Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
The synthesis of a Bi2MoO6/Bi4V2O11 heterojunction photocatalyst with enhanced visible-light-driven photocatalytic activity†
Chol-Nam Ri *ab,
Song-Gol Kima,
Kyong-Sik Juc,
Hyok-Su Ryod,
Chol-Ho Muna and
U-Hyon Kima
*ab,
Song-Gol Kima,
Kyong-Sik Juc,
Hyok-Su Ryod,
Chol-Ho Muna and
U-Hyon Kima
aInstitute for Electronic Materials, Kim Il Sung University, Pyongyang, Democratic People's Republic of Korea
bSchool of Chemistry and Chemical Engineering, Harbin Institute of Technology, Harbin, 150001, China. E-mail: rcn906@yahoo.com
cInstitute of Advanced Science, Kim Il Sung University, Pyongyang, Democratic People's Republic of Korea
dFaculty of Physics, Kim Il Sung University, Pyongyang, Democratic People's Republic of Korea
First published on 31st January 2018
Abstract
A novel Bi2MoO6/Bi4V2O11 heterostructured photocatalyst was successfully fabricated using a facile one-pot solvothermal method. This heterojunction consists of homogeneous dispersed Bi4V2O11 nanocrystals anchored on the surface of Bi2MoO6 nanoflakes, endowing the heterojunction with nanosized interfacial contact. Based on the favorable interfacial contact, the band alignment at the heterojunction effectively facilitated photo-generated carrier transfer, which was verified by photoelectrochemical and photoluminescence measurements. Thereby, in contrast with pristine Bi2MoO6 and Bi4V2O11, the as-synthesized heterojunction with nanoscale contact exhibited significantly enhanced photocatalytic activity towards the degradation of MB and the reduction of Cr(VI). In addition, the as-fabricated Bi2MoO6/Bi4V2O11 heterojunction exhibited good cycling stability for MB degradation after 4 cycles. Finally, a plausible photocatalytic mechanism for MB degradation over the Bi2MoO6/Bi4V2O11 heterojunction was discussed in detail. This work not only reports a highly efficient photocatalyst but also sheds light on the design and optimization of a heterojunction.
1. Introduction
In recent years, water pollution has become a serious problem all around the world. A large number of pollutants, such as organic dyes, heavy metal ions, drugs etc. are discharged into both industrial waste water and domestic sewage. Until now, the conventional water treatment methods such as adsorption, coagulation, microbial degradation, and ultra-filtration were commonly used to treat waste water, however, this approach has the disadvantage of low removal efficiency and difficulty in removing low concentrations of contaminants.1–3 As a kind of green energy technology, semiconductor photocatalytic technology can remove all kinds of pollutants under the irradiation of sunlight and thus, has attracted a large amount of attention. Photocatalysis possesses a number of advantages, such as the room-temperature oxidation of contaminants even at low concentrations, reduced secondary pollution, non-toxicity and low-cost, which is suitable for the degradation of contaminants.4,5 To date, titanium dioxide (TiO2) is undoubtedly considered to be the most exceptional photocatalyst for solar energy conversion and environmental applications under UV illumination (λ < 400 nm). As is well known, among the solar spectrum, ultraviolet-light makes up only less than 5%, while visible-light (760 nm> λ > 400 nm) makes up approximately 40%. However, the major drawback of TiO2 is the wide band gap (3.2 eV), which greatly reduces the efficiency of solar energy utilization and limits its commercial applications.6,7 Therefore, over the past few decades, a great deal of effort has been made to exploit more efficient visible-light-responding photocatalysts in which Bi-based semiconductors have attracted a substantial amount of attention due to their peculiar electronic structure and the low cost of raw materials.8Recently, as an example of a Bi-based semiconductor, Bi2MoO6 has been regarded as a promising photocatalyst because of its narrow band gap (2.5–2.8 eV), high chemical stability and non-toxicity. Unfortunately, the rapid recombination of photo-induced electron–hole pairs and sluggish charge transport in pristine Bi2MoO6 greatly restrict its photocatalytic activity.9,10 To overcome the obstacles of rapid charge carrier recombination, many strategies have been developed, such as controlling the morphology,11 doping12 and constructing a heterojunction. Among them, the method of constructing a heterojunction has been the most popular, since it can boost charge carrier separation and transfer efficiently originating from the as-introduced built-in electric field at the heterostructured interface. To date, different types of Bi2MoO6-based composite photocatalysts, such as g-C3N4/Bi2MoO6,13 BiOCl/Bi2MoO6,14 BiOBr/Bi2MoO6,15 BiOI/Bi2MoO6,16 Bi2MoO6/TiO2,17 α-Fe2O3/Bi2MoO6,18 Bi2O3/Bi2MoO6,19 Bi2S3/Bi2MoO6,20 CdS/Bi2MoO6 (ref. 21) Bi2MoO6/Bi2WO6 (ref. 22) etc. have been fabricated with enhanced photocatalytic efficiency.
Very recently, Bi4V2O11 with a peculiar layered structure that has intrinsic oxygen vacancies in perovskite (VO3.5□0.5)2− (□ represents the intrinsic oxygen vacancies) slabs sandwiched between (Bi2O2)2+ layers, has been considered as an excellent photocatalyst for oxygen evolution and water purification.23–25 Because of the narrow band gap (∼2.1 eV) and excellent charge carrier transport originating from its unique crystalline structure, Bi4V2O11 has been employed to construct heterojunction photocatalysts, such as BiVO4/Bi4V2O11 (ref. 26) and Bi24O31Br10/Bi4V2O11.27 All of above heterojunctions realize considerably enhanced photocatalytic activity due to the effective separation and transfer of photo-induced charge carriers. However, to the best of our knowledge, the construction of a Bi2MoO6/Bi4V2O11 heterojunction has not been reported, which might enhance the photocatalytic properties of Bi2MoO6 considerably.
In this study, a Bi2MoO6/Bi4V2O11 heterojunction was fabricated using a one-pot solvothermal method. This heterojunction was constructed from Bi4V2O11 nanocrystals anchored on the Bi2MoO6 nanoflakes surface, achieving a nanosized interfacial contact. The photocatalytic performance of the as-fabricated pristine Bi2MoO6, Bi4V2O11 and different Bi2MoO6/Bi4V2O11 heterojunctions was evaluated by the photocatalytic degradation of methylene blue (MB) and photocatalytic reduction of Cr(VI) under visible-light illumination. The results indicated that the Bi2MoO6/Bi4V2O11 heterojunction exhibited superior photocatalytic activity than pristine Bi2MoO6 and Bi4V2O11. Furthermore, the as-fabricated Bi2MoO6/Bi4V2O11 heterojunction exhibited good cycling stability for MB degradation after 4 cycles. Finally, the enhanced photocatalytic mechanism of the Bi2MoO6/Bi4V2O11 heterojunction has also been discussed.
2. Experimental
2.1 Photocatalysts preparation
All chemicals reagents were received from Aladdin Chemical Co. Ltd. and used without further purification.2 mmol of Bi(NO3)3·5H2O was added into 15 mL of ethylene glycol and magnetically stirred in a water bath at 80 °C to form a clear solution. Then, appropriate stoichiometric amounts of (NH4)6Mo7O24·4H2O and NH4VO3 were added into the abovementioned bismuth nitrate solution, which was continuously stirred for 20 min at 80 °C to obtain a transparent solution. Subsequently, the pH of the above solution was adjusted to about 8 by the slow dropwise addition of 2 M sodium hydroxide solution. Then, the abovementioned solution was poured into a 25 mL Teflon-lined stainless autoclave, which was subsequently sealed and maintained at 160 °C for 16 h in an oven. After the reactor cooled down to room temperature, the as-obtained product was centrifuged three times using water and absolute ethanol.
Finally, the photocatalyst was obtained after drying at 80 °C in air for 4 h. According to the abovementioned method, Bi2MoO6/Bi4V2O11 heterojunction photocatalysts with different molar amounts of Mo and V at 0.2 mmol and 0.8 mmol (denoted as BMV-28), 0.4 mmol and 0.6 mmol (denoted as BMV-46), 0.5 mmol and 0.5 mmol (denoted as BMV-55), and 0.6 mmol and 0.4 mmol (denoted as BMV-64), respectively, were prepared. In addition, pristine Bi2MoO6 and Bi4V2O11 were also fabricated for the purpose of comparison.
2.2 Characterization
The crystalline structures of the as-fabricated samples were analyzed using X-ray diffraction (XRD) on a Rigaku D/max-2000 diffractometer with Cu Kα radiation (λ = 1.5406 Å) in the range of 2ϑ = 20–90° at a scanning rate of 4 °C min−1 with a scan width of 0.02°. The morphology of the samples was observed using field emission scanning electron microscopy (FE-SEM, HELIOS NanoLab 600i) at an accelerating voltage of 20 kV. The transmission electron microscopy (TEM) and high-resolution TEM (HRTEM) analyses were carried out on a JEM-2100 transmission electron microscope at an accelerating voltage of 200 kV. The X-ray photoelectron spectroscopy (XPS) was conducted on a Thermo Scientific ESCALAB 250Xi X-ray photoelectron spectrometer coupled with a pass energy of 20.00 eV and an Al Kα excitation source (1486.6 eV). The UV-vis diffuse reflectance spectra (DRS) were obtained on a spectrophotometer (HITACHI UH-4150) using BaSO4 as the reflectance standard. Photoluminescence (PL) analysis was accomplished on a HORIBA FluoroMax-4.2.3 Photocatalytic activity and photoelectrochemical measurements
The photocatalytic performance of the as-fabricated samples was evaluated by the degradation of methylene blue (MB) and reduction of Cr(VI) under visible-light illumination using a 300 W Xe lamp (Trusttech PLS-SXE 300, Beijing) with a UV cut-off filter (λ ≥ 400 nm). In a typical photocatalytic process, 0.05 g of the as-fabricated sample was added into 100 mL of MB solution (10 mg L−1) or Cr(VI) solution (10 mg L−1, which was based on Cr in a dilute K2Cr2O7 solution). The photocatalyst was dispersed in the above solution under ultrasonic treatment for 10 min, and the mixed solution was magnetically stirred in the dark for 30 min to achieve an adsorption–desorption equilibrium between the photocatalyst and the pollutants. When the photodegradation experiment began, 4 mL of the solution was collected from the suspension at fixed-time intervals, centrifuged at 104 rpm for 5 min to remove catalyst powders, and the MB and Cr(VI) concentrations were determined using a HITACHI UH-5300 UV-vis spectrometer at 664 and 352 nm, respectively. The photoelectrochemical measurements were carried out on a CHI604C electrochemical workstation using a standard three-compartment cell with the Bi4V2O11, Bi2MoO6 and Bi2MoO6/Bi4V2O11 samples coated on FTO glass used as the working electrode, a piece of Pt sheet as the counter electrode, standard Ag/AgCl in saturated KCl as the reference electrode, and a 0.5 M Na2SO4 aqueous solution as the electrolyte. Converting between the measured potential (vs. Ag/AgCl) and NHE was achieved using eqn (1).28| ENHE = EAg/AgCl + 0.1976 (25 °C) | (1) |
The light source employed was a 300 W Xe lamp. The Mott–schottky measurements were carried out at a frequency of 100 Hz and an amplitude of 10 mV.
3. Results and discussion
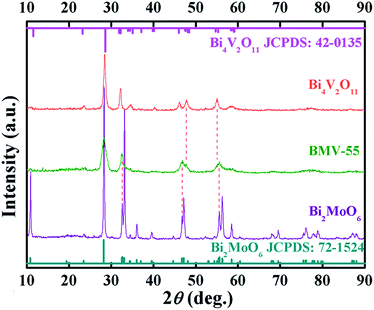
Fig. 1 shows the XRD patterns of the as-prepared pristine Bi2MoO6 and Bi4V2O11 samples, and the BMV-55 composite sample. The diffraction peaks of the pristine Bi2MoO6 and Bi4V2O11 samples can be well indexed to the orthorhombic phase Bi2MoO6 (JCPDS No. 72-1524) and the orthorhombic phase Bi4V2O11 (JCPDS No. 42-0135), respectively. The XRD pattern of the BMV-55 composite contains both Bi2MoO6 and Bi4V2O11 phases, indicating the coexistence of the two phases in the BMV-55 composite in which the extremely weak diffraction intensity of Bi4V2O11 can be ascribed to its tiny size and uniform dispersion on the surface of Bi2MoO6. In addition, the XRD patterns of the BMV-28, BMV-46 and BMV-64 samples are shown in Fig. S1 in the ESI.† It can be seen that the diffraction peaks of BMV-28 and BMV-46 samples belong to the characteristic peaks of Bi4V2O11, while no peaks corresponding to Bi2MoO6 can be detected, which might be attributed to the relatively lower content of Bi2MoO6 in the samples. Meanwhile, the diffraction peaks of BMV-64 samples correspond to those of Bi4V2O11; however, the peak intensities are significantly weakened relative to those of pristine Bi4V2O11. The morphology and microstructure of the samples are investigated using SEM, TEM and HRTEM. Fig. 2 shows the SEM images of the as-fabricated samples. The FESEM image in Fig. 2a shows that pristine Bi2MoO6 possesses an irregular flake-like morphology with a thickness of ca. 55 nm. In contrast, pristine Bi4V2O11 (Fig. 2b) displays hierarchical microspheres with diameters of approximately 1–2 μm. In addition, Fig. 2c shows the morphology of the BMV-55 composite, showing that the BMV-55 composite has a fluffy structure stacked in a disorderly manner by the nanoflakes. Moreover, the SEM images of the BMV-28, BMV-46 and BMV-64 samples are shown in Fig. S2 in the ESI.† To obtain a more detailed structural information about the BMV-55 composite, TEM analysis has been carried out. The TEM images of the BMV-55 composite are shown in Fig. 3. The TEM image of the BMV-55 composite at low magnification (Fig. 3a) illustrates that the BMV-55 composite is comprised of nanoflakes with a thickness of about 10 nm and length of about 100–200 nm, which is consistent with the SEM results described above. Interestingly, as observed from the magnified TEM image (Fig. 3b), nanocrystals with a size of ca. 10 nm are homogeneously dispersed and tightly adhered to the surface of the nanoflakes. By observing the HRTEM image of the BMV-55 composite (Fig. 3c), it can be further confirmed that the nanoflakes are Bi2MoO6 and the as-anchored nanocrystals are Bi4V2O11 because the observed interplanar spacings of 0.811 nm and 0.312 nm correspond well to the (020) plane of the orthorhombic Bi2MoO6 and the (113) plane of the orthorhombic Bi4V2O11, respectively. These results suggest that the Bi4V2O11 nanocrystals are embedded on the Bi2MoO6 nanoflakes in the BMV-55 composite, forming a Bi2MoO6/Bi4V2O11 heterojunction with a nanosized interfacial contact. With the aim of further verifying the coexistence of Bi2MoO6 and Bi4V2O11, surface electronic states analysis of the BMV-55 composite have been performed using the X-ray photoelectron spectroscopy (XPS). Fig. 4 shows the high-resolution XPS spectra of the as-fabricated BMV-55 composite photocatalyst. The full survey spectra and C 1s spectra of BMV-55 are shown in Fig. S3 in the ESI.†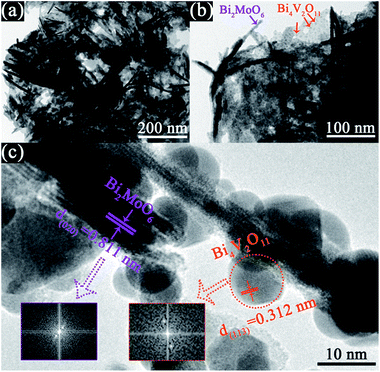 | ||
| Fig. 3 TEM images of the BMV-55 composite. The (a) TEM image at low magnification, (b) magnified TEM image, and (c) HRTEM image of the BMV-55 composite. | ||
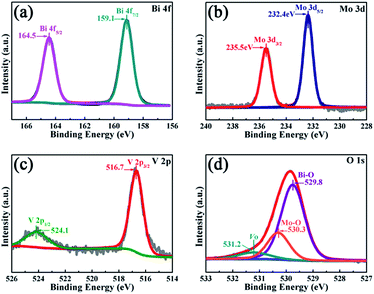
The peak positions in all of the XPS spectra were calibrated using the C 1s peak (284.6 eV) as the reference. First, the two characteristic peaks located at 164.5 and 159.1 eV in the Bi 4f spectra (Fig. 4a) were assigned to Bi 4f5/2 and Bi 4f7/2, respectively, which corresponded to Bi3+.29 Then, in the Mo 3d XPS spectra (Fig. 4b), the binding energies for Mo 3d3/2 and Mo 3d5/2 located at 235.5 and 232.4 eV, respectively were assigned to Mo6+ in Bi2MoO6.9,29 Furthermore, for the V 2p peaks (Fig. 4c), the two peaks located at 524.1 and 516.7 eV were assigned to V 2p1/2 and V 2p3/2, respectively and belonged to V5+ in Bi4V2O11.27,30 Moreover, as shown in Fig. 4d, the O 1s spectra could be divided into three peaks, and the binding energies located at 529.8, 530.3 and 531.2 eV could be assigned to the lattice oxygen in Bi–O,13 lattice oxygen in Mo–O,18 and intrinsic oxygen vacancy (Vo) in Bi4V2O11,31 respectively. The XPS results further indicated the simultaneous existence of Bi2MoO6 and Bi4V2O11 species in the BMV-55 composite photocatalyst. In a bid to investigate the optical absorption capacity of the as-prepared photocatalysts, UV-vis diffuse reflection spectra (DRS) analysis was carried out and is displayed in Fig. 5. As can be seen from Fig. 5a, the absorption edges of pristine Bi2MoO6, Bi4V2O11 and the BMV-55 composite were observed at about 475, 624 and 600 nm, respectively. Obviously, the absorption edge of the BMV-55 composite exhibited a red shift compared with that of pristine Bi2MoO6, which illustrated that the introduction of Bi4V2O11 was conducive to enhancing the light absorption capacity of the BMV-55 composite. In addition, the band gaps of pristine Bi2MoO6 and Bi4V2O11 were calculated using the Kubelka–Munk function (eqn (2)).32
| αhν = A(hν − Eg)n/2 | (2) |
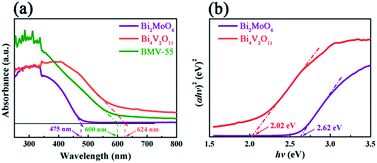 | ||
| Fig. 5 The (a) UV-vis diffuse reflectance spectra (DRS) and (b) (αhν)2 vs. hν curves of the pristine Bi2MoO6 and Bi4V2O11 samples, and the BMV-55 composite. | ||
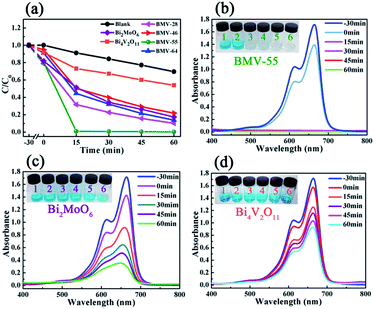
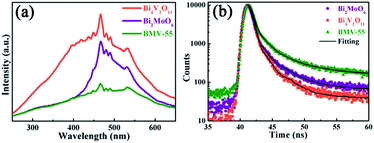
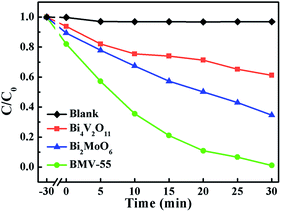
Obviously, the photocatalytic activity of BMV-55 in the absence of H2O2 is much lower than that found upon adding H2O2 into the system of which the degradation rate is only 75% after 90 min, indicating that H2O2 plays a key role in our photocatalytic reactions. To further study the photocatalytic application of the as-fabricated photocatalysts, we have also used a toxic heavy metal ion, Cr(VI), as a model pollutant, and we have conducted the photocatalytic reduction of Cr(VI) over the pristine Bi2MoO6 and Bi4V2O11 samples, and the BMV-55 composite samples with the results illustrated in Fig. 7. As can be seen from the figure, the BMV-55 composite shows a distinctly enhanced photocatalytic activity for the reduction of Cr(VI) compared with pristine Bi2MoO6 and Bi4V2O11, demonstrating that our heterojunction photocatalysts have a good application in wastewater purification. It is speculated that the highest activity of the BMV-55 composite could be associated with the higher separation and transport efficiency of the photo-induced carriers originating from the construction of the heterojunction between the Bi2MoO6 nanoflakes and Bi4V2O11 nanocrystals. Aiming at confirming this viewpoint, two important approaches, including photoelectrochemical and photoluminescence (PL) analyses, have been used to evaluate the separation and transport efficiency of photo-induced carriers. Fig. 8 illustrates the results of the electrochemical measurements consisting of the photocurrent and electrochemical impedance spectroscopy (EIS) over the as-prepared pristine Bi2MoO6 and Bi4V2O11 samples, and the BMV-55 composite, under visible irradiation. As demonstrated in Fig. 8a, the BMV-55 composite exhibits a remarkably enhanced photocurrent response in striking contrast with the pristine Bi2MoO6 and Bi4V2O11 samples. In detail, the photocurrent intensity of the BMV-55 composite is approximately 8- and 18-fold larger than that of pristine Bi2MoO6 and Bi4V2O11, implying that the separation and transport of the photoexcited charge carriers can be effectively promoted by the construction of the heterogeneous structure by anchoring the Bi4V2O11 nanocrystals on the Bi2MoO6 nanoflakes. Fig. 8b shows the EIS Nyquist plots. Apparently, the BMV-55 composite exhibits a smaller arc radius relative to that of the pristine Bi2MoO6 and Bi4V2O11 samples, indicating the significantly enhanced interfacial charge carrier transport ability of the BMV-55 composite. These results can furnish the valid evidence that the BMV-55 composite possesses facilitated charge carrier separation and transport properties compared with pristine Bi2MoO6 and Bi4V2O11. To shed light on the recombination rate and fluorescence lifetime of the photo-induced charge carriers, PL analysis has been performed, as shown in Fig. 9. As depicted in Fig. 9a, the BMV-55 composite exhibits a lower PL emission intensity compared with pristine Bi2MoO6 and Bi4V2O11 samples, manifesting that the recombination of the photo-induced charge carriers is effectively suppressed in the Bi2MoO6/Bi4V2O11 heterojunction. Moreover, Fig. 9b presents the time-resolved fluorescence decay spectra monitored at 460 nm under an excitation wavelength of 345 nm and the corresponding fitting curve of pristine Bi2MoO6 and Bi4V2O11, and the BMV-55 composite. Obviously, the decay kinetics of the BMV-55 composite are slower when compared to those of pristine Bi2MoO6 and Bi4V2O11. Consequently, the radiative lifetimes are extracted by means of a reconvolution fit using a 3-exponential model (eqn (3)).34
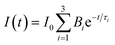 | (3) |
![[small tau, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0d4.gif) is calculated using eqn (4).34
is calculated using eqn (4).34
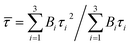 | (4) |
 | ||
| Fig. 7 The photocatalytic Cr(VI) reduction plots over pristine Bi2MoO6 and Bi4V2O11, and the BMV-55 composite samples under visible-light irradiation. | ||
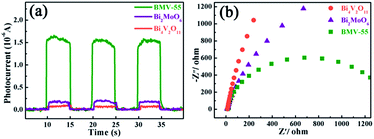 | ||
| Fig. 8 The (a) transient photocurrent plots and (b) electrochemical impedance spectroscopy (EIS) Nyquist plots obtained for the pristine Bi2MoO6 and Bi4V2O11, and the BMV-55 composite samples. | ||
The best fitting curve and kinetic parameters observed for pristine Bi2MoO6 and Bi4V2O11, and the BMV-55 composite are displayed in Fig. S5 in the ESI.† In detail, the average-lifetime of carriers in pristine Bi2MoO6 and Bi4V2O11 were shortened to ca. 1.983 and 0.487 ns, respectively. In contrast, the average-lifetime of the BMV-55 composite was distinctly prolonged to 7.059 ns, indicating that the formation of the Bi2MoO6/Bi4V2O11 heterojunction is tremendously beneficial to prolong the radiative lifetimes of the photo-induced charge carriers, and the greatly prolonged lifetime of the charge carriers could be closely related to the high separation efficiency of the charge carriers. The results of the PL spectra and time-resolved fluorescence decay spectra analysis further reveal that the BMV-55 composite exhibits a significantly enhanced separation and transport efficiency for the photo-induced charge carriers relative to those of pristine Bi2MoO6 and Bi4V2O11, which is very consistent with the results of the photoelectrochemical analysis.
From a practical point of view, the cycle stability of the photocatalysts is a very important factor in evaluating the photocatalytic performance. To evaluate the cycle stability, a cycling experiment for the photodegradation of MB over the BMV-55 composite upon adding 0.1 mL of H2O2 was carried out. Prior to the next cycle experiment, the previously used photocatalyst was subjected to ultrasonic cleaning with distilled water and drying at 60 °C. Fig. 10 depicts the results of the recycling experiment after 4 cycles. It was be clearly seen that the photodegradation rate of MB for 15 minutes remained above 95% after 4 cycles, which indicated that the as-fabricated BMV-55 composite photocatalyst possessed good cycle stability. For the purpose of unraveling the mechanism of the enhanced photocatalytic activity of the Bi2MoO6/Bi4V2O11 heterojunction, the energy band structures of pristine Bi2MoO6 and Bi4V2O11 were investigated by determining their band gaps and flat-band potentials. Using the above UV-vis diffuse reflection spectra (DRS) analysis (Fig. 5b), the band gaps of pristine Bi2MoO6 and Bi4V2O11 were determined to be 2.62 and 2.02 eV, respectively.
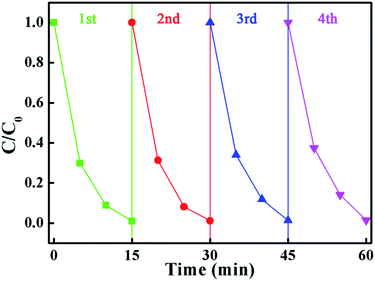 | ||
| Fig. 10 The results of the recycling experiment after 4 cycles for the photodegradation of MB in the presence of H2O2 over the BMV-55 composite sample. | ||
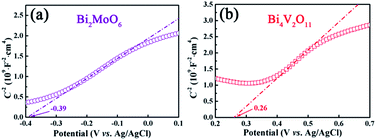
In addition, the flat-band potentials of pristine Bi2MoO6 and Bi4V2O11 are determined via Mott–Schottky analysis, and the results are shown in Fig. 11. It can be seen that both of them show a positive slop, indicating that they are all n-type semiconductors. In addition, the flat-band potentials of pristine Bi2MoO6 and Bi4V2O11 are ca. −0.39 and 0.26 V, respectively, vs. Ag/AgCl (−0.19 and 0.46 V vs. NHE), which indicates that the flat-band potential of Bi2MoO6 is more negative than that of Bi4V2O11 by 0.65 V. According to the semiconductor theory, the conduction band potential (ECB) of n-type semiconductors is very close to (0.1–0.2 V more negative) their flat-band potential.35 Therefore, it can be concluded that the CB position of Bi2MoO6 is more negative than that of Bi4V2O11. Based on above results, possible photo-induced electron–hole pair separation and the photocatalytic degradation of MB mechanism over the Bi2MoO6/Bi4V2O11 heterojunction with the assistance of H2O2 as an electron-trapping agent are proposed, as shown in Scheme 1. On one hand, because of the fact that the CB potential of Bi2MoO6 is more negative than that of Bi4V2O11, the photo-induced electrons can easily migrate from the CB of Bi2MoO6 to the CB of Bi4V2O11 driven by the built-in electric field at the heterostructured interface; it could be then captured by H2O2 and reacted to generate hydroxide radicals (˙OH) with strong oxidizing ability, which will completely degrade MB. On the other hand, since the VB potentials of Bi2MoO6 (2.43 V) and Bi4V2O11 (2.48 V) are very close to each other, the migration of the photo-induced holes is very difficult, leading to the holes staying in their valence band positions.36 In this way, the rapid migration of the photo-induced electrons and the immobilization of the photo-induced holes can lead to the effective separation of the photo-induced electrons and holes over the Bi2MoO6/Bi4V2O11 heterojunction, which ultimately results in the enhanced photocatalytic activity observed during the photodegradation of MB.
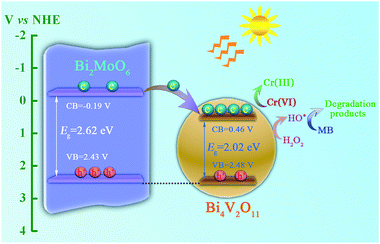 | ||
| Scheme 1 A schematic illustration of the photo-induced charge carrier separation and photocatalytic reaction mechanism over the Bi2MoO6/Bi4V2O11 heterojunction. | ||
4. Conclusions
In summary, a novel visible-light responsive Bi2MoO6/Bi4V2O11 heterojunction photocatalyst with a nanosized interfacial contact has been successfully fabricated using a facile one-pot solvothermal method. The Bi2MoO6/Bi4V2O11 heterojunctions show excellent photocatalytic MB degradation efficiency under visible-light illumination. In particular, the BMV-55 composite exhibits significantly enhanced photocatalytic activity during the photodegradation of MB and photoreduction of Cr(VI) compared with pristine Bi2MoO6 and Bi4V2O11. In addition, the Bi2MO6/Bi4V2O11 heterojunctions also display an enhanced transient photocurrent response, lower electrochemical impedance, lower PL intensity and greatly prolonged lifetime, which indicates that the photo-induced charge carriers are more effectively separated and transferred in the Bi2MoO6/Bi4V2O11 heterojunctions. The significantly enhanced photocatalytic activity and charge carrier separation effect are attributed to the formation of a heterojunction with a nanosized interfacial contact between the Bi2MoO6 nanoflakes and Bi4V2O11 nanocrystals. Moreover, the Bi2MoO6/Bi4V2O11 heterojunctions exhibit excellent cycle stability after 4 cycles. This work may be further extended to the research and development of a novel semiconductor heterojunction containing a new kind of Bi4V2O11 photocatalyst for water purification and related applications.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by the National Nature Science Foundation of China (21501035).References
- S. Vadivel, D. Maruthamani, A. Habibi-Yangjeh, B. Paul, S. S. Dhar and K. Selvam, J. Colloid Interface Sci., 2016, 480, 126 CrossRef CAS PubMed.
- H. Y. Li, S. Y. Gan, H. Y. Wang, D. X. Han and L. Niu, Adv. Mater., 2015, 27, 6906 CrossRef CAS PubMed.
- S. Y. Dong, J. L. Feng, M. H. Fan, Y. Q. Pi, L. M. Hu, X. Han, M. L. Liu, J. Y. Sun and J. H. Sun, RSC Adv., 2015, 5, 14610 RSC.
- J. W. Tang, Z. G. Zou and J. H. Ye, Angew. Chem., 2004, 116, 4563 CrossRef.
- A. D. Paola, E. García-López, G. Marcì and L. Palmisano, J. Hazard. Mater., 2012, 211–212, 3 CrossRef PubMed.
- L. Zhao, X. F. Chen, X. C. Wang, Y. J. Zhang, W. Wei, Y. H. Sun, M. Antonietti and M. M. Titirici, Adv. Mater., 2010, 22, 3317 CrossRef CAS PubMed.
- B. Huang, W. J. Yang, Y. W. Wen, B. Shan and R. Chen, ACS Appl. Mater. Interfaces, 2015, 7, 422 CAS.
- R. A. He, S. W. Cao, P. Zhou and J. G. Yu, Chin. J. Catal., 2014, 35, 989 CrossRef CAS.
- Y. S. Xu and W. D. Zhang, Dalton Trans., 2013, 42, 1094 RSC.
- M. Y. Zhang, C. L. Shao, J. B. Mu, X. M. Huang, Z. Y. Zhang, Z. C. Guo, P. Zhang and Y. C. Liu, J. Mater. Chem., 2012, 22, 577 RSC.
- G. H. Tian, Y. J. Chen, W. Zhou, K. Pan, Y. Z. Dong, C. G. Tian and H. G. Fu, J. Mater. Chem., 2011, 21, 887 RSC.
- Z. Dai, F. Qin, H. P. Zhao, J. Ding, Y. L. Liu and R. Chen, ACS Catal., 2016, 6, 3180 CrossRef CAS.
- H. P. Li, J. Y. Liu, W. G. Hou, N. Du, R. J. Zhang and X. T. Tao, Appl. Catal., B, 2014, 160–161, 89 CrossRef CAS.
- D. Yue, D. M. Chen, Z. H. Wang, H. Ding, R. L. Zong and Y. F. Zhu, Phys. Chem. Chem. Phys., 2014, 16, 26314 RSC.
- S. Y. Wang, X. L. Yang, X. H. Zhang, X. Ding, Z. X. Yang, K. Dai and H. Chen, Appl. Surf. Sci., 2017, 391, 194 CrossRef CAS.
- T. Yan, M. Sun, H. Y. Liu, T. T. Wu, X. J. Liu, Q. Yan, W. G. Xu and B. Du, J. Alloys Compd., 2015, 634, 223 CrossRef CAS.
- M. Y. Zhang, C. L. Shao, J. B. Mu, Z. Y. Zhang, Z. C. Guo, P. Zhang and Y. C. Liu, CrystEngComm, 2012, 14, 605 RSC.
- J. Zhao, Q. F. Lu, M. Z. Wei and C. Q. Wang, J. Alloys Compd., 2015, 646, 417 CrossRef CAS.
- Y. S. Xu, Z. J. Zhang and W. D. Zhang, Mater. Res. Bull., 2013, 48, 1420 CrossRef CAS.
- J. L. Zhang, L. S. Zhang, N. Yu, K. B. Xu, S. J. Li, H. L. Wang and J. S. Liu, RSC Adv., 2015, 5, 75081 RSC.
- Y. Feng, X. Yan, C. B. Liu, Y. Z. Hong, L. Zhu, M. J. Zhou and W. D. Shi, Appl. Surf. Sci., 2015, 353, 87 CrossRef CAS.
- F. J. Zhang, S. F. Zhu, F. Z. Xie, J. Zhang and Z. D. Meng, Sep. Sci. Technol., 2013, 113, 1 CAS.
- X. F. Chen, J. B. Liu, H. Wang, Y. L. Ding, Y. X. Sun and H. Yan, J. Mater. Chem. A, 2013, 1, 877 CAS.
- Z. Y. Jiang, Y. Y. Liu, M. M. Li, T. Jing, B. B. Huang, X. Y. Zhang, X. Y. Qin and Y. Dai, Sci. Rep., 2016, 6, 22727 CrossRef CAS PubMed.
- S. Kumar and P. D. Sahare, Nano, 2013, 8, 1350007 CrossRef.
- C. D. Lv, G. Chen, J. X. Sun, Y. S. Zhou, S. Fan and C. M. Zhang, Appl. Catal., B, 2015, 179, 54 CrossRef CAS.
- Z. S. Liu, J. N. Niu, P. Z. Feng, Y. W. Sui and Y. B. Zhu, RSC Adv., 2014, 4, 43399 RSC.
- X. Yang, X. J. Lian, S. J. Liu, J. Tian, C. P. Jiang and G. Wang, Int. J. Electrochem. Sci., 2013, 8, 3721 CAS.
- J. Di, J. X. Xia, M. X. Ji, H. P. Li, H. Xu, H. M. Li and R. Chen, Nanoscale, 2015, 7, 11433 RSC.
- Y. T. Lu, Y. F. Pu, J. Wang, C. X. Qin, C. L. Chen and H. J. Seo, Appl. Surf. Sci., 2015, 347, 719 CrossRef CAS.
- K. K. Banger, Y. Yamashita, K. Mori, R. L. Peterson, T. Leedham, J. Rickard and H. Sirringhaus, Nat. Mater., 2011, 10, 45 CrossRef CAS PubMed.
- J. Cao, B. Y. Xu, H. L. Lin, B. D. Luo and S. F. Chen, Dalton Trans., 2012, 41, 11482 RSC.
- W. S. D. Santos, L. D. Almeida, A. S. Afonso, M. Rodriguez, J. P. Mesquita, D. S. Monteiro, L. C. A. Oliveira, J. D. Fabris and M. C. Pereira, Appl. Catal., B, 2016, 182, 247 CrossRef.
- H. L. Gao, S. C. Yan, J. J. Wang, Y. A. Huang, P. Wang, Z. S. Li and Z. G. Zou, Phys. Chem. Chem. Phys., 2013, 15, 18077 RSC.
- C. M. Li, G. Chen, J. X. Sun, H. J. Dong, Y. Wang and C. D. Lv, Appl. Catal., B, 2014, 160–161, 383 CrossRef CAS.
- J. Su, X. X. Zou, G. D. Li, X. Wei, C. Yan, Y. N. Wang, J. Zhao, L. J. Zhou and J. S. Chen, J. Phys. Chem. C, 2011, 115, 8064 CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7ra12766a |
| This journal is © The Royal Society of Chemistry 2018 |