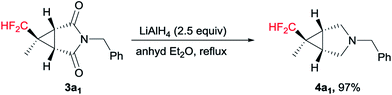Open Access Article
Open Access ArticleSynthesis of CHF2-substituted 3-azabicyclo[3.1.0]hexanes by photochemical decomposition of CHF2-pyrazolines†
Yang Zhenga,
Xinling Yua,
Songyang Lva,
Pavel K. Mykhailiuk bc,
Qiang Maa,
Li Hai*a and
Yong Wu
bc,
Qiang Maa,
Li Hai*a and
Yong Wu *a
*a
aKey Laboratory of Drug-Targeting of Education Ministry, Department of Medicinal Chemistry, West China School of Pharmacy, Sichuan University, Chengdu 610041, China. E-mail: smile.hl@hotmail.com; wyong@scu.edu.cn
bEnamine Ltd., Matrosova 23, 01103 Kyiv, Ukraine. E-mail: Pavel.Mykhailiuk@mail.enamine.net; Web: www.enamine.net
cTaras Shevchenko National University of Kyiv Chemistry Department, Volodymyrska 64, 01601 Kyiv, Ukraine. E-mail: Pavel.Mykhailiuk@gmail.com
First published on 29th January 2018
Abstract
A practical synthesis of CHF2-substituted 3-azabicyclo[3.1.0]hexanes was developed for the first time. The key step was photochemical decomposition of CHF2-substituted pyrazolines. This protocol has the advantages of simple operation, and mild conditions, as well as excellent functional group tolerance, giving the desired products in moderate to excellent yields.
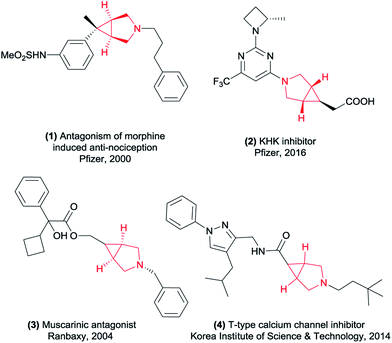
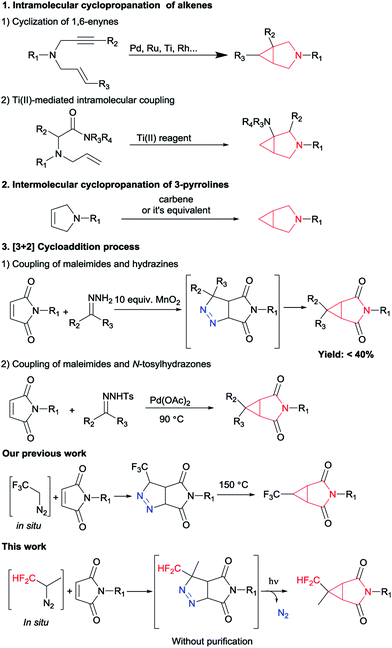
The 3-azabicyclo[3.1.0]hexyl ring system as a conformationally constrained bicyclic isostere for the piperidine motif displays diverse biological activities and great potential in the pharmaceutical industry.1 Representative examples of this type of application are potent μ opioid receptor antagonist 1 for the treatment of pruritus,2 the ketohexokinase (KHK) inhibitor 2 for the treatment of non-alcoholic fatty liver disease (NAFLD),3 muscarinic receptor antagonist 3 (ref. 4) and T-type calcium channel inhibitor 4 (ref. 5) (Fig. 1). In this context, considerable effort has been devoted to developing general and efficient methods for the synthesis of the 3-azabicyclo[3.1.0]hexane scaffolds (Scheme 1). The elegant early studies focused on the intramolecular cyclopropanation, such as metal-catalyzed oxidative cyclization of 1,6-enynes6 and cyclopropanation of N-allylamino acid dimethylamides using Ti(II) reagents.7 Furthermore, the intermolecular cyclization of 3-pyrrolines and metal carbenoids was also a useful tool to construct this core structure.8 Although these known procedures had their merits, they were also associated with some drawbacks. For example, requiring long routes for the starting materials preparation and/or using expensive metal catalytic systems. In the last few years, [3 + 2] cycloaddition process has been the most popular method to construct this cyclopropane ring of 3-azabicyclo[3.1.0]hexanes. The coupling of maleimides and hydrazines has been reported by Lunn's group.9 And Jiang and co-workers have disclosed a method of palladium-catalyzed cyclopropanation of maleimides and N-tosylhydrazones.10 To match the increasing scientific and practical demands, it is still of continued interest and great importance to explore new and straightforward methods to access these highly rigid cyclopropanes with more simple operation.
On the other hand, decoration of organic molecules with fluorinated groups often affects their physicochemical and biological properties such as metabolic stability and lipophilicity,11 so organofluorine compounds are widespread in pharmaceuticals, agrochemicals, and advanced functional materials. Among all fluorine-containing groups, difluoromethyl group can develop special effects on molecules: it can be used as a bioisostere of a carbinol moiety and as a more lipophilic hydrogen bond donor.12 However, protocols for the synthesis of difluoromethylated azabicyclo[3.1.0]hexanes remain to be underexplored. In line with previous work from our group dealing with the preparation of difluoromethyl-substituted pyrazolines using in situ generated difluoromethyl diazomethane,13 we report herein the development of a simple and efficient method to synthesize CHF2-substituted 3-azabicyclo[3.1.0]hexane derivatives from commercially available maleimides.
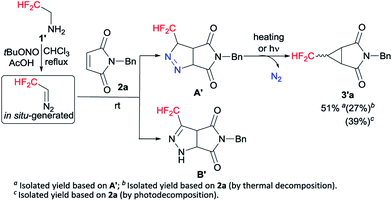
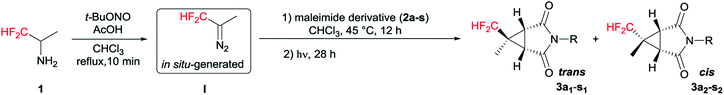
Following our previously established protocol,14 we initially examined the thermal decomposition of pyrazoline A′, and the desired 3′a was observed in 51% yield, but 3′a was obtained only in 27% yield based on 1-benzyl-1H-pyrrole-2,5-dione 2a (see Scheme S1 and S2†). Considering the low yield and the tedious operation, we tried to develop a one-pot cascade approach (see Table S1†). Accidentally, we discovered that photochemical process was more effective than thermal decomposition. As we all know, photochemistry is considered as one of the simplest manifolds of chemical reactivity and photochemical reactions are the key for the synthesis of many reactive intermediates.15 Accordingly, this phenomenon attracted our attention. On the other hand, considering the quantitative formation of the isomeric Δ2-pyrazoline B′ in the [3 + 2] cycloaddition process, we decided to prevent the formation of the byproduct B′ by taking the simplest higher homologue – CF2H(CH3)CNH2 (Scheme 2).
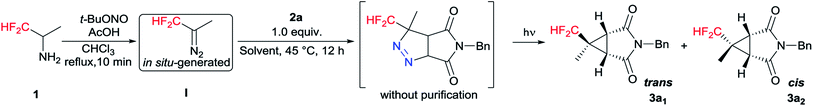
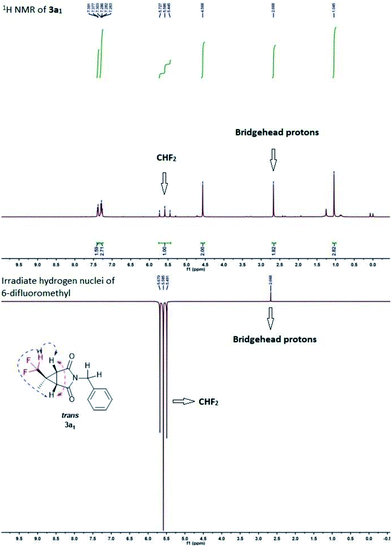
Initial tests were done on CF2H(CH3)CHN2 I generated in situ and 1-benzyl-1H-pyrrole-2,5-dione 2a as the model substrates.16 To our delight, both trans (3a1) and cis (3a2) products were isolated separately and the yields of 3a1 and 3a2 were 39% and 12%, respectively (Table 1, entry 1). The stereochemistry was determined on the basis of the results from NMR spectroscopy (see Fig. S1†), and an example of which was shown for 3a1 in Fig. 2. The bridgehead protons were correlated through space to the nearby difluromethyl protons, indicating they were on the same convex side of the bicyclic system. Encouraged by this result, we further optimized the reaction conditions, and the results were showed in Table 1. When the amount of 1 was increased to 3.0 equiv., increasing yield of 3a was obtained, but further increases resulted in dramatically decreasing yield (entries 2 and 3). Further examination of the solvents indicated that MeCN was the optimum solvent, and the other solvents, such as THF, DMSO·Et2O, (i-Pr)2O and i-PrOMe gave inferior results (entries 4–8). When MeCN was used as the solvent, the desired products 3a1 and 3a2 were produced in 56% and 14% yields, respectively. Lamp power was also a crucial factor in this system. Increasing the lamp power to 1000 W could significantly promote the result of this reaction, and the total yield of 3a could reach to 80% (entries 10–13). At the same time, the reaction time was optimized (entries 14–16), and 28 hours was found to be optimum affording 3a1 and 3a2 in 66% and 16%, respectively. The experimental results also revealed that changing the reaction conditions had little effect on the diastereomeric ratio.
| Entry | 1 (equiv.) | t-BuONO (equiv.) | AcOH (equiv.) | Solvent | Lamp power (W) | Time (h) | Yieldb (%) | drc | |
|---|---|---|---|---|---|---|---|---|---|
| 3a1 | 3a2 | ||||||||
| a Reaction conditions: a solution of 1-methyl-2,2-difluoroethanamine 1 in CHCl3 and t-BuONO and HOAc were added in turn. After 10 min heating, the obtained yellow solution was cooled down to a room temperature by external water bath. Then 2a was added into the reaction mixture and stirred at 45 °C. After removing CHCl3, the residue was dissolved in 5 mL solvent and transferred into a quartz tube which was irradiated with a high-pressure mercury lamp (250–720 nm).b Isolated yield by chromatography on silicagel.c Diastereomeric ratio of 3a1 and 3a2 was based on column chromatography.d This is the maximum power of this lamp. | |||||||||
| 1 | 2.0 | 2.4 | 0.4 | Toluene | 500 | 24 | 39 | 12 | 78![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 22 22 |
| 2 | 3.0 | 3.6 | 0.6 | Toluene | 500 | 24 | 51 | 14 | 78![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 22 22 |
| 3 | 4.0 | 4.8 | 0.8 | Toluene | 500 | 24 | 44 | 14 | 76![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 24 24 |
| 4 | 3.0 | 3.6 | 0.6 | THF | 500 | 24 | 29 | 13 | 69![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 31 31 |
| 5 | 3.0 | 3.6 | 0.6 | DMSO | 500 | 24 | n.d. | n.d. | — |
| 6 | 3.0 | 3.6 | 0.6 | Et2O | 500 | 24 | 52 | 14 | 79![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 21 21 |
| 7 | 3.0 | 3.6 | 0.6 | (i-Pr)2O | 500 | 24 | 36 | 12 | 75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25 25 |
| 8 | 3.0 | 3.6 | 0.6 | i-PrOMe | 500 | 24 | 51 | 14 | 78![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 22 22 |
| 9 | 3.0 | 3.6 | 0.6 | MeCN | 500 | 24 | 56 | 14 | 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 20 |
| 10 | 3.0 | 3.6 | 0.6 | MeCN | 400 | 24 | 50 | 16 | 76![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 24 24 |
| 11 | 3.0 | 3.6 | 0.6 | MeCN | 600 | 24 | 58 | 15 | 79![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 21 21 |
| 12 | 3.0 | 3.6 | 0.6 | MeCN | 800 | 24 | 61 | 16 | 79![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 21 21 |
| 13 | 3.0 | 3.6 | 0.6 | MeCN | 1000d | 24 | 64 | 16 | 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 20 |
| 14 | 3.0 | 3.6 | 0.6 | MeCN | 1000 | 20 | 63 | 16 | 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 20 |
| 15 | 3.0 | 3.6 | 0.6 | MeCN | 1000 | 28 | 66 | 16 | 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 20 |
| 16 | 3.0 | 3.6 | 0.6 | MeCN | 1000 | 32 | 64 | 17 | 79![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 21 21 |
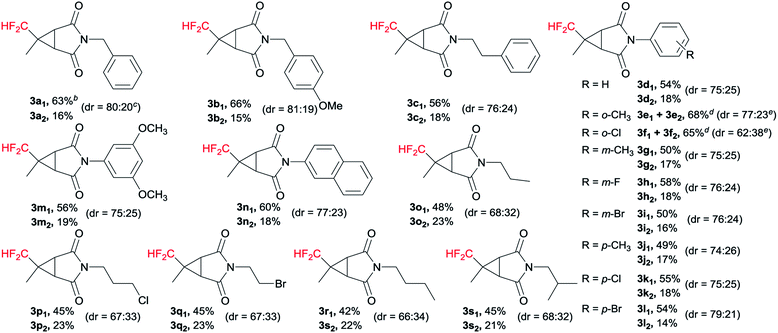
With the optimized reaction conditions in hand, the generality of maleimides in this cyclopropanation reaction was examined, and the results were summarized in Table 2. It was worth mentioning that both of the diastereoisomers could be easily isolated by silica gel chromatography. Benzyl, p-methoxybenzyl and phenylethyl groups were compatible with the present conditions, obtaining excellent to good yields and diastereoselectivity (3a1–c1 and 3a2–c2). Various electron-donating (2e, 2g, 2j, 2m) and electron-withdrawing groups (2f, 2h, 2i, 2k, 2l) at the different positions on the phenyl ring of maleimides 2 were fully tolerated. For instance, 1-(3,5-dimethoxyphenyl)-1H-pyrrole-2,5-dione (2m) gave the diastereoisomers 3m1 and 3m2 in 56% and 19% yields, respectively. And we were pleased to observe that a number of maleimides having alkyl substituents at the N-position were highly facile to afford the desired N-protected products (3o1–s1 and 3o2–s2) in good yields and diastereoselectivity. For example, N-(n-propyl) maleimide 2o was 71% in yield and 68![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 32 in diastereomeric ratio.
32 in diastereomeric ratio.
| a Reaction conditions: a solution of 1-methyl-2,2-difluoroethanamine 1 (0.1 M, 3.0 eq.) in CHCl3 and t-BuONO (3.6 eq.) and HOAc (0.6 eq.) were added in turn. After 10 min heating, the obtained yellow solution was cooled down to a room temperature by external water bath, and 2 (1.0 eq.) was added immediately. The reaction mixture was stirred at 45 °C for 12 h. After removing CHCl3, the residue was dissolved by acetonitrile (5 mL) and transferred into a quartz tube which was irradiated with a 1000 W high-pressure mercury lamp for 28 h.b Isolated yield.c Diastereomeric ratio of trans and cis products was based on column chromatography.d Isolated yield combined trans and cis products.e Diastereomeric ratio was based on 1H NMR. |
|---|
 |
We further explored its application for the synthesis of the 3-azabicyclo[3.1.0]hexane scaffold. To our satisfaction, the representative reduction of the carbonyl groups in 3a1 with LiAlH4 in diethyl ether smoothly gave the pyrrolidine 4a1 in nearly quantitative yield (Scheme 3).14 It is worth mentioning that no cyclopropane ring cleavage was observed during this reaction.
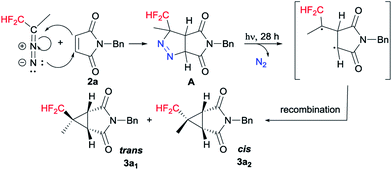
Based on the experimental results and the previous literature,17 a plausible mechanistic pathway is depicted in Scheme 4 with 2a as a model substrate. Mechanistically, [3 + 2] cycloaddition is carried out with CF2H(CH3)CHN2 and 2a to generate pyrazoline A. Subsequently the photodenitrogenation of pyrazoline occurs by the stepwise cleavage of the two C–N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–C bonds to give a 1,3-biradical. Finally, the 1,3-biradical recombines to give cyclopropane diastereoisomers 3a1 and 3a2.
N–C bonds to give a 1,3-biradical. Finally, the 1,3-biradical recombines to give cyclopropane diastereoisomers 3a1 and 3a2.
In summary, we have developed a general and efficient method for the synthesis of CHF2-substituted 3-azabicyclo[3.1.0]hexane derivatives via photochemical process of commercially available maleimides and in situ generated CF2H(CH3)CHN2. It is worth mentioning that both of the diastereoisomers could be easily isolated by silica gel chromatography. This protocol has the advantages of simple operation, mild conditions as well as excellent functional group tolerance. We believe that this method will expand the synthetic arsenal in the field of medicinal chemistry, agrochemistry and organic synthesis.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
We are grateful for support from the National Natural Science Foundation of China (NSFC) (grant number 81373259, 81573286 & 81773577).Notes and references
- (a) T. A. Blizzard, F. DiNinno, J. D. Morgan 2nd, H. Y. Chen, J. Y. Wu, C. Gude, S. Kim, W. Chan, E. T. Birzin, Y. T. Yang, L. Y. Pai, Z. Zhang, E. C. Hayes, C. A. DaSilva, W. Tang, S. P. Rohrer, J. M. Schaeffer and M. L. Hammond, Bioorg. Med. Chem. Lett., 2004, 14, 3861 CrossRef CAS PubMed; (b) G. Lunn, L. R. Roberts, S. Content, D. J. Critcher, S. Douglas, A. E. Fenwick, D. M. Gethin, G. Goodwin, D. Greenway, S. Greenwood, K. Hall, M. Thomas, S. Thompson, D. Williams, G. Wood and A. Wylie, Bioorg. Med. Chem. Lett., 2012, 22, 2200 CrossRef CAS PubMed; (c) A. R. Renslo, H. Gao, P. Jaishankar, R. Venkatachalam, M. Gomez, J. Blais, M. Huband, J. V. Vara Prasad and M. F. Gordeev, Bioorg. Med. Chem. Lett., 2006, 16, 1126 CrossRef CAS PubMed; (d) S. P. Runyon, C. M. Kormos, M. G. Gichinga, S. W. Mascarella, H. A. Navarro, J. R. Deschamps, G. H. Imler and F. I. Carroll, J. Org. Chem., 2016, 81, 10383 CrossRef CAS PubMed; (e) L. M. Simpkins, S. Bolton, Z. Pi, J. C. Sutton, C. Kwon, G. Zhao, D. R. Magnin, D. J. Augeri, T. Gungor, D. P. Rotella, Z. Sun, Y. Liu, W. S. Slusarchyk, J. Marcinkeviciene, J. G. Robertson, A. Wang, J. A. Robl, K. S. Atwal, R. L. Zahler, R. A. Parker, M. S. Kirby and L. G. Hamann, Bioorg. Med. Chem. Lett., 2007, 17, 6476 CrossRef CAS PubMed; (f) Z. Xiang, A. D. Thompson, J. T. Brogan, M. L. Schulte, B. J. Melancon, D. Mi, L. M. Lewis, B. Zou, L. Yang, R. Morrison, T. Santomango, F. Byers, K. Brewer, J. S. Aldrich, H. Yu, E. S. Dawson, M. Li, O. McManus, C. K. Jones, J. S. Daniels, C. R. Hopkins, X. S. Xie, P. J. Conn, C. D. Weaver and C. W. Lindsley, ACS Chem. Neurosci., 2011, 2, 730 CrossRef CAS PubMed.
- S. F. McHardy, S. D. Heck, S. Guediche, M. Kalman, M. P. Allen, M. H. Tu, D. K. Bryce, A. W. Schmidt, M. Vanase-Frawley, E. Callegari, S. Doran, N. J. Grahame, S. McLean and S. Liras, Med. Chem. Commun., 2011, 2, 1001 RSC.
- M. Dowling, D. Fernando, K. Futatsugi, K. Huare, T. V. Magee, B. Raymer, A. Smith, B. Thuma, A. Tsai and M. H. Tu, US pat. 20170183328, 2017Chem. Abstr., 2017, 167, 151561m.
- A. Ray, S. G. Dastidar, R. Shirumalla and S. Malhotra, WO patent 2007045979, 2007Chem. Abstr., 2007, 146, 468545m.
- J. H. Kim and G. Nam, Bioorg. Med. Chem., 2016, 24, 5028 CrossRef CAS PubMed.
- (a) W. F. Wang, J. M. Yang, F. J. Wang and M. Shi, Organometallics, 2011, 30, 3859 CrossRef CAS; (b) F. Monnier, B. C. Vovard-Le, D. Castillo, V. Aubert, S. Derien, P. H. Dixneuf, L. Toupet, A. Ienco and C. Mealli, J. Am. Chem. Soc., 2007, 129, 6037 CrossRef CAS PubMed; (c) D. Qian and J. Zhang, Chem. Commun., 2011, 47, 11152 RSC; (d) W. Wang, J. Yang, F. Wang and M. Shi, Organometallics, 2011, 30, 3859 CrossRef CAS; (e) Z. Zhang and M. Shi, Eur. J. Org. Chem., 2011, 2011, 2610 CrossRef.
- (a) R. F. Boswell and R. G. Bass, J. Org. Chem., 1977, 42, 2342 CrossRef CAS; (b) T. Kimura, N. Wada, T. Tsuru, T. Sampei and T. Satoh, Tetrahedron, 2015, 71, 5952 CrossRef CAS.
- (a) B. Cao, D. Xiao and M. M. Joullie, Org. Lett., 1999, 1, 1799 CrossRef CAS; (b) M. Gensini, S. I. Kozhushkov, D. S. Yufit, J. A. K. Howard, M. Es-Sayed and A. de Meijere, Eur. J. Org. Chem., 2002, 2499 CrossRef CAS.
- G. Lunn, B. J. Banks, R. Crook, N. Feeder, A. Pettman and Y. Sabnis, Bioorg. Med. Chem. Lett., 2011, 21, 4608 CrossRef CAS PubMed.
- P. Chen, C. Zhu, R. Zhu, Z. Lin, W. Wu and H. Jiang, Org. Biomol. Chem., 2017, 15, 1228 CAS.
- (a) S. Purser, P. R. Moore, S. Swallow and V. Gouverneur, Chem. Soc. Rev., 2008, 37, 320 RSC; (b) S. D. Halperin, D. Kwon, M. Holmes, E. L. Regalado, L. C. Campeau, D. A. DiRocco and R. Britton, Org. Lett., 2015, 17, 5200 CrossRef CAS PubMed; (c) J. Miao, K. Yang, M. Kurek and H. Ge, Org. Lett., 2015, 17, 3738 CrossRef CAS PubMed; (d) D. O'Hagan, J. Fluorine Chem., 2010, 131, 1071 CrossRef; (e) S. Purser, P. R. Moore, S. Swallow and V. Gouverneur, Chem. Soc. Rev., 2008, 37, 320 RSC.
- Y. Zafrani, D. Yeffet, G. Sod-Moriah, A. Berliner, D. Amir, D. Marciano, E. Gershonov and S. Saphier, J. Med. Chem., 2017, 60, 797 CrossRef CAS PubMed.
- J. Li, X. L. Yu, J. Cossy, S. Y. Lv, H. L. Zhang, F. Su, P. K. Mykhailiuk and Y. Wu, Eur. J. Org. Chem., 2017, 2017, 266 CrossRef CAS.
- O. S. Artamonov, E. Y. Slobodyanyuk, O. V. Shishkin, I. V. Komarov and P. K. Mykhailiuk, Synthesis, 2013, 45, 225 CAS.
- (a) A. V. Denisenko, T. Druzhenko, Y. Skalenko, M. Samoilenko, O. O. Grygorenko, S. Zozulya and P. K. Mykhailiuk, J. Org. Chem., 2017, 82, 9627 CrossRef CAS PubMed; (b) C.-C. Wang, Y.-C. Ku and G. J. Chuang, J. Org. Chem., 2015, 80, 10979 CrossRef CAS PubMed; (c) A. M. Linsenmeier, C. M. Williams and S. Brase, J. Org. Chem., 2011, 76, 9127 CrossRef CAS PubMed.
- Firstly, CF2H(CH3)CHN2 I was prepared by 2.0 mmol CF2H(CH3)CHNH2 1, based on our previous work. 13 Subsquently, 2a (1.0 mmol) was added and the reaction mixture was stirred at 45 °C for 12 h. After removing CHCl3, the residue was dissolved by toluene (5 mL) and transferred into a quartz tube which was irradiated with a 500 W high-pressure mercury lamp (250–720 nm). See ESI† for more details.
- (a) S. Shiraki, C. S. Vogelsberg and M. A. Garcia-Garibay, Photochem. Photobiol. Sci., 2012, 11, 1929 RSC; (b) M. Abe, J. Ye and M. Mishima, Chem. Soc. Rev., 2012, 41, 3808 RSC; (c) L. Mertens and R. M. Koenigs, Org. Biomol. Chem., 2016, 14, 10547 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7ra13141k |
| This journal is © The Royal Society of Chemistry 2018 |