- M. Manickam, P. Singh and S. Thurgate, et al., Redox behavior and surface characterization of LiFePO4 in lithium hydroxide electrolyte, J. Power Sources, 2006, 158(1), 646–649 CrossRef CAS.
- G. J. Wang, N. H. Zhao and L. C. Yang, et al., Characteristics of an aqueous rechargeable lithium battery (ARLB), Electrochim. Acta, 2007, 52(15), 4911–4915 CrossRef CAS.
- A. Yuan and Q. Zhang, A novel hybrid manganese dioxide/activated carbon supercapacitor using lithium hydroxide electrolyte, Electrochem. Commun., 2006, 8(7), 1173–1178 CrossRef CAS.
- A. Eftekhari, Electrochemical behavior of thin-film LiMn2 aqueous media, Electrochim. Acta, 2001, 47, 495–499 CrossRef CAS.
- J. Lee and S. Pyun, Investigation of lithium transport through LiMn2O4 film electrode in aqueous LiNO3 solution, Electrochim. Acta, 2004, 49(5), 753–761 CrossRef CAS.
- J. Luo and Y. Xia, Aqueous Lithium-ion Battery LiTi2(PO4)3/LiMn2O4 with High Power and Energy Densities as well as Superior Cycling Stability, Adv. Funct. Mater., 2007, 17(18), 3877–3884 CrossRef CAS.
- F. Risacher and B. Fritz, Origin of Salts and Brine Evolution of Bolivian and Chilean Salars, Aquat. Geochem., 2009, 15(1–2), 123–157 CrossRef CAS.
- A. Yaksic and J. E. Tilton, Using the cumulative availability curve to assess the threat of mineral depletion: The case of lithium, Resour. Policy, 2009, 34(4), 185–194 CrossRef.
- Y. Cao, L. Xiao and M. L. Sushko, et al., Sodium Ion Insertion in Hollow Carbon Nanowires for Battery Applications, Nano Lett., 2012, 12(7), 3783–3787 CrossRef CAS PubMed.
- P. Senguttuvan, G. Rousse and V. Seznec, et al., Na2Ti3O7: Lowest Voltage Ever Reported Oxide Insertion Electrode for Sodium Ion Batteries, Chem. Mater., 2011, 23(18), 4109–4111 CrossRef CAS.
- S. Monica and L. L. Shaw, Advances and challenges of sodium ion batteries as post lithium ion batteries, RSC Adv., 2015, 5, 53129–53154 RSC.
- R. Berthelot, D. Carlier and C. Delmas, Electrochemical investigation of the P2-NaxCoO2 phase diagram, Nat. Mater., 2010, 10(1), 74–80 CrossRef PubMed.
- Y. Cao, L. Xiao and W. Wang, et al., Reversible Sodium Ion Insertion in Single Crystalline Manganese Oxide Nanowires with Long Cycle Life, Adv. Mater., 2011, 23(28), 3155–3160 CrossRef CAS PubMed.
- T. H. J. U. Sebastian Wenzel, Room-temperature sodium-ion batteries: Improving the rate capability of carbon anode materials by templating strategies, Energy Environ. Sci., 2011, 4, 3342–3345 Search PubMed.
- S. Komaba, N. Yabuuchi and T. Nakayama, et al., Study on the Reversible Electrode Reaction of Na1−xNi0.5Mn0.5O2 for a Rechargeable Sodium-Ion Battery, Inorg. Chem., 2012, 51(11), 6211–6220 CrossRef CAS PubMed.
- M. Sathiya, K. Hemalatha and K. Ramesha, et al., Synthesis, Structure, and Electrochemical Properties of the Layered Sodium Insertion Cathode Material:NaNi1/3Mn1/3Co1/3O2, Chem. Mater., 2012, 24(10), 1846–1853 CrossRef CAS.
- S. Oh, S. Myung and J. Hassoun, et al., Reversible NaFePO4 electrode for sodium secondary batteries, Electrochem. Commun., 2012, 22, 149–152 CrossRef CAS.
- J. E. C. N. Prabeer Barpanda and A. J. T. Michel Armand, Structural, Transport, and Electrochemical Investigation of Novel AMSO4F (A = Na, Li; M = Fe, Co, Ni, Mn) Metal Fluorosulphates Prepared Using Low Temperature Synthesis Routes, Inorg. Chem., 2010, 49, 7401–7413 CrossRef PubMed.
- H. Zhuo, X. Wang and A. Tang, et al., The preparation of NaV1−xCrxPO4F cathode materials for sodium-ion battery, J. Power Sources, 2006, 160(1), 698–703 CrossRef CAS.
- Y. Kawabe, N. Yabuuchi and M. Kajiyama, et al., Synthesis and electrode performance of carbon coated Na2FePO4F for rechargeable Na batteries, Electrochem. Commun., 2011, 13(11), 1225–1228 CrossRef CAS.
- T. Jiang, G. Chen and A. Li, et al., Sol–gel preparation and electrochemical properties of Na3V2(PO4)2F3/C composite cathode material for lithium ion batteries, J. Alloys Compd., 2009, 478(1–2), 604–607 CrossRef CAS.
- A. Rudola, K. Saravanan and C. W. Mason, et al., Na2Ti3O7: an intercalation based anode for sodium-ion battery applications, J. Mater. Chem. A, 2013, 1(7), 2653 CAS.
- C. Y. Y. L. Wei Wang, Single crystalline Na2Ti3O7 rods as an anode material for sodium-ion batteries, RSC Adv., 2013, 3, 1041–1044 RSC.
- L. Zhao, J. Zhao and Y. Hu, et al., Disodium Terephthalate (Na2C8H4O4) as High Performance Anode Material for Low-Cost Room-Temperature Sodium-Ion Battery, Adv. Energy Mater., 2012, 2(8), 962–965 CrossRef CAS.
- C. Chen, F. Kun and L. Yao, et al., Use of a tin antimony alloy-filled porous carbon nanofiber composite as an anode in sodium-ion batteries, RSC Adv., 2015, 5, 30793–30800 RSC.
- M. Xu, C. Cheng and Q. Sun, et al., A 3D porous interconnected NaVPO4F/C network: preparation and performance for Na-ion batteries, RSC Adv., 2015, 5(50), 40065–40069 RSC.
- V. O. Palomares, P. Serras and I. Villaluenga, et al., Na-ion batteries, recent advances and present challenges to become low cost energy storage systems, Energy Environ. Sci., 2012, 5, 5884–5901 CAS.
- M. Choi, K. Kang and H. Kim, et al., The effect of titanium in Li3V2(PO4)3/graphene composites as cathode material for high capacity Li-ion batteries, RSC Adv., 2015, 5(7), 4872–4879 RSC.
- M. Ren, Z. Zhou and Y. Li, et al., Preparation and electrochemical studies of Fe-doped Li3V2(PO4)3 cathode materials for lithium-ion batteries, J. Power Sources, 2006, 62(2), 1357–1362 CrossRef.
- Y. Chen, Y. Zhao and X. An, et al., Preparation and electrochemical performance studies on Cr-doped Li3V2(PO4)3 as cathode materials for lithium-ion batteries, Electrochim. Acta, 2009, 54(24), 5844–5850 CrossRef CAS.
- C. Dai, Z. Chen and H. Jin, et al., Synthesis and performance of Li3(V1−xMgx)2(PO4)3 cathode materials, J. Power Sources, 2010, 195(17), 5775–5779 CrossRef CAS.
- Q. Kuang, Y. Zhao and X. An, et al., Synthesis and electrochemical properties of Co-doped Li3V2(PO4)3 cathode materials for lithium-ion batteries, Electrochim. Acta, 2010, 55(5), 1575–1581 CrossRef CAS.
- S. M. Stankov, I. Abrahams and A. Momchilov, et al., Effect of Ti-doping on the electrochemical performance of lithium vanadium(III) phosphate, Ionics, 2015, 21(6), 1501–1508 CrossRef CAS.
- A. R. Burke, C. R. Brown and W. C. Bowling, et al., Ignition mechanism of the titanium–boron pyrotechnic mixture, Surf. Interface Anal., 1988, 11(6–7), 353–358 CrossRef CAS.
- M. Choi, K. Kang and H. Kim, et al., The effect of titanium in Li3V2(PO4)3/graphene composites as cathode material for high capacity Li-ion batteries, RSC Adv., 2015, 5(7), 4872–4879 RSC.
- G. Li, D. Jiang and H. Wang, et al., Glucose-assisted synthesis of Na3V2(PO4)3/C composite as an electrode material for high-performance sodium-ion batteries, J. Power Sources, 2014, 265, 325–334 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2018 |